Abstract
This study attempts to apply translational science currently limited to medical and clinical applications to the evolving regulatory science. The study provides a brief review of fundamental aspects of translational medical science and generalization of translational science process including key elements of Best Available Regulatory Science(BARS) and Metrics for Evaluation of Scientific Claims (MERSC). Subsequently a brief review of regulatory science is provided including a brief description of the phases of regulatory science consisting of initial, exploratory and standard operational phases. In the next section elements of translational regulatory science are described. The process starts with conventional scientific assessment during the next “verification” key requirements of BARS/MERSC are identified requiring the identification of all assumptions, judgments, exclusion of societal objective in scientific assessment and related items and application of Jeffersonian Principle by translating scientific issues in a language that is understandable to the affected community. The example of linear non-threshold (LNT) process is used to demonstrate this step. In the third step “translation” the results of the verification are provided to the public particularly the stakeholders. Once these are done, the promulgation step is simplified as the scientific issues are separated from policy issues and science is translated in a language intended for the affected community.
Introduction
Regulatory science is a reasonably new scientific discipline with potentially wide-range of applications. Similarly, translational science is reasonably new but provides a process to enhance the application of scientific findings. This paper attempts to demonstrate that the translational science process as currently used in medicine is also applicable to regulatory science.
There are two distinct categories of translational science. The first category consists of translating scientific materials in a language that is understandable to a specific audience. A traditional example of this category is translating science for inclusion in high school textbooks that includes science of various complexity. As we will see later in this paper, this category is also applicable to the second category of translational science. In contrast to the first category, the second category of translational science (or sciences) emphasizes clinical medicine. The recognition of the significance of translational science in medicine has caused a rapid development of education, research, and development as well as government and private funding support; formation of several professional societies; and establishment of a significant number of scientific publications including several journals devoted to the translational science. As described in the next section of this paper, translational science as currently used applies almost entirely to the medical discipline
Regulatory science addresses how science is used in regulatory and other policy-related processes. Although the regulatory science discipline is traceable to the late 1970s, the first relevant scholarly organization was by the Institute for Regulatory science founded in 1985, and the first regulatory agency to officially use that term was the Food and Drug Administration (FDA) sometimes around 2010. As indicated by, Johannesen et al. (2014), translational process can also be used to expedite the approval of new drugs addressing heart toxicity [1]. One of the first studies that attempted to apply principles of translational science to areas outside of medicine was presented by Dunmire and Moghissi (2015) addressing corrosion issues that the Department of Defense establishment is facing [2]. This paper resulted from the participation of students in a newly established regulatory science program at Georgetown University and starts by briefly describing translational science to be followed with a summary of regulatory science. Subsequently, an attempt is made to describe translational regulatory science.
Translational science
There is a voluminous literature on the fundamentals and application of translational science (or sciences) in medicine, often referred to as translational clinical and related issues. According to Curry (2008) the evolution of translational science goes back to the 1990s [3]. However, it was Elias Zerhouni (2003, 2005, 2006), the then director of the National Institutes of Health (NIH) who established a research program in translational science at NIH and supported its application [4-6]. Eventually Francis Collins [7] who followed Zerhouni as the Director of NIH, established the National Center for the Advancement of Translational Sciences (NCATS), within NIH awarding grants for research and related activities (Austin 2013) [8].
Recognizing the significance of translational science there are more than a dozen scientific journals and several professional societies devoted to translational science including the following:
- International Society for Translational Medicine
- Association of Clinical and Translational Medicine
- European Society for Translational Medicine
Furthermore, virtually all medical schools in the United States have a program in translational science. For example, the University of Texas system has an established a PhD program and several universities outside of the United States have also degrees in translational science including Oxford and Cambridge Universities in the UK, and University of Helsinki, and Aalborg University in Denmark. Similarly, there are several research institutions that include translational science in their research.
Definition of Translational Science: A cursory literature search indicated a significant confusion on the definition of translational science. A generalized definition of translational science is provided by NCATS:
Translational science is the field of investigation focused upon understanding the scientific and operational principles underlying each step of the translational process.
A more practical definition of translational medicine is provided by the European Society for Translational Medicine (Curry 2017) [9] is as follows:
Translational medicine is an interdisciplinary branch of biomedical field supported by three main pillars: Bench-side, bedside, and community
There is a reasonable agreement that translational medical science follows a four- step process expressed as T1 to T4 (Figure 1). During the first step (T1) basic research is translated to its application to humans or “from bench research to patient bedside”. The next step (T2) provides proof of concept by translating research results to new methods of diagnosis, treatment and prevention, in effect expanding the T1 to a larger population. The third step (T3) attempts to translate the results of the second step (T2) to patients by performing controlled studies leading to effective treatment. During the final step (T4) the results of previous steps notably the third step (T3) are translated to the general market including general and global applications.
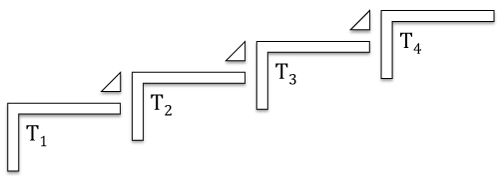
Figure 1: The four steps of translational science
Generalization of translational science: A generalized definition of translational science is as follows:
Translational science attempts to identify a scientific idea, verify its usefulness, and reduce the time to translate the verified idea to its introduction into the market
The generalization of translational science would require the evaluation of lessons learned from the translational experience of the medical profession. Note that the definition of translational science as provided by NCATS is applicable to virtually all scientific disciplines. The information on the four steps included in the description of figure 1 can be generalized by identifying four key elements as shown in (Figure 2):
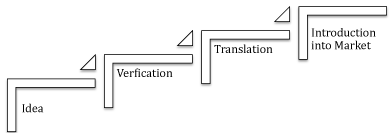
Figure 2: Generalized Translational Science
I: Idea: The process starts by identification of an idea consisting of:
- The results of research as published in peer reviewed scientific journals or peer reviewed reports.
- Information includes the reports that were not subjected to independent peer review.
- Opinions provided by members of the relevant profession.
- Any other accessible relevant information.
II: Verification of the Idea: Probably the most cumbersome part of translational science is the verification process. Key problems of this part are:
- There is sufficient evidence indicating that the results of published research including those published in peer-reviewed journals are not necessarily reproducible. There are several organizations such as Retraction Watch (2017) that regularly publish retraction of published papers in peer-reviewed journals [10]. The verification process ensures the reproducibility of the research results. The verification process is also applicable to an idea included in reports, expressed opinions, and other ideas. It is likely that this latter group is less reproducible than information published in peer reviewed journals.
- Another key issue is the evaluation of the usefulness of the idea. As described above, only a small fraction of ideas pass the usefulness test
III: Translational Activities: One of the key elements of translational science is the translation of the verified idea to a useful product consisting of a device, a drug, a useful chemical agent, a regulation, or any other useful item. As described above the participation of the relevant community is a key to the success of this element.
IV: Introduction into the Market: Once the previous steps are properly managed, the last step; introducing the product into the market; can be implemented reasonably fast.
Regulatory science
Regulatory science is an evolving scientific discipline. It consists of the application of existing scientific disciplines to the regulatory process. In the US the evolution of the regulatory process started in early 20th Century leading to the identification of many applied scientific disciplines. For example, applied toxicology includes regulatory, environmental, forensic, and occupational toxicology to mention a few. In recent years several publications have attempted to identify key elements of regulatory science [11-12]. A generalized version of the definition by the Food and Drug Administration FDA is as follows: Regulatory science is a scientific discipline consisting of the development and application of scientific methods, tools, approaches, and other relevant processes derived from various scientific disciplines used in regulatory and other policy processes including decisions.
A generic definition of regulatory science is as follows: Regulatory science consists of applied versions of various scientific disciplines used in the regulatory process
Although regulatory science covers the applied version of many scientific disciplines that are used in the regulatory process, there are certain key elements ad tools that are common to virtually all regulatory science disciplines:
Best Available Regulatory Science: Metrics for Evaluation of Regulatory Science Claims (MERSC) derived from Best Available Regulatory Science (BARS) are a key element of regulatory science. Among the five principles of BARS (Open-mindedness, Skepticism, Scientific Rules, Ethical Rules, and Reproducibility) the Ethical Rules Principle is particularly relevant to translational regulatory science. Key elements of Ethical Rules Principle are:
These elements rely heavily upon the Jeffersonian Principle [13]. Jeffersonian Principe requires that scientific information used in the regulatory process be translated into a language that, at a minimum is understandable to knowledgeable non-specialists, (also known as educated) and preferably to the entire population.
As shown in (Figure 3), there are four categories of standards of regulatory science claims: Proven regulatory science claims consist of scientific laws and their reproducible applications. In contrast, most regulations rely upon evolving regulatory science. This category includes assumptions and many other items and processes that are necessary to predictive events that are common in many regulations. The Borderline regulatory science is based on the judgment or speculation that almost has no scientific foundation. The item designated as Fallacious is often called junk science and is used by those who want to promote their ideology.
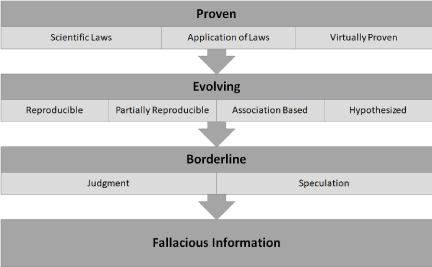
Figure 3: Categorization of regulatory science claims
Another important and often violated requirement is the exclusion of non-scientific issues such as societal objective, ideology, and faith in regulatory science.
Finally, the BARS/MERSC system also addresses the level of maturity and reliability of science that is used in the regulatory process as determined by independent peer review and independent scientific assessment that constitute another key element of regulatory science.
Regulatory Science Tools: Key tools of regulatory science include the application of Jeffersonian Principles to communicate scientific uncertainties, regulatory science ethics, risk assessment and management, cost benefit analysis, and stakeholder participation.
Three Phases of Regulatory Science: The initial requirements for promulgation of a regulation is based on a the desire of legislators or courts to avoid a potential problem. The process to accommodate the decision makers follows multiple steps as follows:
2021 Copyright OAT. All rights reserv
Initial Phase: Often the needed scientific prerequisite to accommodate the mandate are inadequate or non-existent. During this period, the regulators use their judgment in identifying best available materials. For obvious reasons, the judgment of the regulators is at least partially based on the political vision.
Exploratory Phase: During this phase, an attempt is made to gather relevant scientific information. A key element of this phase is the evaluation of the effects of the promulgated regulation. In addition, the scientific community provides results of relevant scientific research including new tools and verification of regulatory science in the original regulation.
Standard Operational Phase: This phase provides an opportunity to apply the findings in the Exploratory Phase to reevaluate initial- phase decisions or revaluate any decision. Obviously, the scientific advancements provide the opportunity to reevaluate decisions that were made during the Standard Operating Phase. In effects, the second and the third phases can be repeated based on the desire of the regulators or a legal mandate.
Translational regulatory science
As described above Translational medicine attempts to identify a good idea and develop a process to bring the resulting product to the market as quickly as possible. Typically, there is a mandate by a law, a judicial decision, or other requirements leading to a decision to act. In effect, the regulator must initiate the development of regulation. The four steps of translational regulatory science are shown in (Figure 4). In many respects, the translational regulatory science follows the four steps identifies under generalized translational science.
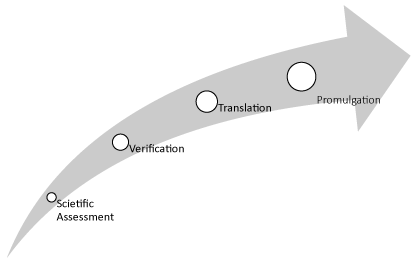
Figure 4: The four-steps of translational regulatory science
Scientific assessment
The first step as shown in figure 4 follows conventional process used in promulgation of regulations. It consists of the assessment of scientific foundation of the desired regulation leading to the development of a document that is often called scientific assessment. Due to the predictive nature of regulations, scientific assessments include reasonably small to large uncertainties.
Verification
This step may also be called the application of BARS/MERSC to verify the scientific claims included in scientific assessment. It requires compliance with the Ethical Rules Principle of BARS/MERSC. Currently many scientific assessments include assumptions, judgments, and other processes that are based on the ideology of the government. Consequently, when a new government with a different ideology is elected, to the extent that is legally possible, an attempt is made to revise the scientific assessment. There must be a clear separation of science from policy. Therefore, it is imperative that the affected as well as the scientific community is provided with information that addresses the following:
- Identification of the level of maturity of scientific claims included in scientific assessment and assumptions that make it possible to apply evolving science in the decision process
- Justification for judgments and assumptions made in the assessment document.
- Description of the alternatives for the chosen Judgments and assumptions and identification of the consequences of alternatives.
- If default data are used, justification for the choice, identification of potential alternatives, and the consequences of selection of alternatives.
- Identification of inclusion of societal objectives (e. g. Being conservative, protective etc.) in assumptions, judgements, choice of default data, and related subjects.
Translation
The third step in translational regulatory science attempts to translate the results of the second step to a product that results in the promulgation of a regulation with significantly less problems than is currently the case. Current problems start with proposed regulations to be followed by many court cases subsequent to the publication of regulations. More importantly often the affected community expresses significant opposition to the regulations. It is likely that many of these problems are reduced for the following reasons:
- During the second step based on Jeffersonian Principle, science used in the regulation is translated into a language that is understandable to the affected community.
- Similarly, societal objectives including the ideology of the operating government are excluded in the scientific assessment.
- Uncertainties including judgements, inclusion of default data, and other issues are identified, and the selected options are justified, and the consequences of the alternatives are described.
- The regulators must justify their decision to include societal objectives in the science part of regulation but belong to policy segment of regulation.
- The regulators must also justify their policy choices including those included in the science part of regulations.
Recall that the presumed success of translational medicine was based on the participation of governmental, academic, industrial organizations as well as practitioners of medicine. The same process applies to the translational regulatory science. Consequently, it is necessary that not only the affected community but many others such as scientific, legal, and other knowledgeable individuals and groups participate in the process. The participation of the public is mandated by the Administrative Procedures Act (APA). In contrast to the “public”, in most cases, based on the current practice a stakeholder is whoever wants to be. Traditionally, regulatory agencies rely upon those individuals and organizations that express an interest in an action, thus become stakeholders. Currently, once the Advanced Notice of Proposed Rulemaking or Proposed Rulemaking is announced either the regulators contact certain stakeholders or they are aware and keep track of relevant events. In most cases these “stakeholders” are advocacy organizations who are supported by specific communities, rather than those who are personally or directly impacted by a decision. In fact, as descried by Love et al. in many cases, the directly/ personal stakeholders do not participate in the decision process [14]. Love et al identify several categories of stakeholders and suggest that the most important category consists of those who are directly or personally impacted by a potential regulation. Often the following example is used to demonstrate the point. Imagine that a factory is being planned in one of the midwestern states in the US. The views of the population in the vicinity of the factory are significantly more relevant than individuals in New York city or San Francisco. However, the current process is using those who have a vision of the desirability or lack of desirability of that factory.
Impact of Translational on Second Phase of Regulatory Science: Translational regulatory science is likely to significantly impact the operational aspects of the regulatory process by limiting the role of science to scientific issues and expanding the role of the policy makers to certain areas that are currently covered in regulatory science. As described above, regulatory science evolved in three phases: initial, exploratory and standard operation.
Historically, during the initial phase, the regulators were mandated to develop regulations although in many cares there was insufficient scientific information. Consequently, in most cases members of the scientific community (regulatory scientists) were asked to rely upon their judgment.
During the exploratory (second) phase, significant advances were made not only in relevant scientific disciplines buts also in methods, processes, and approaches that address unique nature of regulatory science. For example, a report by the National Academies (1983) [15] provided guidance on risk analysis by separating risk assessment, a scientific process from risk management, the role of regulators. Recognizing the importance of peer review in 2001 Congress enacted the so-called Emerson Amendment or Data Quality Act also known as Information Quality Act mandating that Office of Management Budget prepares a manual that all government agencies would have to follow [16]. In another case in 2010 the Food and Drug Administration (FDA) identified regulatory science, an existing scientific discipline as one of its key scientific areas. The implementation of the third step (translation) of translational regulatory science provides a new process in the exploratory phase of regulatory science and is likely to significantly impact the regulatory process.
Promulgation
Once the key elements of the translation are implemented the promulgation of the regulation will be impacted as follows:
- Legal actions that currently include science are significantly reduced.
- As the regulators must justify their policy decisions during the third step(translation), it is likely that they are more careful in their choices
- More importantly, disagreements on regulations promulgated by one government are almost entirely based on policies rather than science.
Application of ethical rules principles
Scientific Assessments that are used by regulatory agencies are largely written for specialists. The translational regulatory science would require that scientific information included in scientific assessments must follow the Jeffersonian Principle and written in a language that is at least understandable to knowledgeable non-specialists and preferably to the entire affected community.
The following examples demonstrate the point:
Implementation of translational regulatory science
Translational regulatory science, once implemented would profoundly impact promulgation of regulations. The example of regulatory toxicology can be used to demonstrate the point. As shown in (Figure 5), currently regulatory toxicology as applied to the regulatory process consists of three parts or categories addressing the effects of exposure to an agent as follows:
Figure 5: Three categories of regulatory toxicology
- Reproducible adverse effects consist of adverse effects such as death of the exposed individuals. Identification of this category is entirely within the purview of science
- The second category may cause an adverse affect, however, there are uncertainties in the relevant science. Again here, the identification of this category is within the purview of science. However, the input of policy makers would be desirable during the application of precautionary principle.
- The third category, currently included in scientific assessment is entirely outside the purview of science.
Ionizing radiation
The example of carcinogenic assessment by exposure to ionizing radiation may be used to demonstrate the point. Currently the carcinogenic assessment of exposure to ionizing radiation follows a process known as liner non-threshold (LNT) implying that exposure to ionizing radiation causes cancer at any exposure level. As described by Calabrese (2017) [17], the LNT process was based primarily on the judgment of one individual and was used in the regulatory process during the first phase of regulatory science as the scientific evidence at low exposure levels was inadequate. There are several key problems with regulations based on LNT.
- There are numerous studies indicating that at very ow levels the exposure to ionizing radiation is beneficial, a process known as hormesis.
- If LNT is valid several cities in the United States such as Albuquerque and Denver would violate the legal standards and thus these and other cities would legally and morally require to be evacuated.
Chromates
Another example of application of translational regulatory science is assessment of hexavalent chromium (Cr-VI) used as an anti-corrosion agent. Currently, the EPA (1998) [18] has designated Cr-VI as a carcinogen and the Department of Defense (DoD) [19] has published regulations (2011) seeking elimination of Cr-VI from DoD activities. However, Cr-VI is currently the primary anticorrosion agent for aluminum, a widely- used metal particularly in airplanes and many other equipment. In large number of cases there is no substitute for Cr-VI and the estimated costs of elimination Cr-VI are exceedingly high. The application of the three categories of regulatory toxicology, as described above, is likely to indicate the exposure to Cr-VI is in the third category [20].
Conclusion
Translational regulatory science provides an opportunity to move regulatory science from the explanatory phase to the standard operating phase. In addition, it has international implications by attempting to separate science from the application of science the in regulatory process. Once implemented, major disagreements among countries with different cultures will be reduced as science used in the regulation will be identical. But how science is used in developing regulations depends upon the culture, faith, and numerous other parameters.
References
- Johannesen L, Vicengte J, Gray RA, et al. (2014) (Improving the assessment of heart toxicity for all new drugs through translations regulatory science. Clinical Pharmacology & Therapeutics 95: 501-508
- Dunmire DA, Moghissi AA (2017) Optimization of corrosion management. Presented at DoD-Allied Nations Technical Corrosion Conference, Birmingham, AL, USA.
- Curry SH. Translational science: past, present and future. BioThechniques 44: I-VII
- Zerhouni EA (2003) The NIH Roadmap. Science 302: 63–72
- Zerhouni AA (2005) Translational and Clinical Science: Time for a new vision New England Journal of Medicine 353: 1621-1623
- Zerhouni EA (2006) Clinical and Translational Science Awards: A framework for a national research agenda. Translational Research 148: 4-5
- Collins FS (2011) Reengineering translational science: The time is right. Science: Translational Medicine 3: 1-6
- Austin CP (2013) National Center for Advancing Translational Sciences: Catalyzing translational innovation; PowerPoint presented at Meeting 3: IOM Committee to Review the CTSA Program at NCATS; Washington, DC, USA. http://www.iom.edu/~/media/Files/Activity%20Files/Research/CTSAReview/2013-JAN-24/Chris%20Austin.pdf.
- EUSTM (European Society for Translational Medicine) About us. http:/eutranslationalmedicine.org/about-us. accessed September 1, 2017
- Retraction Watch. Retractionwatch.com. Accessed September 1, 2017
- Moghissi AA, Straja, SR, Love BR, McBride DK, and Stough RR. Innovation in regulatory science: Evolution of a new scientific discipline. Technology and Innovation 16:155-165
- Moghissi AA, Calderone RA, McBride DK, and Jaeger L. Innovation in Regulatory Science: Metrics for evaluation of regulatory science claims based on best available regulatory science. Journal of Regulatory Science 5: 50-59.
- Moghissi AA, Calderone RR, Azam F, Nowak T, Sheppard S, et al. (2018) Regulating ionizing radiation based on metrics for evaluation of regulatory science claims. Dose Response: In Press
- National Academies (1983) Risk assessment in the federal government: Managing the process. Washington DC National Academy Press 1983
- Calabrese EJ (2017) Flaws on the LNT single-hit model for cancer risk: An historical assessment. Environmental Research 158: 773-788
- Information Quality Act (also known as Data Quality Act. Public Law515: 106-554
- EPA (Environmental Protection Agency) Toxicological review of hexavalent chromium. Washington DC, EPA 1998
- DoD (Department of Defense) (2011) Defense Federal Acquisition Regulations: Minimizing the use of materials containing hexavalent chromium (DFARS case 2009-D004). Federal Register 76; 25569-25576
- Berglund L, Tarantal A (2009) Strategies for innovation and interdisciplinary translational research: Removal of barriers through the CTSA mechanism. Journal of Investigative Medicine 57 :474–476
- Blumberg RS, Dittel B, Hafler D, von Herrath M, Nestle FO, et al. (2012) Unraveling the autoimmune translational research process layer by layer. Nature Medicine18: 35–41





