Background: Brainstem gliomas in adults are a rare and heterogeneous group of brain tumors that vary with regard to underlying pathology, radiographic appearance, clinical course and prognosis. Diffuse intrinsic pontine gliomas represent the most common subtype. In the literature there are not many cases of spinal metastatic glioblastoma from previous intracranial high- grade glioma.
We report the clinical presentation, immunohistochemistry (IHC), radiological presentation and the operative management of a cervical intramedullary spinal glioblastoma from a previous brainstem anaplastic astrocytoma, but also a brief review of the literature about this topic.
Case presentation: A 25-year-old male patient underwent in 2016, a stereotactic biopsy for a brainstem lesion. Anatomopathologic analysis revealed a diffuse anaplastic astrocytoma. He then received chemotherapy with Temozolomide and radiotherapy. Clinical and radiological evolution has been quite good, but three years later, he developed a worsening of the right hemiparesis, a new onset of left hemiparesthesia and left lower limb hemi anaesthesia.
A full spine MRI showed a C2-C3 nodular intramedullary lesion. A surgical resection of this lesion was performed. A glioblastoma was diagnosed. Further radio-chemotherapy was given and the follow- up showed no recurrence 6 months after.
Conclusion: Intramedullary metastasis from intracranial glioma are rare but possible. Despite the progress in the management of glioblastoma, this pathology should be recognized. Clinical physicians should be aware about the significance that secondary spinal glioblastoma might ensue in patients with intracranial gliomas.
anaplastic glioma, intramedullary glioblastoma, spinal matastasis, gfap expression
Glial tumors represent 30% of primary tumors of the central nervous system (CNS) in adults and 81% of malignant CNS tumors diagnosed in the USA [1]. Glioblastoma multiforme (GBM) was first described by Rudolph Virchow in 1863 and represents the most malignant tumor of the cerebral hemispheres in adult population [2]. These tumors are associated with a very poor prognosis with actually a median survival between 15-18 months [3]. GBM is usually localized in the supratentorial parts of the brain and infratentorial localization are rare situations [4]. Anaplastic astrocytoma also pertains to aggressive behaviour and the potential of malignant transformation into glioblastoma, with 23% of 5‑year survival rate [5]. Brainstem tumors occur more frequently in children (10-20% of all tumors) and 90% of them are gliomas [6]. In adults, brainstem tumors account for only 3-5% [7]. In virtue of their deep location and the presence of vital neural centers within, they are associated with a poor prognosis. The common causes of death are loco regional extension and / or recurrence and, less frequently, leptomeningeal dissemination [8].
Brainstem tumors are more commonly encountered in children and represent 10% of all paediatric brain tumors. They represent only 1% to 2% of all brain tumors in adults and commonly involve the pons (60%-63% of tumors). They are also identified in the medulla oblongata (25% of tumors) and the midbrain (12%-15% of tumors) [22]. Spinal metastatic glioblastoma, which is a very infrequent clinical condition, is usually the result of tumoral cells dissemination from a previous intracranial glioblastoma via the cerebrospinal fluid seeding pathway [5]. Here we report very rare case of metastatic spinal glioblastoma originated from a previous brainstem high grade glioma in a young male patient. We present the pathology, the immunohistochemistry and the management of the disease.
Medical history and examination
A 25-year-old male patient, without systemic disease, presented to the department of neurosurgery at the Hospital of Namur in Belgium, in August 2016 for right hemiparesia, right facial palsy, dysarthria and diplopia. A full cerebral magnetic resonance imaging (MRI) showed a large pontine infiltrating tumor, with surrounding oedema compatible with a brainstem glioma (Figure 1A and 1B).
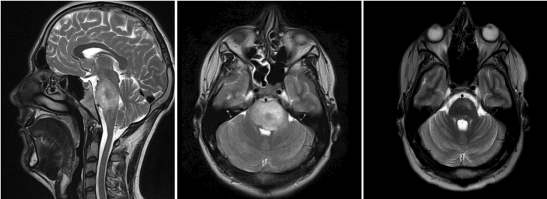
Figure 1. Preoperative cervical MRI showing a diffuse glial lesion developed in the annular protuberance of the brainstem (white arrow) on sagittal T2-weighted sequence (A), axial T2-weighted sequence (B) and axial T2-weighted sequence 2 years after multimodal treatment of the pontine glioma (C).
A stereotactic biopsy of this lesion was performed in September 2016. The histopathology demonstrated an anaplastic astrocytoma (grade III OMS Classification), with a Ki67 around 10-15%. IHC analysis showed positive glial fibrillar acidic protein (GFAP) (Figure 2), high Ki-67 labeling index (10 – 15%), positive tumor protein P53 (TP53) mutation and a negative finding of isocitrate dehydrogenase-1 (IDH-1). A K27M mutation in histone H3.3 was also found.
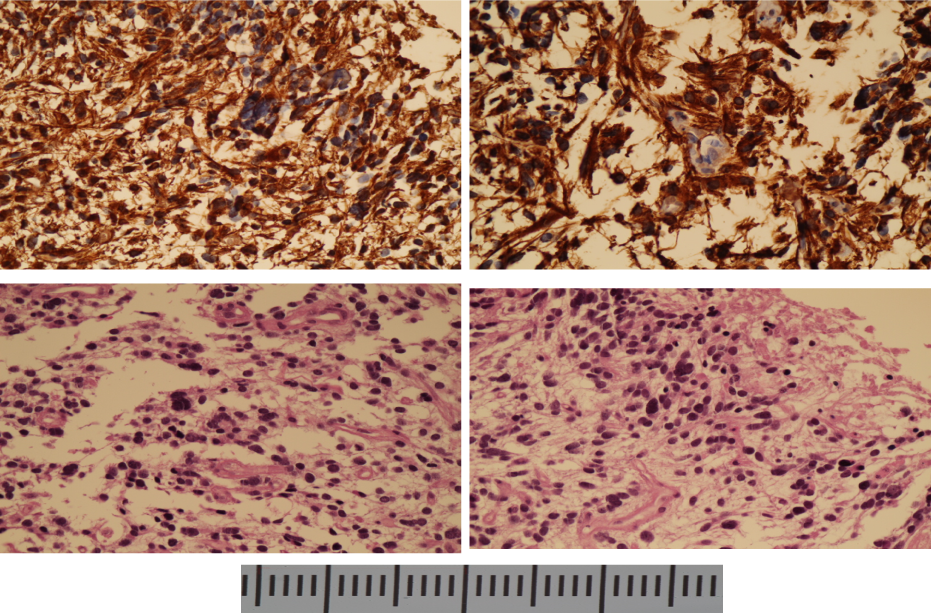
Figure 2. Biopsy of the brainstem high grade glioma with A and B GFAP-positive cells in IHC; C and D hematoxylin and eosin staining showing microvascular proliferation and many mitoses (40X magnification)
He then received combined radiotherapy in total of 54 Gy distributed in 1.8 Gy/day and chemotherapy with Temozolomide (75mg/m2). This treatment resulted in a quite good clinical and radiological evolution (Figure 1C), with improvement of motor deficit.
Despite the persistence of right facial palsy (House-Brackmann [4]), the patient was satisfied with the result and started working again.
Three years later, he developed a worsening of his neurological condition. He was readmitted on October 2019, owing to this new onset of symptoms for a check-up.
The neurological examination revealed a worsening of the right hemiparesis, a left-sided hemiparesthesia and left lower limb anaesthesia.
Cranial and whole spinal magnetic resonance imaging (MRI) examinations were performed. Cranial MRI showed at C2-C3 level, a nodular process measuring 36 × 10 × 9 MM, hyperintense on T2-weighted and on Flair sequences and heterogeneously enhanced in periphery after injection of gadolinium (Figure 3).
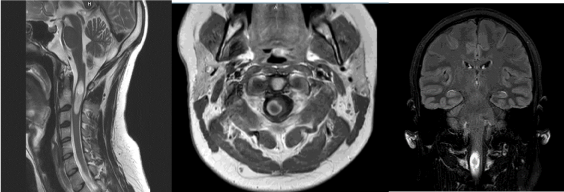
Figure 3. Preoperative cervical MRI showing an intramedullary C2-C3 lesion on T2-Weighted cervical MRI (A), axial contrast-enhanced T1-weighted (B) and Flair sequence (C). Note that this lesion is associated with a cranial kystic cavity and a medullar oedema
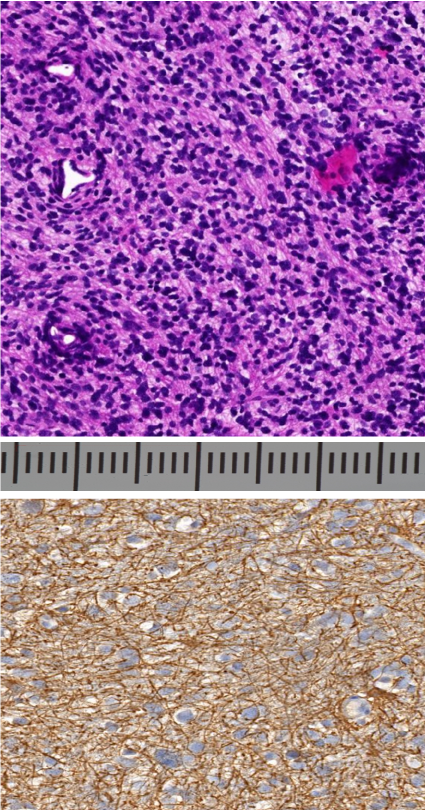
Figure 4. Histologic studies of the intramedullary lesion confirming a GBM with: A: increased cellularity and frequent mitoses and necrosis on hematoxylin and eosin staining (9 X magnification) and B: GFAP-positive cells in IHC (20 X magnification)
Surgery and pathology
The surgical management of the cervical metastatic lesion consisted in a gross total resection (GTR) under general anaesthesia. Histopathologic diagnosis and safe maximal resection of the lesion were the goals of the suggested surgery.
To facilitate tumoral removal, fluorescence-guided surgery (FGS) using 5-aminolevulinic acid (5-ALA) was used. That means the patient received three hours before reaching the operating room, an oral solution of 5 – aminolevulinic acid (5-ALA) also known as Gliolan.
The patient was placed in prone, with the head fixed in a Mayfield headrest, perfectly exposing the upper cervical region. The incision location was defined using a fluoroscopy, guided by the occipito-cervical landmarks. We realized a midline posterior approach and then a laminectomy of C1, C2 and C3 was performed. On the MRI imaging, the lesion seems to be right laterally located within the spinal cord.
We perform under microscope a right paramedian linear opening of the dura-mater, allowing immediate visualization of a light red coloured lesion inside the right hemi medulla flowering under the thickened arachnoid.
The arachnoid was opened and a lateral incision of the medulla just in front of the emergence of the posterior roots was done. There seemed to be a boundary between the tumor and the medulla. The tumor was progressively aspirated and removed largely using an Ultrasonic Surgical Aspirator (Figure 5).
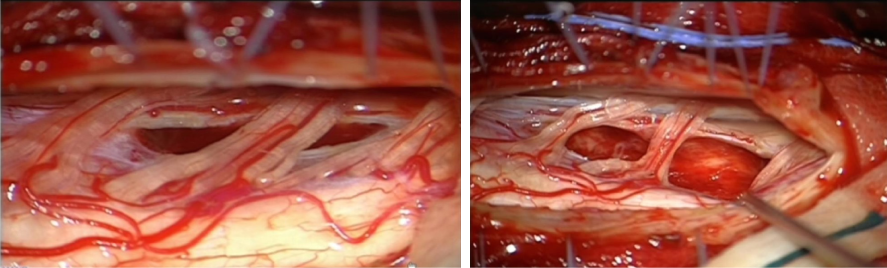
Figure 5. The dura mater (DM) has been opened allowing the visualization of the medulla and the cervical roots. A: Part of the tumor have been removed. The blue arrow shows a remnant of this light red coloured tumor. B: Intramedullary cavity after total removal of the tumor.
Unfortunately, the tumoral cells showed no fluorescence with Gliolan uder microscope. Nevertheless, we performed a GTR . Histopathologic examination revealed a glioblastoma (grade IV OMS classification). IHC studies showed a high rate of MGMT mutation (30%), a strong expression of glial fibrillar acidic protein (GFAP) (Figure 4). There was no expression of isocitrate dehydrogenase 1.
The patient received a further concurrent chemoradiotherapy (CCRT) following the STUPP scheme. The evolution was quite good, and the patient recovered from his right hemiparesis so that he could walk without any additional help. The follow up was done using cerebral and full spine MRI examination and the last MRI, performed 6 months after the surgery revealed no residual tumor or tumoral recurrence (Figure 6). Currently he has recovered from his neurological deficits and thus he pursues a normal life and a satisfactory professional activity.
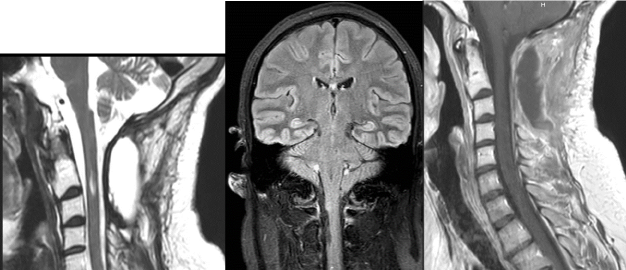
Figure 6. Postoperative MRI images. A: Sagittal T2-weighted sequence showing a little intramedullary cavity filled by LCR, and no residue or recurrence of the C2-C3 lesion. B: Coronal Flair sequence showing no sign of intramedullary lesion and a right-side intramedullary resection cavity (Blu arrow). C: Sagittal contrast- enhanced T1-weighted sequence showing no enhancement in the medulla.
Our young patient presented a brainstem anaplastic glioma responsible of a metastatic cervical spinal glioblastoma. The relationship between this brainstem lesion and the spinal cord tumor might seem uncertain. In this case we tried to reassemble the criteria proposed by Weiss in 1955 to establish an acceptable metastatic link between the primary tumor and the spinal metastatic glioblastoma. The Weiss criteria are 1. the presence of a single histological characteristic tumor of the central nervous system; 2. a clinical history which demonstrate that the tumor accounted for the initial symptoms; 3. a complete necropsy to exclude any other primitive tumor ; 4. identical morphology of the primary lesion and the metastasis. In our case, the majority of the criteria were fulfilled except for the third one because the patient is alive and the check-up didn’t show any other tumoral location.
The spinal glioblastoma might be consider as induced by radiotherapy. This hypothesis is invalidated because the GBM occurred out of the margin of the previously irradiated field. In fact, the planned target volume included the brainstem tumor as defined by the T2-weighted MRI images with a margin of 1cm (Figure 7A).
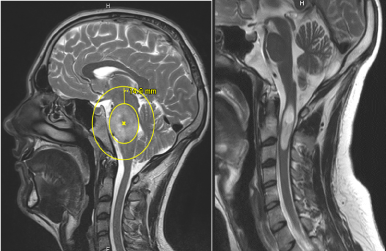
Figure 7. T2-weighted MRI showing A: the planned target volume including the brainstem tumor with a margin of more than 1 cm. The cervical region is out of irradiation field. B: The fatty changes in the occipital and clivus narrow bone, due to previous radiotherapy (blue arrows).
Moreover, we can notice fatty changes in clivus bone narrow (Figure 7A), corresponding to the irradiation field of brainstem tumor. It’s clearly not the case of the cervical vertebrae. It is the confirmation that the upper cervical region was not within or at the margin of the field of irradiation. Better still, the same fatty changes of bone narrow are now visible in the cervical vertebrae after irradiation of the tumoral bed (Figure 6A).
High grade brainstem glioma is a less frequent entity in adults than in children (<2%) (9,22) and it is not that usual to deal with intramedullary metastasis originated from this type of tumors [10]. The most common location of brainstem gliomas is known to be the pons followed by medulla and midbrain [11,22].
Glioblastoma, contrarily are rarely encountered in the brainstem. Clinically, brainstem gliomas present with symptoms like multiple cranial nerve palsy and long tract signs [12]. It was clearly the case of our patient who presented with right hemiparesia, right facial palsy, dysarthria and diplopia.
Guillamo et al. [13] have classified brainstem gliomas in these groups : adult diffuse infiltrative low-grade gliomas, adult malignant brainstem glioma, focal tectal brainstem glioma and others.
Otherwise, Epstein and Farmer [14] presented another classification of brainstem gliomas : they separated them into diffuse, focal, exophytic and cervico-medullary. According to these classifications, we can deduce that the primary lesion we refer to in this case report was an adult diffuse malignant brainstem glioma.
As much as it is clear that the management of glioblastoma includes the administration of radiotherapy and concurrent temozolomide, that have been shown to improve the survival in patients; in case of high grade brainstem glioma, radiotherapy remains the standard treatment for adult brainstem gliomas [22]. However, in exophytic lesions, a debulking as large as possible, avoiding brainstem dysfunction and preventing impairment of quality of life as well is indicated [22]. In order to make an accurate diagnosis, it is recommended to perform a surgical biopsy if possible, in high grade diffuse gliomas [22]. A positive impact on overall survival have been shown in patient who has undergone a surgical procedure. In our case the location, the diffuse character and infiltrative behaviour did not allow a surgical treatment. Our therapeutic strategy consisted in a stereotactic biopsy followed by a radio-chemotherapy, with a good evolution over more than three years.
According to the literature, non-malignant brainstem glioma has a better prognosis in adults. This statement is clearly not true for malignant gliomas, which prognosis is around 11 to 17 months [12,16].
Spinal cord GBM represent 1-5% of all the GBM and 1-3% of all the spinal cord tumors [5]. It is important to distinguish primary GBM from secondary ones. The difference between the two cases relies on the presence or not of a pre-existing less aggressive glioma [5]. In the present case, the patient was treated for a previous high grade glioma in the brainstem and then he was diagnosed for a spinal metastatic glioblastoma.
According to the literature, 20% of patient presenting high grade intracranial gliomas develop later leptomeningeal and intramedullary dissemination [10]. A malignant transformation of an anaplastic intracranial astrocytoma into a spinal GBM is less frequent. It is usually a local recurrence or a dissemination along the white matter tract [2,5].
Spinal metastasis are more frequently seen in younger patients [17] and that can be explained by the simple fact that elderly patients succumb more quickly to neoplastic pathologies than younger patient, who live longer even with malignant glioma.
The common ways for tumoral cells to spread are thought to be via the cerebrospinal fluid (CSF) pathway either from direct spread into the subarachnoid space or from iatrogenic spread following surgical manipulation [18,19]. The usual locations of intraspinal metastases are lower thoracic and lumbar spine [18]. Exceptionally our patient presented a cervical metastasis.
The most common symptoms of a spinal metastasis are radicular or local pain, paresthesia’s and other sensory symptoms followed by motor weakness [18]. Paresthesia, dysesthesia and right hemiparesis were the symptoms we observed, which motivated our investigations.
Despite the primary brainstem disease was under control this young patient developed an intramedullary metastasis.
James Powell [18] Reported cases of intramedullary metastases in patients with stable intracranial disease and that’s why it’s important to perform a regular follow up of these patients with MRI imaging, especially when they are young.
The GFAP (Glial Fibrillar Acid protein) has been found to be the most reliable marker of astrocytic tumors differentiation [10]. According to K Onda [20], in a clinicopathological study of 14 cases examined by complete autopsy, glial tumors with GFAP negative cells tend to be locally less invasive but they disseminate vigorously through CSF, whereas the GFAP positive cells gliomas tended to be invasive and showed infiltrative behaviour at the primary tumoral site.
Another retrospective review on 157 patients conducted by Arita et al. [19] concluded in similar findings. They found less GFAP expression in both leptomeningeal lesion and intracranial primary tumor of all the patients who presented leptomeningeal dissemination.
For this case review, we manage to find tissues samples of both spinal secondary glioblastoma and previous brainstem glioma. The immunohistochimical analysis showed in both cases a high expression of the GFAP. These findings support the hypothesis that highly differentiated astrocytic tumor like our brainstem high grade glioma, show more conventional infiltrative behavior and are more likely to induce intramedullary metastases, contrarily to poorly differentiated glioma cells which frequently disseminate via the CSF pathway.
The immunohistochemistry of the brainstem tissue samples revealed a K27M mutation in histome H3.3.
A K27M mutation in histone 3 has been described to identify high-grade midline gliomas associated with a particularly unfavorable prognosis [15].
This case presentation underlines the significance of keeping in mind spinal metastasis as a delayed complication of high-grade glioma. In general, earlier diagnosis of dissemination can lead to improvement
We presented a rare case of an intramedullary C2-C3 metastatic glioblastoma from a previous anaplastic brainstem astrocitoma (WHO grade III) in a 25-year-old male patient. We clearly demonstrate that the spinal lesion was not induced by radiotherapy but a real metastasis. Despite the progress in management of high-grade gliomas, recurrences and dissemination are not rare events.
Cervical spinal cord metastasis is not usual as second localization site. Regardless of the treatment, even if this clinical condition occurs more frequently in young patients, the prognosis is generally poor and its presence tends to signalize end-stage cancer [21]. So, it is fundamental to investigate the full spinal cord in order to find earlier spinal metastatic lesions, even in healthy, young patients with lower grade gliomas with clinical and radiological signs of remission.
- Brainstem glioma is a very rare entity in adults (1-2%).
- Spinal cord GBM represent 1-5% of all the GBM and 1-3% of all the spinal cord tumors.
- Intramedullary metastatic lesion should be highly suspected in all patients with a history of intracranial high-grade glioma who complain about local spinal symptoms and signs or symptoms not explained by the primary lesion.
- The ways of dissemination are via the CSF pathways, from direct spread into the subarachnoid space or from iatrogenic spread following surgical manipulation.
- GFAP expression is a one of the most important determinants of the dissemination of glial tumoral cells.
We are very thankful to the pathologists of UZ Brussel Institute of anatomo-pathology and Gosselies Institute of Pathology and Genetics (IPG) for providing us the histopathological analysis our patient.
- 1. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, et al. (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131: 803–20. [Crossref]
- 2. Birbilis TA, Matis GK, Eleftheriadis SG, Theodoropoulou EN, Sivridis E (2010) Spinal Metastasis of Glioblastoma Multiforme: An Uncommon Suspect? Spine (Phila Pa 1976) 35: E264–9. [Crossref]
- 3. Jayamanne D, Wheeler H, Cook R, Teo C, Brazier D, et al. (2018) Survival improvements with adjuvant therapy in patients with glioblastoma. ANZ J Surg 88: 196–201. [Crossref]
- 4. Stark AM, Maslehaty H, Hugo HH, Mahvash M, Mehdorn HM (2010) Glioblastoma of the cerebellum and brainstem. J Clin Neurosci 17: 1248–51. [Crossref]
- 5. Lieu A-S, Tsai F-J, Chen Y-T, Liang P-I, Kuo K-L (2015) Intramedullary spinal glioblastoma metastasis from anaplastic astrocytoma of cerebellum: A case report and review of the literature. Asian J Neurosurg 10: 268. [Crossref]
- 6. Chen F, Li Z, Weng C, Li P, Tu L, et al. (2017) Progressive multifocal exophytic pontine glioblastoma: a case report with literature review. Chin J Cancer 36: 34. [Crossref]
- 7. Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, et al. (2014) CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007-2011. Neuro Oncol 16: iv1–63. [Crossref]
- 8. Lefranc F, Brotchi J, Kiss R (2005) Possible future issues in the treatment of glioblastomas: special emphasis on cell migration and the resistance of migrating glioblastoma cells to apoptosis. J Clin Oncol 23: 2411–22. [Crossref]
- 9. Kwon T-H, Moon H-J, Chotai S, Kim J-H, Kim J-H (2012) Primary glioblastoma multiforme of medulla oblongata: Case report and review of literature. Asian J Neurosurg 7: 36. [Crossref]
- 10. Maslehaty H, Cordovi S, Hefti M (2011) Symptomatic spinal metastases of intracranial glioblastoma: clinical characteristics and pathomechanism relating to GFAP expression. J Neurooncol 101: 329–33. [Crossref]
- 11. Reyes‐Botero G, Mokhtari K, Martin‐Duverneuil N, Delattre J, Laigle‐Donadey F (2012) Adult Brainstem Gliomas. Oncologist 17: 388–97. [Crossref]
- 12. Das KK, Bettaswamy GP, Mehrotra A, Jaiswal S, Jaiswal AK, et al. (2014) Dorsally exophytic glioblastoma arising from the medulla oblongata in an adult presenting as 4th ventricular mass. Asian J Neurosurg 12: 224-227. [Crossref]
- 13. Guillamo JS, Monjour A, Taillandier L, Devaux B, Varlet P, et al. (2001) Brainstem gliomas in adults: prognostic factors and classification. Brain 124: 2528–39. [Crossref]
- 14. Epstein FJ, Farmer J-P (1993) Brain-stem glioma growth patterns. J Neurosurg 78: 408–12. [Crossref]
- 15. Doyle J, Khalafallah AM, Yang W, Sun Y, Bettegowda C, et al. (2019) Association between extent of resection on survival in adult brainstem high-grade glioma patients. J Neurooncol 145: 479–86. [Crossref]
- 16. Spurgeon A, Le V, Konakondla S, Miller DC, Hopkins T, et al. (2016) High-Grade Glioma of the Ventrolateral Medulla in an Adult: Case Presentation and Discussion of Surgical Considerations. Case Rep Neurol Med 2016: 1–9. [Crossref]
- 17. Schwaninger M, Patt S, Henningsen P, Schmidt D (1992) Spinal canal metastases: a late complication of glioblastoma. J NeuroOncol 12: 93-8. [Crossref]
- 18. Powell J (2012) Symptomatic Spinal Metastases from Intracranial High-grade Glioma – Report of Four Cases and Review of the Literature. European Oncology & Haematology 8: 213.
- 19. Arita N, Taneda M, Hayakawa T (1994) Leptomeningeal dissemination of malignant gliomas. Incidence, diagnosis and outcome. Acta neurochir 126: 84–92. [Crossref]
- 20. Onda K, Tanaka R, Takahashi H, Takeda N, Ikuta F (1989) Cerebral glioblastoma with cerebrospinal fluid dissemination: a clinicopathological study of 14 cases examined by complete autopsy. Neurosurgery 25: 533–40. [Crossref]
- 21. Sung W-S, Sung M-J, Chan JH, Manion B, Song J, et al. (2013) Intramedullary Spinal Cord Metastases: A 20-Year Institutional Experience with a Comprehensive Literature Review. World Neurosurg 79: 576–84. [Crossref]
- 22. Eisele SC, Reardon DA (2016) Adult brainstem gliomas. Cancer 122: 2799–809. [Crossref]







