Introduction
Tachyarrhythmia and tachycardia have been shown to be associated with prolonged hospitalization, higher complication rates, and increased mortality [1-3]. Beta-blockers constitute an essential part in the therapeutic control of heart frequency, heart rhythm and in heart failure therapy. However, treatment with beta-adrenoreceptor antagonists may be delayed or withheld in catecholamine-dependent patients with reduced cardiac contractility due to concerns regarding negative inotropic and hypotensive side effects [4,5]. With landiolol and esmolol, two ultra-short acting, highly selective beta-1 adrenoreceptor antagonists are available. Compared to other beta-adrenoreceptor antagonists, landiolol and esmolol are characterized by a rapid onset of action, short half-life of 3-5 min and 10 min, respectively, and high cardioselectivity [6]. The latter results in low impairment of cardiac contractility and arterial blood pressure [7]. In Japan, landiolol has been clinically approved for over ten years, while approval was granted for most European countries only in 2016 [8-12].
Constrictive pericarditis (CP) is an unusual form of diastolic heart failure with primarily preserved systolic function [13]. The prevalence of CP is not known conclusively but is observed in 0.2-0.4% of patients who have undergone cardiac surgery or have had pericardial inflammation, and the aetiology of the disease is very variable. The most common is viral and idiopathic pericarditis, followed by postoperative pericarditis after cardiac surgery and mediastinal radiotherapy [13]. A reduced ejection fraction after pericardectomy is also described [14,15].
The American College of Cardiology/American Heart Association guidelines recommend the perioperative use of beta-blockers in patients with a positive cardiac stress test result as a predictor of subsequent development of angina pectoris or even the occurrence of a major cardiac event. It is recommended to start beta-blocker therapy weeks before surgery for protection against major cardiac events [16]. Nevertheless, beta-blockers are often started intraoperatively for the treatment of newly occurring tachyarrhythmia and tachycardia [11]. As a beta-adrenoreceptor antagonist, esmolol may be best suitable for patients without reduced cardiac contractility, with high selectivity and short half-life. However, patients with reduced cardiac output may experience hypotension under esmolol due to its negative inotropic effect [7]. Here, landiolol presents an interesting alternative due to the increased beta-1 selectivity [6,12]. In contrast to esmolol, landiolol appears to be free of renin effects and does not exhibit undesirable membrane effects due to its lower lipophilicity [17,18].
We describe the first case of a hemodynamically vulnerable patient after pericardectomy where both ultra-short beta blockers, esmolol and landiolol, were used to treat sinus tachycardia. Esmolol and landiolol elicited an equipotent negative chronotropic effect, however, landiolol had a lesser negative inotropic effect than esmolol.
Case Report
A male patient (52 years, 56 kg bodyweight) presented with intra- and postoperative sinus tachycardia of 120 bpm after undergoing pericardiectomy for chronic idiopathic constrictive pericarditis with recurrent right ventricular failure. After induction of anaesthesia, low dose levosimendan was administered continuously (2.5 mg over 8 h) to improve lusitropy, to increase right ventricular inotropy, and to reduce the perioperative need for other inotropic agents [19]. Pericardectomy was successfully performed without cardiopulmonary bypass, and during surgery, a mean arterial pressure >65 mmHg was maintained by maximum rates of epinephrine at 0.2 mg·h-1 (0.06 µg·kg-1·min-1), norepinephrine at 0.8 mg·h-1 (0.24 µg·kg-1·min-1), and vasopressin at 1 IE·h-1 (add 0.016 IE·min-1). Postoperatively, the patient was transferred to the ICU with epinephrine at 0.1 mg·h-1 (0.03 µg·kg-1·min-1), norepinephrine at 0.7 mg-1·h-1 (0.20 µg·kg-1·min-1), vasopressin at 1 IE·h-1 (add 0.016 IE·min-1) and a sinus tachycardia of 120 bpm.
Transesophageal echocardiography showed no sign of restricted right or left ventricular function (LV ejection fraction 40%). Normovolemia was achieved by administration of balanced crystalloid volume under echocardiographic monitoring, which allowed for reduction of vasopressors to norephinephrine at 0.4 mg·h-1 (0.12 µg·kg-1·min-1) and vasopressin at 0.5 IE·h-1 (add 0.008 IE·min-1). Despite the discontinuation of epinephrine, extubation, restitution of normovolemia and sufficient analgesia, tachycardia persisted (115 bpm). Hemodynamic parameters were monitored with continuous pulse contour cardiac output (PiCCO) analyses.
Landiolol was administered at 24 mg·h-1 (7.14 µg·kg-1·min-1) at a cardiac index (CI) of 3.4 L·min-1·m-2, which reduced the heart to 97 bpm and lowered CI to 3.0 L·min-1·m-2 (Figure 1). After heart rate control was achieved, landiolol was reduced to 12 mg·h-1 (3.57 µg·kg-1·min-1) with a consecutive increase of CI to 3.3 L·min-1·m-2 at a heart rate of 93 bpm. Landiolol therapy was terminated after 16 h. Within 2 hours, the patient relapsed into sinus tachycardia up to 108 bpm. Since CI measurements were within reference range and for economic considerations, not landiolol but esmolol treatment was started at a rate of 100 mg·h-1 (1.78 mg·kg-1·h-1 = 30 µg·kg-1·min-1). We observed a rapid decrease in CI from 3.3 L·min-1·m-2 to 2.3 L·min-1·m-2, while the heart rate showed a similar decrease compared to landiolol treatment, from 108 to 89 bpm. After reduction of esmolol to 50 mg·h-1 (0.89 mg·kg-1·h-1 = 15 µg·kg-1·min-1), CI increased from 2.3 L·min-1·m-2 to 3.4 L·min-1·m-2 (Figure 1).
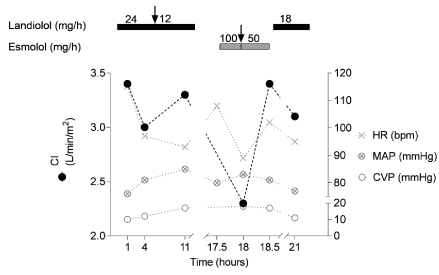
Figure 1. Effect of landiolol or esmolol on inotropy. Landiolol (black bar) or esmolol (grey bar) were administered at indicated concentrations, and cardiac index (CI), heart rate (HR), mean arterial pressure (MAP) and central venous pressure (CVP) were monitored over time upon ICU admission
Despite the increase in CI with the reduced dosage of esmolol, this lower dosage did not result in the same decrease of heart frequency (102 bmp), compared to the higher dose of esmolol (32 µg·kg-1·min-1, 100 mg·h-1) or landiolol at both concentrations of 12 mg·h-1 (3.57 µg·kg-1·min-1) and 24 mg·h-1 (7.14 µg·kg-1·min-1) (Figure 1).
Relevant changes in mean arterial pressure (MAP) or the need of higher vasopressors were not detected at any time. Similarly, no considerable changes in central venous pressure (CVP) were observed. Due to the lack of validity in extubated patients, stroke volume variance (SVV) was not included in our analyses. Since tachycardia reoccurred after discontinuation of esmolol, landiolol therapy was reassumed at 18 mg·h-1 (5.36 µg·kg-1·min-1). Compared to esmolol, we observed no relevant decline in CI, but a decrease in heart rate to 95 bpm (Figure 1). After a cumulative dosage of 1200 mg landiolol, bisoprolol was started 48 hours after surgery at 1.25 mg·d-1 and increased to 2.5 mg·d-1 after 72 hours, when the patient was transferred to a standard ward with a heart rate of 86 bpm.
Discussion
Measurement of CI under the influence of the two ultra-short beta-blockers landiolol and esmolol within the same patient allowed for the observation of the lack of negative inotropy of landiolol compared to esmolol. With comparable effects on heart frequency, hemodynamic parameters improved under landiolol but worsened during esmolol administration (Figure 1).
Beta-adrenoreceptor antagonists are an established therapeutic option for frequency control of atrial fibrillation and tachycardia [11]. However, they are rarely used in cases of concomitant restricted left ventricular ejection fraction and catecholamine requirement, due to their negative inotropic and hypotonic effects [20]. A further deficit is the long half-life of most beta-blockers and the associated poor adjustability of therapy, especially in critically ill patients. Until 2016, amiodaron was the gold standard for patients with supraventricular arrhythmia, but amiodaron is not indicated for the treatment of sinus tachycardia. However, with the 2020 published guidelines of the European Society of Cardiology for treatment of atrial fibrillation, the therapy recommendation has changed. Beta-blocker administration now is recommended in patients with impaired cardiac ejection fraction [5]. The mechanism of lower negative inotropic and chronotropic effects of landiolol are thus of high interest. The reasons why landiolol shows hemodynamic advantages over esmolol in tachycardic patients may be explained by several of its properties. First, landiolol is more beta-1 selective than esmolol, causing less beta-2 receptor blockade, which plays an important role in heart failure [6,7,21]. Second, since esmolol is more lipophilic than landiolol, it penetrates the cell membrane [4]. This triggers an undesirable effect on sodium, calcium, and potassium channels, reducing action potential duration and calcium influx and thus, inotropy [7,22].
Because esmolol, unlike landiolol, also induces a greater reduction in blood pressure through its renin action, it may be suggested that reflex tachycardia reduces the heart rate-lowering effect of esmolol [18]. The Japanese guidelines include landiolol for the treatment of atrial fibrillation, together with carvedilol and bisoprolol, as first-line therapy for heart rate control in patients with heart failure and reduced cardiac output [5,23].
Landiolol at a low dosage (1-10 µg·kg-1·min-1) is usually sufficient to rapidly control heart rate, which is associated with an earlier and higher rate of conversion to sinus rhythm and faster control of heart rate compared to other beta-blockers. The potent chronotropic effect of landiolol could be highlighted by the fact that a successive reduction of the dosage did not result in massive heart rate elevations. This approach to the appropriate dosage depending on the clinical parameters is essential and helpful also to reduce pharmacological side effects and to establish a positive pharmacoeconomic balance [24].
For patients after myocardial infarction, atrial ablation or acute heart failure with or without impaired left ventricular ejection fraction, landiolol at 1-10 µg·kg-1·min-1 is recommended [5,23]. The tolerance of landiolol at a lower dosage (3-5 µg·kg-1·min-1) may allow to initiate prophylactic use during surgery and postoperatively. Recently, low dose landiolol has been shown to be beneficial in patients with sinus tachycardia who received catecholamine support after cardiovascular interventions. Similar to the presented case, a safe reduction of heart rate with simultaneous improvement of haemodynamic parameters could be achieved [25].
This case report suggests that low-dose landiolol (<8 µg·kg-1·min-1) represents a safe option to control heart rate in catecholamine-dependent patients after cardiac surgery without a clinically relevant negative impact on CI. In this direct comparison of landiolol and esmolol, landiolol was associated with a lower negative inotropic effect compared to esmolol. To our knowledge, this is the first direct comparison of the effects of landiolol and esmolol on heart rate and CI in the same patient. Thus, based on its superselectivity, landiolol may be more beneficial than esmolol for treatment or prophylaxis of postoperative tachycardia and arrhythmia [26].
Consent
Written informed consent was obtained from the patient for publication of this case report. A copy of the written consent is available for review by the editor of the Journal of Cardiothoracic and Vascular Anesthesia.
Conflict Of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Summary
Tachyarrhythmia and tachycardia are associated with increased complication rates, prolonged hospitalization, and higher mortality. However, treatment with beta-adrenoreceptor antagonists, first line drug for rate control, may be delayed or withheld in catecholamine-dependent patients with reduced cardiac contractility due to concerns regarding negative inotropic and hypotensive side effects. Here, we describe the case of a 52-year-old male patient with idiopathic constrictive pericarditis undergoing pericardyectomy. Landiolol and esmolol were administered postoperatively to control sinus tachycardia. Hemodynamic effects of these two ultra-short acting beta-blockers showed striking differences with regard to cardiac output alterations. Landiolol was associated with a lower negative inotropic effect compared to esmolol. To our knowledge, this is the first comparison of the two ultra-short acting beta-blockers esmolol and landiolol in a clinically setting in a human.
References
- Greenberg JW, Lancaster TS, Schuessler AB, Melby SJ (2017) Postoperative atrial fibrillation following cardiac surgery: a persistent complication. Eur J Cardiothorac Surg 4: 665-672. [Crossref]
- Crimm A, Severance HW, Coffey K, McKinnis R, Wagner GS, et al. (1984) Prognostic significance of isolated sinus tachycardia during first three days of acute myocardial infarction. Am J Med 6: 983-988. [Crossref]
- Hasegawa D, Sato R, Prasitlumkum N, Nishida N, Takahashi K, et al. (2021) Effect of Ultrashort-Acting β-Blockers on Mortality in Patients With Sepsis With Persistent Tachycardia Despite Initial Resuscitation: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Chest 6: 2289-2300. [Crossref]
- Couffignal C, Amour J, Ait-Hamou N, Cholley B, Fellahi JL, et al. (2020) Timing of β-Blocker Reintroduction and the Occurrence of Postoperative Atrial Fibrillation after Cardiac Surgery: A Prospective Cohort Study. Anesthesiology 2: 267-79. [Crossref]
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, et al. (2021) 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 5: 373-498. [Crossref]
- Nasrollahi-Shirazi S, Sucic S, Yang Q, Freissmuth M, Nanoff C, et al. (2016) Comparison of the beta-Adrenergic Receptor Antagonists Landiolol and Esmolol: Receptor Selectivity, Partial Agonism, and Pharmacochaperoning Actions. J Pharmacol Exp Ther 1: 73-81. [Crossref]
- Ikeshita K, Nishikawa K, Toriyama S, Yamashita T, Tani Y, et al. (2008) Landiolol has a less potent negative inotropic effect than esmolol in isolated rabbit hearts. J Anesth 4: 361-366. [Crossref]
- Nagai R, Kinugawa K, Inoue H, Atarashi H, Seino Y, et al. (2013) Urgent Management of Rapid Heart Rate in Patients With Atrial Fibrillation/Flutter and Left Ventricular Dysfunction. Circ J 77: 908 -916. [Crossref]
- Group JCSJW (2014) Guidelines for Pharmacotherapy of Atrial Fibrillation (JCS 2013). Circ J 8: 1997-2021. [Crossref]
- Adachi T, Sato A, Baba M, Hiraya D, Hasegawa T, et al. (2014) Novel use of the ultra-short-acting intravenous β1-selective blocker landiolol for supraventricular tachyarrhythmias in patients with congestive heart failure. Heart Vessels 4: 464-469. [Crossref]
- Poveda-Jaramillo R, Monaco F, Zangrillo A, Landoni G (2018) Ultra-Short-Acting β-Blockers (Esmolol and Landiolol) in the Perioperative Period and in Critically Ill Patients. J Cardiothorac Vasc Anesth 3: 1415-1425. [Crossref]
- Sezai A, Osaka S, Yaoita H, Ishii Y, Arimoto M, et al. (2015) Safety and efficacy of landiolol hydrochloride for prevention of atrial fibrillation after cardiac surgery in patients with left ventricular dysfunction: Prevention of Atrial Fibrillation After Cardiac Surgery With Landiolol Hydrochloride for Left Ventricular Dysfunction (PLATON) trial. J Thorac Cardiovasc Surg 4: 957-964. [Crossref]
- Miranda WR, Oh JK (2017) Constrictive Pericarditis: A Practical Clinical Approach. Prog Cardiovasc Dis 4: 369-379. [Crossref]
- Schofield RS, Shoemaker SB, Ryerson EG, Cooper GR, Spotnitz WD (2004) Left ventricular dysfunction after pericardiectomy for constrictive pericarditis. Ann Thorac Surg 4: 1449-1451. [Crossref]
- Senni M, Redfield MM, Ling LH, Danielson GK, Tajik AJ, et al. (1999) Left ventricular systolic and diastolic function after pericardiectomy in patients with constrictive pericarditis. J Am Coll Cardiol 5: 1182-1188. [Crossref]
- Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, et al. (2007) ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery. Circulation 17: 418-500. [Crossref]
- Shibata S, Okamoto Y, Endo S, Ono K (2012) Direct effects of esmolol and landiolol on cardiac function, coronary vasoactivity, and ventricular electrophysiology in guinea-pig hearts. J Pharmacol Sci 2: 255-265. [Crossref]
- Kakuta N, Kawano T, Tanaka K, Oshita S (2005) A comparison of landiolol and esmolol for attenuation of cardiovascular response and plasma renin activity against tracheal intubation with laryngoscopy. Anesthesiology 103.
- Woehrle T, Mehringer L, Juchem G, Dashkevich A, Weis M, et al. (2020) Individualized use of levosimendan in cardiac surgery. Anaesthesist 3: 204-212. [Crossref]
- Zangrillo A, Bignami E, Noe B, Nardelli P, Licheri M, et al. (2021) Esmolol in Cardiac Surgery: A Randomized Controlled Trial. J Cardiothorac Vasc Anesth 4: 1106-1114. [Crossref]
- Werner CM, Bohm M (2008) The therapeutic role of RAS blockade in chronic heart failure. Ther Adv Cardiovasc Dis 3: 167-177. [Crossref]
- Sasao J, Tarver SD, Kindscher JD, Taneyama C, Benson KT, et al. (2001) In rabbits, landiolol, a new ultra-short-acting beta-blocker, exerts a more potent negative chronotropic effect and less effect on blood pressure than esmolol. Can J Anaesth 10: 985-989. [Crossref]
- Okajima M, Takamura M, Taniguchi T (2015) Landiolol, an ultra-short-acting beta1-blocker, is useful for managing supraventricular tachyarrhythmias in sepsis. World J Crit Care Med 3: 251-257. [Crossref]
- Walter E, Heringlake M (2020) Cost-Effectiveness Analysis of Landiolol, an Ultrashort-Acting Beta-Blocker, for Prevention of Postoperative Atrial Fibrillation for the Germany Health Care System. J Cardiothorac Vasc Anesth 4: 888-897. [Crossref]
- Sakai M, Jujo S, Kobayashi J, Ohnishi Y, Kamei M (2019) Use of low-dose beta1-blocker for sinus tachycardia in patients with catecholamine support following cardiovascular surgery: a retrospective study. J Cardiothorac Surg 1: 145. [Crossref]
- Matsuishi Y, Mathis BJ, Shimojo N, Kawano S, Inoue Y (2020) Evaluating the Therapeutic Efficacy and Safety of Landiolol Hydrochloride for Management of Arrhythmia in Critical Settings: Review of the Literature. Vasc Health Risk Manag 111-123. [Crossref]

