Aim: Insulin-like growth factor-2 (IGF2) is known to regulate fetal development and differentiation, but its role in adults is still poorly understood compared with that of IGF1. This study evaluated the expression of IGF2 in obesity with type 2 diabetes mellitus (T2DM) and investigated the effects of IGF2 on adipocyte proliferation, differentiation and lipid deposition.
Methods: A cross-sectional study with pair-matched controls was conducted on 50 adults with obese T2DM and 50 controls in Yuyao people's hospital, China. Serum levels of IGF2 were measured with a commercial ELISA kit. Pre-adipocytes of 3T3-L1 cell viability was determined by an MTS assay. Oil Red O staining and Nile Red staining were performed to evaluate the effects of IGF2 on 3T3-L1 differentiation and lipid deposition.
Results: The expression of IGF2 is up-regulated in obese T2DM and correlates with lipid-related parameters like body mass index (r=0.315, p=0.002), waist circumference (r=0.271, p=0.008), triglyceride (r=0.262, p=0.008) and HDL-c (r=-0.324, p=0.002). Treatment of IGF2 could promote the proliferation of 3T3-L1 cells in a dose-dependent manner. High dose of IGF2 promoted 3T3-L1 differentiation by Oil Red O staining and enhanced the lipid accumulation in differentiated 3T3-L1 adipocytes by Nile Red staining and triglyceride assay.
Conclusion: High level of IGF2 was positively correlated with lipid-related parameters in obeseT2DM. Furthermore, IGF2 could promote the proliferation and differentiation of 3T3-L1 cells and increase the lipid deposition of differentiated 3T3-L1 adipocytes.
Insulin-like growth factor 2, differentiation, lipid deposition, obesity, type 2 diabetes
Type 2 diabetes mellitus (T2DM) and obesity are associated with nonalcoholic fatty liver disease, cardiomyopathy, and cardiovascular mortality. Both show stronger links between ectopic and visceral fat deposition, and an increased cardiometabolic risk compared with subcutaneous fat [1]. Obesity is a popular disease in today's society. Superfluous calories accumulate in the body like an overrunning river. Unless it can be dredged, T2DM will occur. The statistics indicate that the incidence of T2DM has been increasing in recent years in China over the last two decades [2]. According to the epidemiological investigation by Wenying Yang et al, the age-standardized prevalence of total diabetes (which included both previously diagnosed diabetes and previously undiagnosed diabetes) and prediabetes were 9.7% and 15.5%, respectively, accounting for 92.4 million adults with diabetes and 148.2 million adults with prediabetes among Chinese adults in 2008 [3]. The main cause is the change in dietary patterns, accompanied by a lack of exercise and the change in rest and lifestyle habits. The superfluous calories caused by elevated food intake as well as decreased energy expenditure promote weight gain and acquisition of adipose tissue [4]. Excess calories accumulate in the form of fat in the cells. Most obese T2DM patients have excessive visceral and subcutaneous fat accumulation, even “an ectopic fat deposition”, which means increased accumulation of fat at undesired sites such as the liver and skeletal muscles [5]. Research indicates that inhibiting fat cell differentiation and the accumulation of lipid droplets can prevent hereditary obesity ob/ob mouse dyslipidemia and diabetes [6].
There have been numerous studies of lipid metabolism in cultured 3T3-L1 cells (derived from the mouse fibroblast line 3T3). Differentiation is a critical process by which preadipocytes are converted into mature lipid droplet-accumulating adipocytes [7]. When cells of the established pre-adipose line 3T3-L1 enter a resting state, they accumulate triglycerides and convert to adipose cells. The conversion is brought about by a large increase in the rate of triglyceride synthesis, as measured by the incorporation rate of palmitate, acetate, and glucose [8]. As would be expected from their known actions on adipose tissue cells, lipogenic and lipolytic hormones and drugs affect the rate of synthesis and accumulation of triglyceride by 3T3-L1 cells. Insulin markedly increases the rate of synthesis and accumulation of triglyceride by 3T3-L1 adipocytes [8].
Insulin-like growth factor-2 (IGF2) is a growth-promoting polypeptide that shares a high degree of structural homology with insulin, a widely expressed peptide hormone in cell division [9]. IGF2 is a key factor regulating cell proliferation, growth, migration, differentiation and survival. IGF2 is involved in many biological processes, such as growth, lipid metabolism, tumorigenesis and development [10]. IGF2 is synthesized primarily by the liver, but it is also produced locally by many tissues, where it acts in an autocrine or paracrine manner [11]. Although it is abundant in serum, understanding of its physiological and pathological role is limited compared with that of IGF1 [12]. Altered IGF2 expression has been observed in metabolic conditions such as obesity, diabetes and polycystic ovary syndrome [13,14]. It has been long known that IGF2 levels increase dramatically during adipogenesis. Autocrine/paracrine effects of IGF2 are a major mechanism controlling adipose growth and differentiation [15,16].
In recent years, the relationship between IGF2 and diseases such as type 2 diabetes, polycystic ovary syndrome and cancer has attracted great attention. However, the relationship between IGF2 and obesity is far from clear. This study aims to determine the expression pattern of IGF2 in obese T2DM compared with normal controls and to elucidate its role in cell proliferation, adipogenic differentiation and lipid regulation in adipose cells.
Study population
Subjects were selected from a population based cross-sectional survey, which was conducted from March 2010 to May 2010 in the department of endocrinology, Yuyao people's hospital, Zhejiang, China. The serum samples were collected from random 1:1 pairing of 50 obesity with T2DM patients and 50 control subjects. Obesity and T2DM were defined according to the WHO criteria as fasting plasma glucose >7.0 mmol/l and/or 2h OGTT ≥11.0 mmol/l with a BMI of 30 or higher. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Yuyao people's hospital. Obese T2DM patients and control subjects with impaired renal function, malignancy, connective tissue disease, or chronic inflammatory disease were excluded.
Clinical evaluation and laboratory investigation
All participants were interviewed by trained medical staff, completing a standardized questionnaire regarding demographic data, lifestyle, present and past illness, medical therapy and other health-related information. Anthropometric data included measurements of height, weight, waist circumference (WC), hip circumference, heart rate (HR) and blood pressure (BP). Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters (kg/m2). WC was measured at the midpoint between the lowest rib and the iliac crest and hip circumference was measured at the widest point of the hips in the standing position. Waist-to-hip ratio (WHR) was calculated by dividing WC by hip circumference. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were calculated as the average of three measurements. The percent body fat was assessed using a Tanita Body Composition Analyzer TBF-300 (Tanita Corporation, Tokyo, Japan). All the participants conducted a 75g oral glucose tolerance test. FPG and 2hPG, triglyceride (TG), total cholesterol (TC), low density lipoprotein-cholesterol (LDL-c), and high-density lipoprotein-cholesterol (HDL-c) were measured with an auto-analyzer (Aeroset, Chicago, IL, USA). Serum insulin levels were measured by radioimmunoassay using an insulin detection kit (Beijing North Institute Biological Technology, China). Glycosylated hemoglobin A 1c (HbA 1c) values were tested using ion-exchange high-performance liquid chromatography (Hemoglobin Testing System; Bio-Rad, Hercules, CA, USA).
Measurements of IGF2 serum levels by ELISA
Blood samples were collected from the antecubital vein between 7:00 and 8:00 am after an overnight fast. The blood samples were drawn into a 5 ml ethylene diamine tetraacetic acid (EDTA) tubes and centrifuged at 4000×g for 5 min to separate the plasma content. Determination of IGF2 concentrations were evaluated using enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Sunred Biological Technology Co., Ltd.), following the manufacturer’s instructions. All measurements were taken in duplicate and averaged. The recombinant human IGF2 was from Novozymes GroPep Ltd., (Adelaide, Australia)
Culture and differentiation of 3T3-L1 preadipocytes
3T3-L1 preadipocytes were purchased from the American Type Culture Collection (ATCC). They were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS, Bio-rad) and 100IU/ml penicillin/streptomycin at 37°C, 5% CO2. The differentiation of 3T3-L1 preadipocytes was induced by the “Cocktail” method. Two days post-confluence, 3T3-L1 cells (designated as Day 0) were induced to differentiate into adipocytes via addition of differentiation mixture with DMEM containing 10% FBS, 10μg/ml insulin (TOCRIS, USA), 0.5mM 3-isobutyl-1-methylxanthine (IBMX, Sigma, USA) and 1μM dexamethasone (Sigma, USA). Two days later, culture medium was changed to DMEM supplemented with 10% FBS and 10μg/ml insulin for two days. Medium was subsequently replaced every other day with DMEM containing 10% FBS for different periods until day ten.
Cell Proliferation Assay
For testing, 3T3-L1 cells were first seeded on a 96-well plate at a density of 5×104 cells/ml and allowed to adhere in an incubator at 37°C and 5% CO2 for 24 hours. After incubation, the culture medium was replaced with IGF2 at different concentrations in fresh media (0、10、50 and 100 ng/ml). The cells were then incubated for 96 hours. Cell proliferation was measured after 24, 48, 72 and 96 hours of incubation by adding methyl tetrazolium salt (MTS) solution 20μl/well (Promega, Southampton, United Kingdom) for 4 hours and measuring the absorbance at 490 nm. The cell density of each sample was calculated with a calibration curve plotted by OD against known cell numbers.
Oil Red O staining
To evaluate the effects of IGF2 on 3T3-L1 differentiation, the cells were cultured with DM in the presence of various concentrations of IGF2 (0、10、50 and 100 ng/ml). Ten days after induction of 3T3-L1 pre-adipocyte differentiation, cells were washed twice in D-Hank’s solution, fixed in 4% formaldehyde for 30 min, and washed thrice with water. Then, cells were stained with Oil Red O (Sigma, USA) for 15 min. Following three washes in water, lipid droplets were observed and photographed under a microscope (TE2000-E; Nikon, Japan).
Nile Red staining
After preparing cells in 6-well plates with oleic acid-induced lipid deposition, 1µl Nile Red working solution (Solarbio, China) was diluted in 1ml residual volume in the plates and then incubated at room temperature for 30 min without light. Next Nile Red working solution was removed and replaced with 100 µl of DAPI nuclear (Yeasen, China). After incubation for 5 min at room temperature, the Olympus Fluorescence Microscope was used to measure the red fluorescence of Nile red at Ex/Em=552/636 nm and blue fluorescence intensity at Ex/Em=360/460 nm to detect DAPI stained nuclei.
Triglyceride Assay
TG was determined by GPO-PAP method according to the instructions from the manufacturer (catalogue no. A110; Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Statistical analysis
Normally distributed variables were expressed as mean ± standard deviation (SD), while variables with a skewed distribution were reported as median value (inter-quartile range). Categorical variables were represented by frequency and percentage. For comparison, the independent sample t-test was used to identify differences between continuous variables, while the Pearson Chi-square analysis was performed for categorical variables. Spearman correlation analysis and linear regression was used to analyze the correlation between IGF2 and physical examination and biochemical indexes. Odds ratios (ORs) and 95% confidence intervals (CIs) were simultaneously estimated by logistic regression model to evaluate IGF2 and metabolic syndrome or its five components. The SPSS ver. 17 (Chicago, IL, USA) was used for statistical analysis. Differences were considered statistically significant at a p value of <0.05.
Baseline participant characteristics
The anthropometric and metabolic characteristics of the study population at baseline were presented in table 1. The average age of the participants was 55.30 (±6.84) years, and 51.25% of the participants were male. As expected, participants with obese T2DM had more cardiovascular risk factors at baseline compared to those without obesity and T2DM, including significantly higher BP, waist‑to‑hip ratio, body fat, fasting insulin levels, as well as obese T2DM indicators, including significantly higher BMI, WC, blood glucose, HbA1c,TG, and significantly lower HDL-c (p<0.05 for all parameters). Furthermore, The ELISA assay was performed to examine the concentration of IGF2 in the sera samples. The results revealed that participants with obese T2DM had higher serum IGF2 levels compared to those without obesity and T2DM [379.43±169.17 vs 285.65±149.76 ng/ml, p = 0.014].
Table 1. Clinical characteristics of the participants included in the validation study. Data are presented as the mean ± standard deviation, the median with 25‑75% interquartile range or n (%). BMI, body mass index; WC, waist circumference; WHR, waist‑to‑hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; ALT, alanine transaminase; AST, aspartate transaminase; FPG, fasting plasma glucose; FINS, fasting serum insulin; TC, total cholesterol; LDL‑c, low‑density lipoprotein‑cholesterol; HDL‑c high density lipoprotein‑cholesterol; TG, triglyceride
Variables |
Total |
Controls |
Obese T2DM |
p for trend |
N |
100 |
50 |
50 |
|
Serum IGF2 (ng/mL) |
340.05±174.15 |
285.65±149.76 |
379.43±169.17 |
0.014 |
Age (years) |
55.30±6.84 |
54.45±6.40 |
56.23±6.85 |
0.638 |
Male, n(%) |
41(51.25) |
18(45.00) |
23(57.50) |
0.532 |
Current smoker, n(%) |
34(42.5) |
8(10) |
26(32.5) |
<0.001 |
Alcohol drinker, n(%) |
25(31.25) |
13(16.25) |
12(15) |
0.117 |
BMI(kg/m2) |
23.78±3.04 |
21.99±2.24 |
25.28±2.50 |
<0.001 |
WC (cm) |
80.54±8.45 |
73.00±7.23 |
88.50±6.92 |
<0.001 |
WHR |
0.88±0.07 |
0.85±0.62 |
0.95±0.56 |
<0.001 |
Fat% (%) |
28.68±6.47 |
26.26±5.65 |
30.87±6.39 |
0.001 |
SBP (mm Hg) |
123.41±17.85 |
116.67±16.34 |
134.67±17.34 |
<0.001 |
DBP (mm Hg) |
81.34±9.21 |
78.32±7.88 |
84.10±8.71 |
<0.001 |
HbA1C (%) |
5.65±1.02 |
5.50±0.48 |
6.40±1.15 |
0.002 |
ALT (U/L) |
18.00(14.00-26.00) |
17.70(10.90-25.50) |
29.10(16.90-33.30) |
0.001 |
AST (U/L) |
19.00(16.00-23.00) |
19.70(14.70-24.70) |
23.50(17.50-29.50) |
0.174 |
FPG (mmol/L) |
5.13(4.50-5.82) |
5.71(5.44-6.40) |
6.42(4.85-6.81) |
0.002 |
2h PG (mmol/L) |
6.23(4.82-8.11) |
6.58(5.4-7.4) |
9.17(7.65-12.26) |
<0.001 |
FINS (μU/ml) |
12.66(10.18-15.87) |
11.48(9.48-14.17) |
17.23(13.18-19.87) |
<0.001 |
2h INS (μU/ml) |
59.25(47.32-87.80) |
54.35(36.39-83.57) |
81.68(49.75-165.18) |
<0.001 |
TC (mmol/L) |
5.53±1.02 |
5.37±0.88 |
5.84±1.07 |
0.308 |
LDL-c (mmol/L) |
2.25(2.00-2.64) |
2.34(1.90-2.78) |
2.29(1.71-2.87) |
0.807 |
HDL-c (mmol/L) |
1.31(1.03-1.66) |
1.75(1.35-1.98) |
1.10(0.86-1.34) |
<0.001 |
TG (mmol/L) |
1.57(0.88-2.21) |
1.03(0.61-1.45) |
3.23(1.81-3.26) |
<0.001 |
Expression of serum IGF2 is up-regulated in obese T2DM and correlates with lipid-related parameters
Associations between serum IGF2 levels and parameters related to adiposity, insulin resistance, lipid profiles and hepatic enzymes were further assessed. Spearman correlation analyses of serum IGF2 with metabolic risk were performed to find the correlation between serum IGF2 levels and metabolic parameters at baseline. After adjustment for gender, age, smoking and drinking, the levels of serum IGF2 displayed some degree of correlation with indexes of lipid-related factors, like body mass index (the relevant index r=0.315, p=0.002), waist circumference (r=0.271, p=0.008), percentage of body fat (Fat%) (r=0.254, p=0.018), low HDL-c (r=-0.324, p=0.002) and TG (r=0.262, p=0.008). Serum IGF2 levels were also correlated with SBP, FPG and FINS (all p<0.05) (Table 2). Linear regression analysis was performed to determine the association of TG and low HDL-c with serum IGF2 levels (Figure 1A).
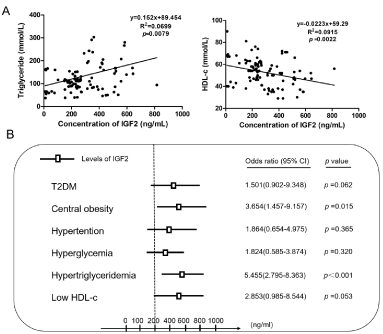
Figure 1. Expression of serum IGF2 and its relationship with lipid-metabolism related parameters in obese T2DM patients compared to control subjects. (A) Scatterplot and Spearman correlation analyses of serum IGF2 levels with triglyceride and high-density lipoprotein-c. (B) Binary logistic regression analysis for the relationship between dyslipidemia and other metabolic subgroups (Central obesity, hypertension, hyperglycemia, hypertriglyceridemia, and low HDL-c) associated with serum IGF2 levels
Table 2. Spearman correlation analyses of serum IGF2 with lipid-related parameters
Variables |
Unadjusted |
Age, sex, smoking, and drinking adjusted |
|
r |
p value |
r |
p value |
BMI(kg/m2) |
0.334 |
<0.001 |
0.315 |
0.002 |
WC (cm) |
0.350 |
<0.001 |
0.271 |
0.008 |
WHR |
0.247 |
0.013 |
0.212 |
0.066 |
Fat% (%) |
0.302 |
0.009 |
0.254 |
0.018 |
SBP (mm Hg) |
0.331 |
0.003 |
0.370 |
0.001 |
DBP (mm Hg) |
0.098 |
0.386 |
0.087 |
0.457 |
ALT (U/L) |
0.023 |
0.818 |
0.047 |
0.653 |
AST (U/L) |
-0.002 |
0.980 |
-0.009 |
0.929 |
FPG (mmol/L) |
0.234 |
0.037 |
0.277 |
0.015 |
2h PG (mmol/L) |
0.001 |
0.993 |
0.153 |
0.187 |
FINS (μU/ml) |
0.124 |
0.074 |
0.234 |
0.023 |
2h INS (μU/ml) |
0.107 |
0.026 |
0.175 |
0.056 |
HbA1c (%) |
0.193 |
0.086 |
0.175 |
0.130 |
TC (mmol/L) |
0.054 |
0.633 |
0.014 |
0.905 |
LDL-c (mmol/L) |
0.132 |
0.242 |
0.112 |
0.318 |
HDL-c (mmol/L) |
-0.305 |
<0.001 |
-0.324 |
0.002 |
TG (mmol/L) |
0.307 |
<0.001 |
0.262 |
0.008 |
Risk of dyslipidemia relative to serum IGF2 concentrations
Subsequently, we further assessed whether high levels of serum IGF2 could effectively predict the risk of incidence of metabolism subgroups (T2DM, central obesity, hypertension, hyperglycemia, dyslipidemia, and low HDL-c) in obese T2DM. Multiple stepwise regression analysis using serum IGF2 levels as a dependent variable after adjustment for gender, age, smoking and drinking revealed that serum IGF2 levels were independently related to BMI (β=0.256, p<0.001), low HDL-c (β=-0.152, p=0.002) and TG (β=0.028, p=0.045) (Table 3). The results demonstrated that serum IGF2 levels are associated with lipid profiles in obese T2DM patients. Finally, binary regression analyses demonstrated that a high serum IGF2 level is a risk factor of hypertriglyceridemia [odds ratio (OR) =5.455, 95% confidence interval (CI) =2.795-8.363, p<0.001] and low HDL-c (OR =2.853, 95% CI =0.985-8.544, p = 0.053). The incidence of hypertriglyceridemia in participants with a high serum IGF2 level was 5.455 times higher in obese T2DM patients compared to those with low serum IGF2 levels. The incidence of low HDL-c in participants with a high serum IGF2 levels was 2.853 times higher in obese T2DM patients compared to those with low serum IGF2 levels after adjusting for age, gender, smoking, and drinking status (Figure 1B).
Table 3. Multiple stepwise regression analyses of independent factors associated with serum IGF2 levels
Independent variables |
β |
p value |
serum IGF2 |
BMI |
0.256 |
<0.001 |
HDL-c (mmol/L) |
0.152 |
0.002 |
TG |
0.028 |
0.045 |
IGF2 promotes 3T3-L1 proliferation, differentiation and lipid accumulation
We performed an MTS assay to analyze the viability of 3T3-L1 pre-adipocytes treated with various concentrations of IGF2 (0、10、50 and 100 ng/ml). The results revealed that treatment of IGF2 exhibited a dose-dependent increase in the absorption at 490nm, after treatment with IGF2 in 100ng/ml for 72 hours, the absorption reached the peak (Figure 2A). According to the “Cocktail” method,3T3-L1 pre-adipocytes were differentiated into mature-adipocytes. During the 3T3-L1 pre-adipocyte differentiation, IGF2 expression increased according to the results of ELISA towards the day 0、4、8 and 12 (Figure 2B). The additional Oil Red O staining assay in differentiated 3T3-L1 cells treated with various concentrations of IGF2 revealed that high dose of IGF2 promoted 3T3-L1 differentiation and increased the lipidosis in differentiated 3T3-L1 cells (Figure 2C). Treatment with 50ng/ml IGF2 could enlarge the lipid droplets as well as enhance the lipid accumulation in 3T3-L1 adipocytes by Nile Red staining (Figure 2D). To assess the level of lipid uptake, intracellular triglyceride levels were measured by triglyceride assays which confirmed that increasing IGF2 levels enhanced the lipid accumulation in differentiated 3T3-L1 adipocytes (Figure 2E). These results suggest that IGF2 drives 3T3-L1 pre-adipocytes into adipogenic differentiation accompanied with lipid accumulation.
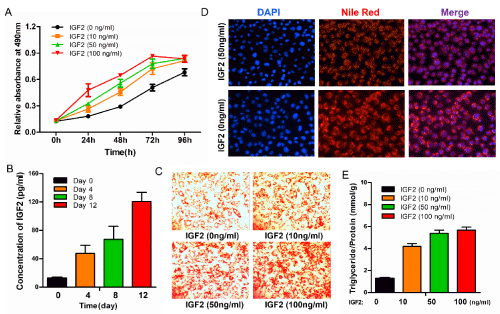
Figure 2. IGF2 promotes 3T3-L1 cell proliferation and increases oil accumulation. (A) Treatment of IGF2 promotes the proliferation of 3T3-L1 cells in a dose-dependent manner. (B) Expression levels of IGF2 during 3T3-L1 pre-adipocyte differentiation. (C) Oil red O staining assay was performed to detect 3T3-L1 pre-adipocyte differentiation and the deposition of lipid droplets with treatment of various concentrations of IGF2 (0、10、50 and 100 ng/ml). Magnification, 20×. (D) Intracellular fat droplets were stained with Neil red and nuclei were stained with DAPI. Scale bar=100 μm. (E)The histogram shows quantities of intracellular triglycerides measured by triglyceride assay
It has been reported that the serum IGF2 concentration in diabetic obese patients had increased and was positively correlated with BMI. In one study, the total serum IGF2 concentration of the obese group was higher than that of lean control subjects, and the free IGF2 showed a parallel increase, indicating that the biological activity of IGF2 was also improved [17].This is probably an appropriate physiological response that promotes energy storage and thus increases fat supplies. The reversibility of this change supports this argument. In the case of weight loss, total serum IGF2, IGF2R and pro-IGF2 are reduced, which is independent of the type of diet [18]. The previous study also showed that the expression of IGF2 in serum samples of patients with metabolic syndrome was higher than that of the healthy control group, and the expression of IGF2 was also higher in obese mice with a high-fat diet and mice with abnormal glucose tolerance than that of mice with normal feeding, suggesting that IGF2 plays a potential role in obesity, diabetes and metabolic syndrome [19]. The present study showed that when obese subjects suffer from Type 2 diabetes, serum IGF2 was significantly elevated, when compared to control subjects.
Obese subjects are prone to develop Type 2 diabetes and in T2DM, IGF2 is in an abnormal regulatory state. In studies of obese individuals (with or without type 2 diabetes), serum IGF2 and IGF2R were observed to be higher in obese individuals than in control subjects. In obese patients with T2DM, IGF2 and IGF2R concentrations are higher and decrease with weight loss [20,21]. Epidemiological studies have shown that continued growth of IGF2 is harmful [22]. Among African-American women, there is a strong association between diabetes and elevated breast cancer risk. This suggests that excessive IGF2 is more likely to lead to diabetes. Therefore, excessive IGF2 increases the incidence of cancer and diabetes [23]. Our results also showed that high levels of serum IGF2 is a risk factor of hypertriglyceridemia and low HDL-c. The incidence of hypertriglyceridemia in participants with a high serum IGF2 level was 5.455 times higher in obese T2DM patients compared to those with low serum IGF2 levels. The incidence of low HDL-c in participants with a high serum IGF2 levels was 2.853 times higher in obese T2DM patients compared to those with low serum IGF2 levels
Adipose tissue is an important energy storage site in the body, which accounts for about one-third of the fat cells in adipose tissue, adipocyte differentiation disorders could lead to an increased in abnormal, excessive lipid accumulation, which in turn may lead to obesity, diabetes, fatty liver disease, hyperlipidemia, atherosclerosis and other diseases [18,21,24]. With the discovery of Leptin, people gradually realized that adipose tissue also played an active role in endocrine functions, regulating various activities of the body. Leptin is a hormone that is released mainly by adipocytes. One of the roles of leptin in animals is regulation of the energy balance by decreasing food intake and increasing energy expenditure. It is also believed that this hormone takes part in the regulation of hematopoietic, endocrine (other than reproductive) and sympathetic system functioning, and is involved in pathogenesis of arterial hypertension and diabetes [25]. Adipose tissue produces various cytokines, called adipokines that can potentially alter peripheral insulin sensitivity [26]. Adipokines such as leptin, adiponectin, resistin, visfatin, inteleukin-6, and tumor necrosis factor alpha have multiple functions and have been found to affect insulin sensitivity, satiety, carbohydrate and fat metabolism [27]. Increases in adipose tissue mass and obesity can occur due to enlargement of existing fat cells (hypertrophy), increased proliferation (hyperplasia), and increased rate of differentiation of adipocytes from their precursor cells [28]. There is evidence that IGF2 plays a major role in adipose tissue, skeletal muscle, and ovaries [9]. All the experimental data, obtained in both animal and cellular models, argue that IGF2 may regulate metabolic homeostasis by affecting body composition, particularly regulating the accrual of adipose tissue [29]. Our experimental results demonstrated that IGF2 can promote the proliferation and differentiation of 3T3-L1 pre-adipocytes and increase lipid accumulation, which may provide reference value for the mechanism of IGF2 in adipose tissue.
However, the regulation of IGF2 in adipocytes is poorly understood. One possible mechanism for the increase in adipose tissue IGF2 expression is increased ICR/H19 DMR methylation [30]. Previous studies have shown that IGF2 methylation is associated with a higher lipid profile in obese children and the IGF2/H19 methylation degree at birth has recently been related to the development of overweight or obesity in early childhood [31]. The IGF2-INS-TH genomic region has been implicated in various common disorders including the metabolic syndrome, type 2 diabetes and coronary heart disease (CHD). Haplotype IGF2-INS-TH is associated with significantly higher fat mass and percentage fat, and with significantly higher diastolic BP: the means for systolic BP and plasma TG are also respectively the highest and second highest for any haplotype [32]. IGF2 develops its function by binding to IGF1 and insulin receptors, recombinant IGF2 increased genes expression associated with lipogenesis and enhanced protein expression on PI3K/AKT/SREBP pathway [33]. IGF2 inhibitor also significantly reduce TG deposition in hepatocytes and hepatic IGF2 overexpression could lead to an increased lipid droplet formation in adult mice [34,35]. These findings support the idea that IGF2 is positively related to hepatic lipogenesis via PI3K/AKT/SREBP pathway.
In this study, we show that serum IGF2 has the potential to be a novel biochemical marker in obese T2DM, and up-regulation of IGF2 is closely linked with lipid metabolism disorders. Therefore, down-regulation of IGF2 might be a new therapeutic target to recover the function of impaired adipocytes in obese T2DM.
LP X, J M, Y M, ZX Y, YM H, CY G and SQ S conceived this study. LP X and LD Y collected and analyzed the data. LP X and SQ S wrote the manuscript. All authors approved the final version.
We thank the staff of the Department of Endocrinology, Yuyao people's hospital for their valuable input and support throughout this study.
This project was supported by grants from the Ningbo Natural Science Foundation (2017A610205).
All authors have read the journal's policy on conflicts of interest. The authors declare no conflict of interest.
- Levelt E, Pavlides M, Banerjee R, Mahmod M, Kelly C, et al. (2016) Ectopic and Visceral Fat Deposition in Lean and Obese Patients with Type 2 Diabetes. J Am Coll Cardiol 68: 53-63. [Crossref]
- Jia W, Weng J, Zhu D, Ji L, Lu J, et al. (2019) Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab Res Rev 35: e3158. [Crossref]
- Yang SH, Dou KF, Song WJ (2010) Prevalence of diabetes among men and women in China. N Engl J Med 362: 2425-2426. [Crossref]
- Trujillo Viera J, El-Merahbi R, Nieswandt B, Stegner D, Sumara G, et al. (2016) Phospholipases D1 and D2 Suppress Appetite and Protect against Overweight. PLoS One 11: e0157607.
- Abranches MV, Oliveira FC, Conceicao LL, Peluzio MD (2015) Obesity and diabetes: the link between adipose tissue dysfunction and glucose homeostasis. Nutr Res Rev 28: 121-32.
- Cheng PT, Mukherjee R (2005) PPARs as targets for metabolic and cardiovascular diseases. Mini Rev Med Chem 5: 741-753. [Crossref]
- Lustig M, Gefen A, Benayahu D (2018) Adipogenesis and lipid production in adipocytes subjected to sustained tensile deformations and elevated glucose concentration: a living cell-scale model system of diabesity. Biomech Model Mechanobiol 17: 903-913.
- Green H, Kehinde O (1975) An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5: 19-27. [Crossref]
- Livingstone C, Borai A (2014) Insulin-like growth factor-II: its role in metabolic and endocrine disease. Clin Endocrinol (Oxf) 80: 773-781. [Crossref]
- Gallagher EJ, LeRoith D (2011) Minireview: IGF, Insulin, and Cancer. Endocrinology 152: 2546-2551. [Crossref]
- Haselbacher G, Humbel R (1982) Evidence for two species of insulin-like growth factor II (IGF II and "big" IGF II) in human spinal fluid. Endocrinology 110: 1822-1824. [Crossref]
- Back K, Brannmark C, Stralfors P, Arnqvist HJ (2011) Differential effects of IGF-I, IGF-II and insulin in human preadipocytes and adipocytes--role of insulin and IGF-I receptors. Mol Cell Endocrinol 339: 130-135. [Crossref]
- Roth SM, Schrager MA, Metter EJ, Riechman SE, Fleg JL, et al. (2002) IGF2 genotype and obesity in men and women across the adult age span. Int J Obes Relat Metab Disord 26: 585-587. [Crossref]
- Narayanan RP, Fu B, Oliver RL (2014) Insulin-like growth factor-II and insulin-like growth factor binding protein-2 prospectively predict longitudinal elevation of HDL-cholesterol in type 2 diabetes. Ann Clin Biochem 51:468-475.
- Alfares MN, Perks CM, Hamilton-Shield JP, Holly J (2018) Insulin-like growth factor-II in adipocyte regulation: depot-specific actions suggest a potential role limiting excess visceral adiposity. Am J Physiol Endocrinol Metab 315: E1098-1098E1107.
- Mercader JM, Liao RG, Bell AD (2017) A Loss-of-Function Splice Acceptor Variant in IGF2 Is Protective for Type 2 Diabetes. Diabetes 66: 2903-2914.
- Buchanan CM, Phillips AR, Cooper GJ (2001) Preptin derived from proinsulin-like growth factor II (proIGF-II) is secreted from pancreatic islet beta-cells and enhances insulin secretion. Biochem J 360: 431-439. [Crossref]
- Frystyk J, Skjaerbaek C, Vestbo E, Fisker S, Orskov H, et al. (1999) Circulating levels of free insulin-like growth factors in obese subjects: the impact of type 2 diabetes. Diabetes Metab Res Rev 15: 314-22.
- Jeyaratnaganthan N, Hojlund K, Kroustrup JP (2010) Circulating levels of insulin-like growth factor-II/mannose-6-phosphate receptor in obesity and type 2 diabetes. Growth Horm IGF Res 20: 185-91.
- Kalla Singh S, Tan QW, Brito C, De Leon M, Garberoglio C, et al. (2010) Differential insulin-like growth factor II (IGF-II) expression: A potential role for breast cancer survival disparity. Growth Horm IGF Res 20:162-170.
- Sandhu MS, Gibson JM, Heald AH, Dunger DB, Wareham NJ (2003) Low circulating IGF-II concentrations predict weight gain and obesity in humans. Diabetes 52: 1403-1408. [Crossref]
- Belobrajdic DP, Frystyk J, Jeyaratnaganthan N (2010) Moderate energy restriction-induced weight loss affects circulating IGF levels independent of dietary composition. Eur J Endocrinol 162:1075-1082.
- Rose DP, Haffner SM, Baillargeon J (2016) Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr Rev 28: 763-777.
- Gude MF, Hjortebjerg R, Oxvig C (2016) PAPP-A, IGFBP-4 and IGF-II are secreted by human adipose tissue cultures in a depot-specific manner. Eur J Endocrinol 175: 509-519.
- Kulik-Rechberger B (2003) Leptin--the metabolic signal from adipose tissue. Przegl Lek 60: 35-39. [Crossref]
- Stefanyk LE, Dyck DJ (2010) The interaction between adipokines, diet and exercise on muscle insulin sensitivity. Curr Opin Clin Nutr Metab Care 13: 255-259.
- Jamurtas AZ, Stavropoulos-Kalinoglou A, Koutsias S, Koutedakis Y, Fatouros I, et al. (2015) Adiponectin, Resistin, and Visfatin in Childhood Obesity and Exercise. Pediatr Exerc Sci 27: 454-462.
- Bol VV, Reusens BM, Remacle CA (2008) Postnatal catch-up growth after fetal protein restriction programs proliferation of rat preadipocytes. Obesity (Silver Spring) 16: 2760-2763.
- Cianfarani S (2012) Insulin-like growth factor-II: new roles for an old actor. Front Endocrinol (Lausanne) 3: 118.
- Sasaki H, Ishihara K, Kato R (2000) Mechanisms of Igf2/H19 imprinting: DNA methylation, chromatin and long-distance gene regulation. J Biochem 127: 711-715. [Crossref]
- Deodati A, Inzaghi E, Liguori A, Puglianiello A, Germani D, et al. (2013) IGF2 methylation is associated with lipid profile in obese children. Horm Res Paediatr 79: 361-367. [Crossref]
- Rodríguez S, Gaunt TR, O'Dell SD, Chen XH, Gu D, et al. (2004) Haplotypic analyses of the IGF2-INS-TH gene cluster in relation to cardiovascular risk traits. Hum Mol Genet 13: 715-725. [Crossref]
- Liu Y, Shen J, Yang X, Sun Q, Yang X (2018) Folic Acid Reduced Triglycerides Deposition in Primary Chicken Hepatocytes. J Agric Food Chem 66: 13162-13172.
- Kessler SM, Laggai S, Van Wonterg E (2016) Transient Hepatic Overexpression of Insulin-Like Growth Factor 2 Induces Free Cholesterol and Lipid Droplet Formation. Front Physiol 7: 147.
- [Crossref] Da Costa TH1, Williamson DH, Ward A, Bates P, Fisher R, et al. (1994) High plasma insulin-like growth factor-II and low lipid content in transgenic mice: measurements of lipid metabolism. J Endocrinol 143: 433-439.


