Background: Measurement of the international normalized ratio (INR) is essential in the monitoring of patients taking vitamin K antagonists (VKAs). High familiarity with this biological indicator is necessary for satisfactory treatment compliance. In France, INR self-testing was first introduced for pediatric indications, but since 2016 it has also been prescribed for patients with mechanical heart valve implants.
Objective and method: A study was conducted to assess relevant knowledge currently possessed by patients being treated with VKAs and feedback from dispensing pharmacists on the utility of self-testing devices for patients. Data were collected from an external assessment questionnaire for patients and a self-assessment questionnaire for pharmacists, in 91 dispensing pharmacies in the Auvergne region belonging to a network hosting 4th year trainee pharmacy students for a week’s training in dispensing practice in October 2017.
Results: In all, 234 patient questionnaires and 154 pharmacist questionnaires were analyzed. According to the dispensing pharmacists, a large majority of patients had good knowledge of their target INR and monitoring schedules. This was confirmed by patient declarations. Pharmacists were also aware of the possible utility of more widespread INR self-testing: 89% considered that public health insurance cover for self-tests ought to be extended in France, 64% to certain patients only.
Conclusion: Pharmacists could join other health professionals in following patients using INR self-testing, as they already do for monitoring VKA treatment through pharmaceutical interview and related to the implementation of pharmacist-led shared medication assessment in France in 2018. This work could advantageously be extended to the national level.
INR self-testing, survey in community pharmacy, VKA treatment, patient knowledge, pharmacist feedback
For many years vitamin K antagonists (VKAs) were the only therapeutic anticoagulants available in France. Since 2008 direct oral anticoagulants (DOAs) have appeared with indications such as atrial fibrillation [1]. The prevention and treatment of deep vein thrombosis and pulmonary embolism are among the indications of both drug classes [1]. In 2015 300 million euros were spent by public health insurance on 5 million boxes of DOAs vs. 34 million euros on 13 million boxes of VKAs dispensed. The additional cost borne by public health insurance for the biological monitoring of patients taking VKAs, by regular measurement of the international normalized ratio (INR), was 73 million euros [2]. Using DOAs thus cost three times more than using VKAs. Meanwhile, the health authorities have often warned about the risks of bleeding and thrombosis linked to the use of VKAs [3,4]. In France, VKAs are the leading cause of drug-related illness requiring hospital admission [3,4]. In addition, in June 2017 the French national drug safety agency (ANSM) alerted prescribers to the immuno-allergic risks linked to Previscan® and recommended beginning treatment with warfarin [5]. Since December 1, 2018, no initiation of treatment with fluindione must be carried out, it is now restricted to the renewal of treatment [5].
In France, to improve the therapeutic care of patients taking anticoagulants, dispensing pharmacists can offer pharmaceutical interviews. These involve talking with the patient about his or her illness (treatment compliance, biological monitoring for VKAs, advice on hygiene and diet, etc.). In addition, self-measurement devices are available for self-monitoring of INR for patients taking VKAs. These were initially prescribed under insurance in pediatric care, but since 2016 have also been made available for patients with mechanical heart valve implants. Two devices commercialized in France, Coaguchek INRange® and Coaguchek XS, prothrombin time, which is converted into INR by an algorithm [6-8]. They are easy to use but still require some theoretical and practical patient training [7,8]. This training is regulated by the Law of 28 July 2017, which provides for initial training by the medical team in the prescribing institution [9].
We note that these costly devices are also sold over-the-counter, priced at 685–790 euros, added to which a box of 24 test strips must be bought, costing more than 100 euros [10-12].
A study was conducted among patients taking VKAs and dispensing pharmacists in Auvergne, France. The aim was to assess (i) the knowledge possessed by patients being treated with VKAs, and (ii) feedback from dispensing pharmacists on the utility of INR self-testing for the patients.
This study was registered with a local correspondent of the Commission on Information Technology, Data Files and Civil Liberty in accordance with French law and declared to the local ethics committee (Comité de Protection des Personnes sud-est 6).
Period, population, location and course of the study
The study was conducted in 91 dispensing pharmacies in the Auvergne region hosting 4th year trainee pharmacy students for a week’s training in dispensing practice (week beginning 16 October 2017). The protocol had been presented to students and their training managers at previous meetings.
The trainee students gave a questionnaire to every patient aged 18 years or over coming to their dispensing pharmacy with a prescription for a VKA (fluindione, warfarin or acenocoumarol), and who had agreed to take part in the study after being asked orally by the pharmacist. Patients aged less than 18 years and those not proficient in French were excluded.
During the same period, at least one pharmacist in the dispensary team filled out a questionnaire for the study. Pharmacists in the Auvergne network who did not host a 4th year trainee pharmacy student, and those outside the host network were excluded.
Data collection
Two questionnaires were drawn up to collect data:
- a patient external assessment questionnaire to evaluate the knowledge possessed by patients taking VKAs, with as main items:
- Data concerning the patient: sex, age, educational level, occupation;
- Data concerning the VKA treatment: duration, indications, target INR, monitoring schedules. We note that to verify the patient’s true knowledge of their target INR according to their health condition, we took an expected target INR of 2–3, including for patients with biological heart valves. However, for patients with a mechanical valve, the declared target INR had to be between 2.5 and 4 (13).
- Data concerning any experience with INR self-testing.
- a pharmacist self-assessment questionnaire to evaluate feedback on self-testing, with as main items:
- Data concerning the pharmacist’s position, whether principal or assistant;
- Data concerning how pharmacists represented patients’ knowledge about their VKA treatment: target INR, monitoring schedules;
- Data concerning the medication consultations on VKAs or DOAs;
- Data concerning INR self-testing: dispensing, utility for the patient
Statistical analysis
The data collected were entered by the community pharmacists teams in a secured academic platform on the RENATER national network and extracted to Microsoft Excel® for later analysis. Statistics were computed using Stata (version 13, Stata Corporation, Collège Station, TX, USA). Results were expressed as frequency and percentage for categorical data and as means, standard deviation and range for continuous data.
Knowledge possessed by patients taking VKAs
Of the 242 external assessment patient questionnaires collected, 8 were incomplete, 234 were analyzable: 104 women and 130 men took part in the study, with a mean age of 73.9 years (± 13), range 18–97 years; 85% (n = 197) of the patients were recorded as retired, 33% (n = 75) had no educational qualifications, 45% (n = 100) had secondary school qualifications and 23% (n = 50) had degrees. The persons who presented the prescription were mostly the patients themselves (n = 180); 37 were proxies (no answer 17).
Of the patients, 95% (n = 223) had been taking a VKA for more than 6 months. In 71% of cases the VKA was fluindione (Previscan®), in 18% warfarin (Coumadine®), and in 11% acenocoumarol (Sintron® or Minisintron®).
The treatment indications declared by the patients were mainly cardiac insufficiency, vein thrombosis, heart valve prosthesis and pulmonary embolism (Figure 1). ‘Other’ was most often antecedent arrhythmia or stroke (Fig.1).
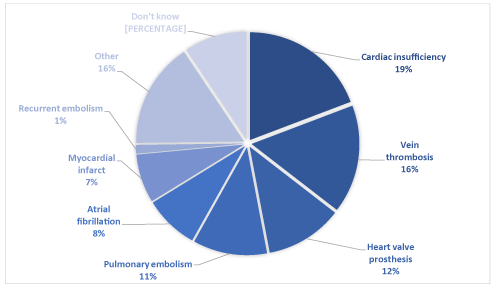
Figure 1.Indications for a VKA treatment declared by patients
Of the 234 patients, 79% (n = 185) claimed to know their target INR. When their responses were analyzed, with respect to the target INR indicated for their health condition, 74% (n = 174) were found to know their correct target INR. Of the patients who claimed to know their target INR, 71% (n = 130) declared that their INR was “usually”, and 18% (n = 34) “always” stable. Concerning the frequency of patient INR monitoring, some 85% of patients claimed to monitor their INR at least once a month, whereas about 15% admitted not monitoring their INR as recommended (at least once a month for a stable INR). A detailed analysis by patient age and sex showed no significant difference for knowledge of target INR and monitoring schedules. Nor was any significant difference observed between treated persons and proxies.
Of the patients taking VKAs, 12% (n = 27) had a heart valve implant, in 90% of cases (n = 24) mechanical, making them eligible for a prescribed self-measurement device covered by public health insurance. Of these patients, only 5 used an INR self-tester, 4 of whom had a mechanical valve. The mean age of the users of self-testers was 72 years, and all indicated that they had received training in the use of the device and knew their target INR. Three of them were satisfied with the use of the device, for its convenience and for better monitoring. One considered the test strips were too costly, and the fifth found their device too old and not very practical.
Feedback from dispensary pharmacists
In all, 154 questionnaires were collected from 86 principal and 68 assistant pharmacists, and all were analyzed. For 68% of the pharmacists (n = 105), the patients knew their target INR “often”, and for 6% (n = 9) “always”; 81% of the pharmacists thought the patients checked their INR at least once a month.
Only 44% of the pharmacists (n = 68) stated that they led pharmaceutical interviews for VKAs or DOAs, the main reasons given for not doing so being lack of time (52% of responses) and unresolved remuneration issues (12% of responses). Of those who did lead pharmaceutical interviews, 91% (n = 62) were ready to help patients with their INR self-testers and 88% (n = 136) wished to have training on their use.
About 10% of them (n = 15) indicated that they had already dispensed these self-testers for both adult patients and children (10 adult patients, of which one kidney transplant, two patients with mechanical heart valves, and five children).
For the pharmacists, the utility of the INR self-tests was, in descending rank, better INR monitoring, prevention of overdoses, prevention of major adverse effects, and improved patient quality of life (Fig. 2).
Finally, 89% of the pharmacists (n = 137) thought that public health insurance coverage of INR self-tests ought to be extended in France; 64% to certain patients only. Among the restrictions expressed was the high cost of the devices and the importance of optimal training to ensure patient autonomy.
According to the dispensing pharmacists, a large majority of the patients had good knowledge of their target INR and monitoring schedules; this opinion was supported by concordant patient responses. Even so, 5% of the patients did not know their target INR and 15% did not follow the monitoring recommendations. It would have been interesting to know the reasons for non-compliance: could INR self-testing have facilitated the biological monitoring of these patients?
The results clearly cannot be extrapolated to the national population even though the sample was relatively representative of patients taking VKAs in France: in 2014, 80% of patients taking VKAs in France were using fluindione (Previscan®), vs. 71% in our study. Our mean age (73.9 years) was very close to the national average (73.7 years for patients taking VKAs). The sex ratio was 55% men in our study vs. 51% nationally (1).
It may seem surprising that most of the patients in our study were taking fluindione. Although warfarin is usually recommended, fluindione is still the most often-used VKA in France [5]. It should be noted that this study predates the recommendations of the ANSM of 2018 [5].
Used in many countries, self-measurement devices have proved useful in care systems internationally, in particular though improving autonomy and quality of life [14-23]. The practice of self-monitoring has also increased time in the target INR therapeutic range and reduced mortality, all causes combined [14-23]. Studies including medico-economic evaluations have shown a reduction of hospitalizations for iatrogenic events linked to VKA [14-23]. Although there is no conclusive evidence showing a reduction in bleeding risk, different studies agree that rates of thrombotic risk has been reduced [14-23]. In France, a different strategy has so far been adopted: biological monitoring of patients taking VKAs is carried out by analytical laboratories, which have a very dense territorial coverage, or via nursing care facilities. Prescribed, insurance-covered self-measurement is restricted to a small number of patients. In our study, with reference to current recommendations, only 7 out of the 15 INR self-testing patients were covered by public health insurance, which was a very small number.
In this study, the pharmacists surveyed were aware of the major advantages of INR self-testing, and emphasized that it would in particular prevent overdoses, improve INR monitoring, and so increase time in the therapeutic range, improve patient quality of life, and reduce major adverse effects. Most of them favored extending their eligibility for public health insurance cover and were ready to be trained and help patients with them. This assistance could strengthen the current pharmaceutical interview by highlighting the following points: reminders on INR measurement frequency, therapeutic range and warning signs, and advice for the use of these devices, on the model of that already given by pharmacists for blood glucose self-monitoring. Summary sheets like that shown in Figure 3 could be given to patients as a basic learning aid. Although major bleeding risks linked to DAOs in atrial fibrillation seem similar to those observed under VKAs, experience of their use is still short [24]. Given this uncertainty and the high cost of DAOs, VKAs are still widely used. In this context, INR self-tests might ensure safer VKA treatment in France. Giving patients easier access to self-measurement devices at reasonable cost and training them for optimal use could help reduce the number of hospitalizations for adverse effects due to VKAs, and lower associated costs, as studies outside France have already shown. Lastly, pharmacists-led shared medication assessment on older patients set up in France in 2018, with the aim of preventing drug-related adverse reactions, could be an additional care aid for patients practicing self-testing or those wanting to use these devices [25]. These pharmacists-led shared medication assessment are designed to record all treatments given to older patients taking multiple medication, and to carry out a pharmaceutical analysis for physicians to consider possible changes in drug prescription that would benefit patients.
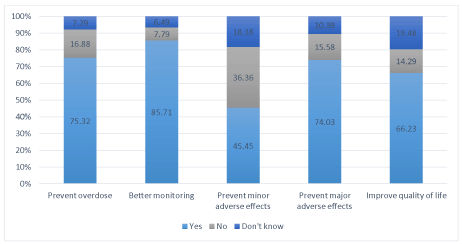
Figure 2. Utility of INR self-tests according to community pharmacists (in %)
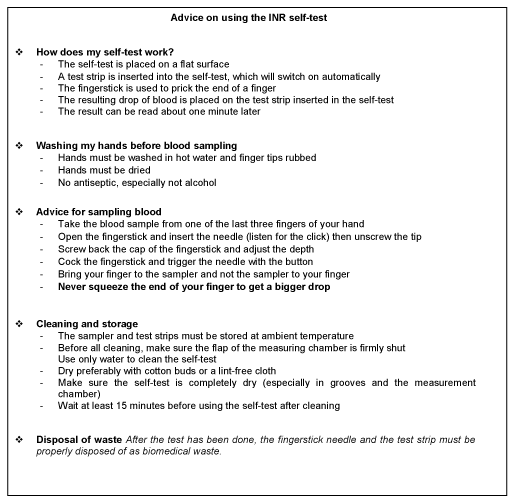
Figure 3. INR self-testing advice sheet
Despite a small sample size, the patients in the study were relatively representative of the French population treated with VKAs. Their knowledge of INR and its monitoring schedules was demonstrated overall. This was an essential prerequisite for a successful therapeutic outcome.
Dispensing pharmacists, who stand at the core of the French healthcare system through their expert skills, good knowledge of their patients, availability and proximity, ensured by a dense territorial coverage, were aware of the potential benefits of INR self-testing for patients taking VKAs, and favored their more extended use in France.
In this context, they could support other healthcare professionals in helping patients use INR self-tests, as they already do for VKA treatment monitoring in pharmaceutical interviews, and in the recent setting-up of pharmacists-led shared medication assessment. These considerations now need to be examined at a national scale.
- Point of view of French dispensing pharmacists on the utility of INR self-testing and need for its extension to other indications to help prevent VKA-related illness.
- Good knowledge among pharmacists of their patients taking VKAs.
- Demand by pharmacists for training in INR self-testing for better patient care.
The authors thank the participating community pharmacists and the students who contributed to our research.
None.
None.
- ANSM. Les anticoagulants en France en 2014: état des lieux, synthèse et surveillance [Internet]. 2014 avr. Available on: http://ansm.sante.fr/Dossiers/Les-Anti-vitamine-K-AVK/Prevention-des-hemorragies-provoquees-par-les-traitements-anticoagulants-anti-vitamine-K-AVK/(offset)/0
- HARO-BRUNET E. Les anticoagulants oraux directs raflent la mise. 18 mars 2017;(Le moniteur des Pharmacies n°3169).
- DREES. Enquête Nationale sur les Événements Indésirables graves associés aux Soins - Description des résultats 2009 - Rapport final [Internet]. 2011 sept [last consultation: 7 june 2018]. Available on: http://drees.solidarites-sante.gouv.fr/IMG/pdf/serieetud110.pdf
- DREES. Les événements indésirables graves liés aux soins observés dans les établissements de santé : premiers résultats d’une étude nationale [Internet]. 2005 [last consultation: 7 june 2018]. Available on: http://www.optimiz-sih-circ-med.fr/Documents/DREES-N%C2%B0398-Ev%C3%A9nementsInd%C3%A9sirables.pdf
- ANSM. Traitement par antivitamines K (AVK): nouvelles informations - PREVISCAN (fluindione) : Prescription restreinte au seul renouvellement de traitement - COUMADINE, PREVISCAN, SINTROM et MINISINTROM (warfarine, fluindione, acénocoumarol) : Contre-indication au cours de la grossesse - Lettre aux professionnels de santé. [last consultation : 2 september 2020]. Avaialble
on:https://www.ansm.sante.fr/content/download/152835/2008141/version/1/file/DHPC_AVK_2018-11-30.pdf
- LASNE Dominique. Place des dispositifs d’automesure de l’INR dans la surveillance des traitements par les antagonistes de la vitamine K ou antivitamine K (AVK) [Internet]. 11/15 [last consultation: 7 june 2018]. Available on: https://www.laboratoires-maymat.fr/contenu/fckn/_AUTOMESURE%20DE%20L%27INR.pdf
- HAS. Avis de la CNEDiMTS - Coaguchek XS [Internet]. 2016 mars [cité 29 mai 2017]. Available on: https://www.has-sante.fr/portail/upload/docs/evamed/CEPP-4918_COAGUCHEK%20XS_08_mars_2016_(4918)_avis.pdf
- HAS. Avis de la CNEDIMTS - Coaguchek INRange [Internet]. [cité 6 juin 2017] p. 10/01/17. Available on: https://www.has-sante.fr/portail/upload/docs/evamed/CEPP-5222_COAGUCHEK%20INRANGE_10_janvier_2017_(5222)_avis.pdf
- PERRUCHON C, WANECQ T. Arrêté du 28 juillet 2017 portant inscription dispositif d’auto-mesure de l’INR COAGUCHEK INRANGE de la société ROCHE DIAGNOSTICS au titre Ier de la liste des produits et prestations remboursables prévue à l’article L. 165-1 du code de la sécurité sociale.
- Assurance Maladie. LPP: Fiche bandelette Coaguchek XS [Internet]. 2012 [last consultation: 7 june 2018]. Available on: http://www.codage.ext.cnamts.fr/cgi/tips/cgi-fiche?p_code_tips=1162147&p_date_jo_arrete=%25&p_menu=FICHE&p_site=AMELI
- Assurance Maladie. LPP: Fiche lecteur Coaguchek XS [Internet]. 2012 [last consultation: 7 june 2018]. Available on: http://www.codage.ext.cnamts.fr/cgi/tips/cgi-fiche?p_code_tips=1117129&p_date_jo_arrete=%25&p_menu=FICHE&p_site=AMELI
- Avis relatif à la tarification de COAGUCHEK INRANGE visé à l’article L. 165-1 du code de la sécurité sociale [Internet]. Available on: https://www.legifrance.gouv.fr/affichTexte.do?cidTexte=JORFTEXT000035330518&categorieLien=id
- HAS. Evaluation de l’autosurveillance de l’INR chez les patients adultes traités par Antivitamines K [Internet]. 2008 oct [cité 8 juin 2017]. Available on: https://www.has-sante.fr/portail/upload/docs/application/pdf/2009-02/rapport_inr_2009-02-11_11-34-20_386.pdf
- Bloomfield HE, Krause A, Greer N, Taylor BC, MacDonald R, et al. (2011) Meta-analysis: effect of patient self-testing and self-management of long-term anticoagulation on major clinical outcomes. Ann Intern Med 154(7): 472‑482. [Crossref]
- Heneghan C, Ward A, Perera R, Bankhead C, Fuller A, et al. (2012) Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. The Lancet 379(9813): 322‑334. [Crossref]
- Siebenhofer A, Hemkens LG, Rakovac I, Spat S, Didjurgeit U, SPOG 60+ Study Group (2012) Self-management of oral anticoagulation in elderly patients - effects on treatment-related quality of life. Thromb Res 130(3): e60-66.
- Cumberworth A, Mabvuure NT, Hallam M-J, Hindocha S (2013) Is home monitoring of international normalized ratio safer than clinic-based monitoring? Interact Cardiovasc Thorac Surg 16(2): 198‑201. [Crossref]
- Jennings I, Kitchen D, Keeling D, Fitzmaurice D, Heneghan C, et al. (2014) Patient self-testing and self-management of oral anticoagulation with vitamin K antagonists: guidance from the British Committee for Standards in Haematology. Br J Haematol 167(5): 600‑7.
- Siebenhofer A, Jeitler K, Horvath K, Habacher W, Schmidt L, Semlitsch T. Self-management of Oral Anticoagulation [Internet]. [cité 25 juin 2017]. Available on: http://www.aerzteblatt.de/int/archive/article?id=153787
- Sharma P, Scotland G, Cruickshank M, Tassie E, Fraser C, et al. (2015) The clinical effectiveness and cost-effectiveness of point-of-care tests (CoaguChek system, INRatio2 PT/INR monitor and ProTime Microcoagulation system) for the self-monitoring of the coagulation status of people receiving long-term vitamin K antagonist therapy, compared with standard UK practice: systematic review and economic evaluation. Health Technol Assess Winch Engl 19(48): 1‑172. [Crossref]
- Sharma P, Scotland G, Cruickshank M, Tassie E, Fraser C, et al. (2015) Is self-monitoring an effective option for people receiving long-term vitamin K antagonist therapy? A systematic review and economic evaluation. BMJ Open 5(6): e007758. [Crossref]
- Chevalier P. Intérêt de l’autocontrôle et de l’autogestion de l’INR [Internet]. Minerva Website. 2016 [last consultation: 7 june 2018]. Available on: http://www.minerva-ebm.be/FR/Analysis/445
- Heneghan CJ, Garcia-Alamino JM, Spencer EA, Ward AM, Pereira R, et al. Self-monitoring and self-management of oral anticoagulation. In: Cochrane Database of Systematic Reviews [Internet]. John Wiley & Sons, Ltd; 2016 [last consultation: 7 june 2018]. Available on: http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD003839.pub3/abstract
- Ha FJ, Barra S, Brown AJ, Begley DA, Grace AA, et al. Continuous and minimally-interrupted direct oral anticoagulant are both safe compared with vitamin K antagonist for atrial fibrillation ablation:An updated meta-analysis. IJCA 2018 (in press).
- USPO, FSPF, UNCAM. Avenant n°12 à la convention nationale organisant les rapports entre les pharmaciens titulaires d’officine et l’assurance maladie [Internet]. Available on: http://www.uspo.fr/wp-content/uploads/2017/11/avenant-12-sign%C3%A9-UNCAM-USPO.pdf



