Background: The human retrosplenial cortex (RSC) is the cortical region of the parietal lobe that lies directly behind the splenium of the corpus callosum. While recent fundamental research in animal models has suggested a very important role for the RSC in orientation, relatively few human cases of lesion to the RSC have been reported in the literature. Furthermore, those few cases have pointed at, in addition to the aforementioned role of this cortical structure in topographical orientation, a potential for other supplementary roles in higher-order cognitive functions such as memory processing and language.
Cases presentation: In this article, we report two remarkably asymptomatic cases of extensive oncological lesions to the RSC in otherwise healthy patients younger than 35 years old. We report their surgical treatments and their release from our hospital. During the entire follow-up, patients never reported any issue with topographical orientation or language, which is remarkable compared to cases previously reported.
Discussion and conclusions: We then discuss the available clinical literature about RSC lesions in human brain and the possible reasons for such a diversity of reported symptoms, or lack thereof, between cases for a seemingly similar radiological entity. Our cases reveal that understanding of the RSC and its lesions remain scarce and needs to be further investigated, both fundamentally and clinically.
retrosplenial cortex, glioblastoma, memory, topographical orientation, asymptomatic
RSC: Retrosplenial cortex; GBM: Glioblastoma; WHO: World Health Organization; MRI: Magnetic resonance images; KPS: Karnofsky Performance Score.
The human retrosplenial cortex (RSC) is the portion of the parietal lobe that lies immediately posteriorly to the splenium of the corpus callosum [1]. This anatomical structure has been reported to have several important connections with other cortical and subcortical regions of the brain [1,2]. Fundamental research in the rat has suggested a very important role of RSC in orientation in both known and unknown environments [3,4] and reported human cases seem to confirm this role [5-7]. However, very interestingly, early on other cases such as the one reported by Valenstein and colleagues [8] seemed to also suggest a role for the human RSC in memory processes, with lesions leading to a so-called ‘retrosplenial amnesia’ and very little is still known on this region from a clinical point of view. Last-but-not-least, RSC lesion has also been associated with language difficulties [9]. To date there are no epidemiological data regarding the prevalence or the age at the occurrence of the lesion of such a region as well as the different symptoms, or lack thereof, associated with it. Moreover, few cases in total have been reported in the entire literature and the majority consists of elderly patients. In this article, we report two new cases of lesion of the RSC, this time in patients younger than 35 years old. Surprisingly and contrarily to all the cases reported previously, no symptoms were reported by the patients in orientation and memory processes. We here describe these two cases and discuss the literature about RSC lesions, emphasizing on the lack of knowledge from the clinical point of view on this extremely complicated region of the human brain.
Case 1
A 33 years old Chinese right-handed woman with no past medical history presented to her local hospital with headache, dizziness, nausea and vomiting for 20 days. Magnetic resonance images (MRI) revealed an intracranial mass (of 2.7 × 2.5 × 2.5 cm) for which she was referred to our department. Upon admission, physical examination and more particularly neurological examination did not reveal any other symptom than the ones described previously with a well-oriented and fluently-speaking patient with Karnofsky Performance Score (KPS) rated at 90. Medical history did not reveal any progressive impairment in cognitive functions in the last year. A new MRI-examination revealed a mass in the right parietal lobe extending to the right lateral ventricle and the splenium of the corpus callosum (Figures 1A and 1B), encompassing 60-70% of the right RSC. Other than that, presence of a cyst in the right maxillary sinus was mentioned. Laboratory tests revealed hypofibrinogenemia and anemia. Removal of the mass was carried as follows: after dura mater opening and exploration, the solid cystic tumor and its blood supply were visualized 2 cm under the cerebral cortex with no clear demarcation between tumor edges and healthy tissue. Upon opening of the cystic component of the tumor, yellow and clear fluid was obtained. Careful separation of the tumor from healthy-appearing tissue with security margins revealed a tumoral extension towards the right parietal lobe, deeply into the occipital horn of the right lateral ventricle and in the corpus callosum. The tumor was fully resected with security margins and final size of resected tissue was 3 × 2.8 × 2.8 cm. Pathological analysis then confirmed the glial origin of the tumor with diagnosis of World Health Organization (WHO) grade 4 glioblastoma. The patient presented no new symptoms or complication and was discharged one week after surgery. At follow-up by phone at one and two months after surgery, patient and her family did not report any new symptom, particularly no impairment in topographical orientation, language or memory skills.
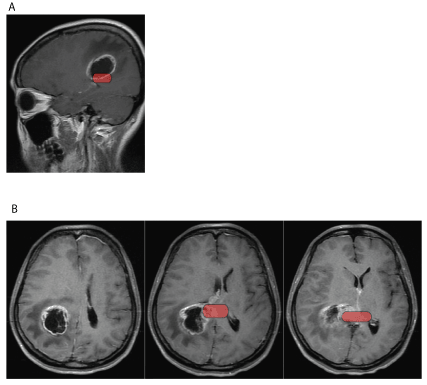
Figure 1. Sagittal (1A) and transversal (1B) gadolinium-injected T1 MRI images of a 33 y.o. female patient showing a (WHO) grade 4 glioblastoma in the right parietal lobe extending to the right lateral ventricle and the splenium of the corpus callosum. In red: position of the RSC
Case 2
A 25 years old Chinese right-handed man with no past medical history (and in particular not indication of a recent worsening in cognitive functions) presented to his local hospital after an episode of rigidity of the left thumb while playing a video game followed by whole body convulsion with screaming and loss of consciousness that lasted for one minute. No mouth foaming nor sphincter incontinence were reported and the episode resolved spontaneously. The patient only presented a post-ictal headache that also resolved before his admission in the hospital. MRI revealed an intracranial mass for which he was referred to our department. Upon admission, physical examination and more particularly neurological examination did not reveal any other symptom with a well-oriented and fluently-speaking patient. A new MRI-examination revealed an expanding mass (measuring 5 × 5 × 4 cm) in the right parietal lobe, extending to the splenium and body of the corpus callosum (Figures 2A and 2B), this time, the lesion was estimated to encompass approximately 50% of the right RSC. Laboratory tests revealed hypofibrinogenemia. Removal of the mass was carried as follows: the solid cystic tumor with blood supply was situated in the right parietal lobe with no clear demarcation between tumor edges and healthy tissue. Inner portion of the tumor was necrotic and hemorrhagic. Tumor was cut along the peritumoral vasogenic brain edema. Examination indicated a cystic portion situated deeper in the brain that prompted opening of the right lateral ventricle, allowing complete removal of the tumor with security margins. Final size of resected tissue was 6 × 6 × 5 cm. Pathological analysis identified the tumor as having two components: partly anaplastic oligoastrocytoma and partly WHO grade 3-4 glioblastoma. During hospitalization following the surgery, the patient presented an infection of the central nervous system at the surgical site that was treated with antibiotics without further complication. He was then finally discharged nine days after surgery. At follow-up by phone at one and two months after surgery, patient and his family did not report any new symptom, particularly no impairment in topographical orientation, language or memory skills.
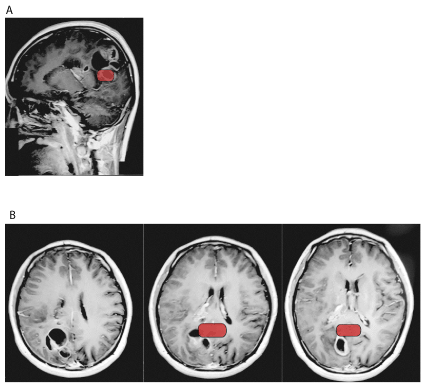
Figure 2. Sagittal (2A) and transversal (2B) gadolinium-injected T1 MRI images of a 25 y.o. male patient showing partly anaplastic oligoastrocytoma and partly WHO grade 3-4 glioblastoma in the right parietal lobe, extending to the splenium and body of the corpus callosum. In red: position of the RSC
The Retrosplenial Cortex (RSC) is described as the portion of the parietal lobe situated directly behind the splenium of the corpus callosum [1] and has been shown to be present in several species including rats, monkeys and humans [1]. This anatomical region has earned great interest in the last decades due to its position, its peculiar organisation at the cellular level and its great number of connections with several cortical and subcortical regions [1,2]. Despite its strong connections to some key structures such as the hippocampus, posterior parietal cortex, subiculum, anterior cingulate and anterior thalamic nuclei and medial entorhinal cortex [10-16], little is still known about the function of this region, particularly in the human brain [1]. Some roles have been suggested by studies conducted in the rats, in which lesion to the RSC has been shown to severely impair ability to navigate within both known and new environment [3]. Several in-vivo electrophysiological studies support this role of RSC in navigation as it was reported to encode head direction [17,18] and, to a lesser degree, spatial position [19-24].
Very interestingly, Pothuizen et al. [4] have shown that the lesion of different regions of the RSC in the rat brought different behavioural abnormalities. In their study, two different lesion groups (lesion of the granular retrosplenial and complete retrosplenial lesion) were both comparably impaired throughout most of radial-arm maze acquisition. However, for one condition (reinforced spatial alternation in a T-maze), the rats with only granular cortex lesions resulted in a larger deficit than the complete RSC lesion [4]. This could be explained by the cellular organization of such different regions, the different connections such regions make with other cortical and subcortical regions or by a combination of those differences and such variety of effect of lesion models warrants further research and development in fundamental research.
Interestingly, when researching the human literature concerning RSC, relatively few cases reporting a lesion in this region and its consequences can be found leading to a surprising lack of certitude regarding the roles of this region in human. However, despite the rarity of these cases, they seem to confirm the suspected roles of the RSC. Indeed, regarding the role of RSC in memory, following Valenstein et al., Takayama et al., Yasuda et al. and Gainotti et al. all reported cases of ‘pure’ retrograde amnesia following lesion in the retrosplenial region after infarction for the former and astrocytoma for the latter [8,25-27]. Such symptoms were also described after lesion of the RSC of the non-dominant hemisphere in a 68 years old patient [28]. Impairment of anterograde memory was also reported after RSC lesion [29-31]. Some authors also reported cases showing an impairment of the components of orientation [5-7]. Alsaadi et al. reported 3 cases of Topographic Disorientation with only one exhibiting a lesion directly in the RSC and the two others showing lesions in others interlinked regions [32] while Greene and colleagues who reported a case of Topographical Heading Disorientation following bilateral lesion of the occipital region including the RSC and argued that the symptoms might arise from a destruction of the connection between RSC and the right posterior parietal lobe [33]. Amnesia and topographic disorientation were even reported concurrently in two cases by Maeshima et al. [34] and Osawa et al. [35]. Finally, a very interesting symptom has been reported in a case by McDonald and colleagues whose patient showed poor verbal encoding scores at testing after a left RSC lesion [9] suggesting a possible role of the left RSC in verbal capabilities.
Glioblastoma (GBM) is the most common primary malignant tumor of the brain (constituting 45-50% of all primary brain tumors), occurs most commonly between the ages of 55 and 85 and is associated with potentially severe symptoms such as epilepsy [36]. Diagnosis of GBM is often suspected by brain imagery (mostly MRI) and confirmed by pathology. Treatment of GBM comprises surgery, radiotherapy, chemotherapy and immunotherapy. However, due to its strong invasiveness and high tendency for recurrence, prognosis of GBM remains poor nowadays, especially in older patients [36,37].
The two cases we present here are relevant because they crystallize the lack of knowledge currently present concerning RSC. Indeed, to our knowledge, there is no known study about the prevalence of RSC lesions in the human brain as well as their type, the age of their occurrence and the associated symptoms. Up till now, human cases seem to confirm the roles suggested by animal research in memory and orientation. However, the number of cases remains too small to draw any definitive conclusion, especially knowing the fact that symptoms can be caused by lesion of other regions (themselves often connected to the RSC). A very interesting point of both our cases is the apparent total absence of the aforementioned symptoms. Indeed, follow-up by phone call at two months post-surgery did not reveal any symptoms in these fields. When asked more specifically, patients did not report any issue with spatial navigation and did not report any condition affecting their quality of life. This could be explained in several ways. First, changes might be far too subtle to be realized by the patients and their family and stricter testing might reveal such a phenotype. This lack of finer neuropsychological testing represents a limitation of our article and it highlights the importance for thorough neuropsychological testing in the case of such brain lesions, both pre- and postoperatively. However, it is very important to note previous cases reported in the literature did not report “slight” but important symptoms greatly reducing quality of life (with patients typically presenting a tendency to lose themselves in previously known environments). Therefore, while we did not finely test spatial navigation, our cases are still relevant, as they did not present with such important impairment in their quality of life.
Another reason might be that time after the lesion is critical; indeed we only followed the patients during two months after the surgery. While majority of RSC lesion case-reports spoke of immediate impairment in the memory or orientation department, symptoms due to the lesion of brain regions functionally related to RSC such as hippocampus have been reported to take time (sometimes many years) to appear [38]. This might point to an evolution with time, possibly due to a modification in the lesion site or a lack of available regional plasticity. The age at the time of the lesion or the type of lesion itself might also be of particular importance. In this article, both our cases were glioblastomas in relatively young patients while majority of the reported symptomatic cases were in older patients with several different types of lesions. Older patients might be more susceptible to lesions of the RSC due to the lowered plasticity of the aged brain. The slow pathological process (compared to acute pathologies such as strokes) might explain why precise inquiring into both patients’ recent medical history did not reveal any cognitive impairment, even a slowly-appearing one as cortical plasticity might have counteracted and mitigated the effect of the tumors on spatial navigation capabilities. Last but not least, as suggested by Pothuizen et al. [4], different RSC regions might be functionally different due to their cellular organization or connections. In the light of this fact, the absence of symptoms in both our cases might come from the fact that the regions lesioned were not eloquent regions or that compensation between regions or hemispheric sides (in this aspect, it is important to note that both our cases suffered from lesion of the right RSC and a predominance of the left RSC cannot be ruled out in these patients, explaining, at least partly, the lack of symptoms). Another explanation for this situation regards the size itself of the lesions, with both patients retaining some intact parts of their RSC. The remaining viable RSC might, indeed, be sufficient to treat spatial and navigational information, leading to an apparent absence of symptoms. This lack of knowledge points to another important point concerning RSC, the risk associated with surgery. Indeed, given the seemingly high complexity of the inter-region connectivity, surgery might present with unexpectedly detrimental outcomes and knowledge of the risks brought by new research might help modify pre-surgical testing, surgical approach and post-surgical follow-up of such patients.
In conclusion, our cases reveal some remaining lack in the knowledge concerning lesions in human RSC. The absence of symptoms of our cases associated to RSC lesions compared to other reported cases is, despite being a very positive outcome, quite astonishing and emphasizes the need for us to not only further research the different roles of the RSC in animal models, but to also develop a more structured approach of this region in humans through anatomical, pathological and behavioural studies. We believe that further fundamental and clinical research in this region is of particular importance to allow better patients’ management in the future.
Ethical approval and consent to participate: all information gathering were conducted in accordance to the institutional ethical guidelines. Patients signed written informed consent for participation in our research.
Competing interests: the authors report no competing interests or any conflict of interests.
Funding: Prof YT (Corresponding Author) was partially supported by the Beijing Municipal Administration of Hospitals’ Youth Program QML2015050; Beijing Natural Science Foundation (7172041). The funding body did not have any role in or influence on the design of the study, collection, analysis, and interpretation of data or in writing the manuscript.
Authors’ contributions: LS, PEC, NL and YT conceived the study. NL and YT identified the patients’ cases and their eligibility. LS and PEC wrote the manuscript and contributed equally to this article. NL contacted the patients and reviewed the medical information. NL, YT and LA critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Acknowledgements: the authors thank Beijing Municipal Administration of Hospitals’ Youth Program and Beijing Natural Science Foundation.
- Vann SD, Aggleton JP, Maguire EA (2009) What does the retrosplenial cortex do? Nat Rev Neurosci 10: 792-802. [Crossref]
- Czajkowski R, Jayaprakash B, Wiltgen B, Rogerson T, Guzman-Karlsson MC, et al. (2014) Encoding and storage of spatial information in the retrosplenial cortex. Proc Natl Acad Sci U S A 111: 8661-8666. [Crossref]
- Maguire EA (2001) The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol 42: 225-238. [Crossref]
- Pothuizen HH, Davies M, Aggleton JP, Vann SD (2010) Effects of selective granular retrosplenial cortex lesions on spatial working memory in rats. Behav Brain Res 208: 566-575. [Crossref]
- Obi T, Bando M, Takeda K, Sakuta M (1992) A case of topographical disturbance following a left medial parieto-occipital lobe infarction. Rinsho Shinkeigaku 32: 426-429. [Crossref]
- Takahashi N, Kawamura M, Shiota J, Kasahata N, Hirayama K (1997) Pure topographic disorientation due to right retrosplenial lesion. Neurology 49: 464-469. [Crossref]
- Ino T, Doi T, Hirose S, Kimura T, Ito J, et al. (2007) Directional disorientation following left retrosplenial hemorrhage: a case report with fMRI studies. Cortex 43: 248-254. [Crossref]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, et al. (1987) Retrosplenial amnesia. Brain 110: 1631-1646. [Crossref]
- McDonald CR, Crosson B, Valenstein E, Bowers D (2001) Verbal encoding deficits in a patient with a left retrosplenial lesion. Neurocase 7: 407-417. [Crossref]
- Vogt BA, Miller MW (1983) Cortical connections between rat cingulate cortex and visual, motor, and postsubicular cortices. J Comp Neurol 216: 192-210. [Crossref]
- van Groen T, Wyss JM (1990) Connections of the retrosplenial granular a cortex in the rat. J Comp Neurol 300: 593-606. [Crossref]
- van Groen T, Wyss JM (1992) Connections of the retrosplenial dysgranular cortex in the rat. J Comp Neurol 315: 200-216. [Crossref]
- Wyss JM, Van Groen T (1992) Connections between the retrosplenial cortex and the hippocampal formation in the rat: a review. Hippocampus 2: 1-11. [Crossref]
- Reep RL, Chandler HC, King V, Corwin JV (1994) Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Exp Brain Res 100: 67-84. [Crossref]
- Van Groen T, Wyss JM (2003) Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol 463: 249-263. [Crossref]
- Czajkowski R, Sugar J, Zhang SJ, Couey JJ, Ye J, et al. (2013) Superficially projecting principal neurons in layer V of medial entorhinal cortex in the rat receive excitatory retrosplenial input. J Neurosci 33: 15779-15792. [Crossref]
- Butler WN, Smith KS, van der Meer MAA, Taube JS (2017) The head-direction signal plays a functional role as a neural compass during navigation. Curr Biol 27: 1259-1267. [Crossref]
- Shinder ME, Taube JS (2019) Three-dimensional tuning of head direction cells in rats. J Neurophysiol 121: 4-37. [Crossref]
- Miller AM, Vedder LC, Law LM, Smith DM (2014) Cues, context, and long-term memory: the role of the retrosplenial cortex in spatial cognition. Front Hum Neurosci 8: 586. [Crossref]
- Nelson AJ, Hindley EL, Haddon JE, Vann SD, Aggleton JP (2014) A novel role for the rat retrosplenial cortex in cognitive control. Learn Mem 21: 90-97. [Crossref]
- Alexander AS, Nitz DA (2015) Retrosplenial cortex maps the conjunction of internal and external spaces. Nat Neurosci 18: 1143-1151. [Crossref]
- Alexander AS, Nitz DA (2017) Spatially periodic activation patterns of retrosplenial cortex encode route sub-spaces and distance traveled. Curr Biol 27: 1551-156. [Crossref]
- Mao D, Kandler S, McNaughton BL, Bonin V (2017) Sparse orthogonal population representation of spatial context in the retrosplenial cortex. Nat Commun 8: 243. [Crossref]
- Mao D, Molina LA, Bonin V, McNaughton BL (2020) Vision and Locomotion Combine to Drive Path Integration Sequences in Mouse Retrosplenial Cortex. Curr Biol 30: 1680-1688. [Crossref]
- Takayama Y, Kamo H, Ohkawa Y, Akiguchi I, Kimura J (1991) A case of retrosplenial amnesia. Rinsho shinkeigaku 31: 331-333. [Crossref]
- Yasuda Y, Watanabe T, Tanaka H, Tadashi I, Akiguchi I (1997) Amnesia following infarction in the right retrosplenial region. Clin Neurol Neurosurg 99: 102-105. [Crossref]
- Gainotti G, Almonti S, Di Betta AM, M.C. S (1998) Retrograde amnesia in a patient with retrosplenial tumour. Neurocase 4: 519-526.
- Maeshima S, Osawa A, Yamane F, Yoshihara T, Kanazawa R, et al. (2014) Retrosplenial amnesia without topographic disorientation caused by a lesion in the nondominant hemisphere. J Stroke Cerebrovasc Dis 23: 441-445. [Crossref]
- Bowers D, Verfaellie M, Valenstein E, Heilman KM (1988) Impaired acquisition of temporal information in retrosplenial amnesia. Brain Cogn 8: 47-66. [Crossref]
- Katai S, Maruyama T, Hashimoto T, Yanagisawa N (1992) A case of cerebral infarction presenting as retrosplenial amnesia. Rinsho shinkeigaku 32: 1281-1287. [Crossref]
- Oka Y, Maeshima S, Morita S, Ishida K, Kunimoto K, et al. (2003) A case of amnesia caused by a subcortical hematoma in the left retrosplenial region. No Shinkei Geka 31: 289-295. [Crossref]
- Alsaadi T, Binder JR, Lazar RM, Doorani T, Mohr JP (2000) Pure topographic disorientation: A distinctive syndrome with varied localization. Neurology 54: 1864-1866. [Crossref]
- Greene KK, Donders J, Thoits T (2006) Topographical heading disorientation: a case study. Appl Neuropsychol 13: 269-274. [Crossref]
- Maeshima S, Ozaki F, Masuo O, Yamaga H, Okita R, et al. (2001) Memory impairment and spatial disorientation following a left retrosplenial lesion. J Clin Neurosci 8: 450-451. [Crossref]
- Osawa A, Maeshima S, Kunishio K (2008) Topographic disorientation and amnesia due to cerebral hemorrhage in the left retrosplenial region. Eur Neurol 59: 79-82. [Crossref]
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, et al. (2016) The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131: 803-820. [Crossref]
- Braun K, Ahluwalia MS (2017) Treatment of glioblastoma in older adults. Curr Oncol Rep 19: 81. [Crossref]
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, et al. (1998) Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia 36: 1217-1238. [Crossref]


