This paper presents the use of non-invasive focused ultrasound surgery (FUS) for persistent high-risk human papillomavirus (HR-HPV) infection and cervical intraepithelial neoplasia (CIN1). Although diagnostic uses of ultrasound are well known, its potential to treat persistent HR-HPV infection and CINI noninvasively is a relatively new area of clinical research interest. Focused ultrasound surgery (FUS) could be a future alternative treatment for HPV and CINI to other current invasive surgical techniques. We aim here to provide an updated review related to the role of FUS in treating persistent HR-HPV infection and CINI, and to outline the progress of research done on this topic. Also included is a review of the safety and effectiveness of FUS in clinical trials.
FUS, persistent HR-HPV, CINI
Even though advances have been made in the diagnosis and treatment of cervical cancer, it is still a common cancer of the female reproductive tract. Cervical intraepithelial neoplasia (CIN) is a premalignant condition of cervical cancer, which has a 20-fold higher risk of developing cervical cancer in HPV infected women than in healthy women [1]. Therefore, timely treatment of CIN is necessary to avoid its progression into invasive cervical cancer [2,3].
Cervical human papillomavirus (HPV) had been found in up to 99.7% of cervical cancers and is implicated as the causative agent for CIN development. HPV genotypes HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59 have been established as the high-risk genotypes [4-6]. Given that HPV-positive cervical cancer is prevalent and that the possibility of HPV reinfection even after CIN treatment is high [5,7], it is necessary to develop effective strategies that minimize the risk of residual disease, as well as to prevent reinfection [4].
At present, the treatment for CIN includes expectant management and surgical treatment [8]. It is generally considered that expectant management is suitable for patients with CIN1 [9]. Regarding the management, women who come for consultation because of borderline or low-grade squamous intraepithelial lesion (LSIL) cytology should undergo a colposcopic assessment with biopsies. The broad consensus used to be that, in women with LSIL, biopsy-proven CIN1, and satisfactory colposcopy, invasive surgical treatment is not indicated. Rather they should be followed by cervical cytology screening. However, for women with high-grade squamous intraepithelial lesion (HSIL) cytology, who had a histologic diagnosis of CINI in targeted biopsies, most guidelines recommend a diagnostic excision of the transformation zone because the risk of associated high-grade disease is high. Then when should CINI be treated? How long should we wait for before we treat persistent CINI with invasive surgical treatment? Of course, there are only a few CIN lesions becoming progressive and require surgical treatment.
On the other hand, most CIN lesions will regress spontaneously. The Issue is what can be done with persistent CINI? Is it cost-effective to follow these women with intensive Pap smear screening over many years? What subgroups can benefit from invasive surgical treatment, and should we apply destructive methods in these women in order to avoid unnecessary morbidity of cancer? These are the hot topics that need further research.
The American Society of Colposcopy and Cervical Pathology (ASCCP) recommends that women with a histological diagnosis of CIN2-3 should undergo ablation or resection treatment [8]. Treatment options for CIN and cervical HPV infection such as diathermy coagulation, cryotherapy, laser ablation, and laser or electrosurgical excision are all invasive methods that may cause adverse side effects. These include bleeding, infection, stenosis of the cervix [10], and even severe complications in subsequent pregnancies, including spontaneous abortion, preterm birth, and low birth weight [11-13]. Radiation and chemotherapy are only used in the management of cervical cancer; therefore, they are inappropriate and not suitable for young patients with pre-cancerous conditions. Thus, it is important to find another efficient alternative and relatively non-invasive method to treat CIN and persistent high-risk HPVs, ideally without much morbidity and compromising the patient’s fertility.
Hoffman et al. [14] in a systematic review of the literature, tried to determine the extent of and prevalence of persistent human papillomavirus (HPV) infection in women following treatment of cervical intraepithelial neoplasia (CIN). From a meta-analysis of 45 studies, they analyzed the data from 6,106 women to determine the persistence rate of HPV after CIN treatment. There was a considerable difference in the types of treatment among these 45 studies: 42% used loop excision; 11% cryotherapy; 7% conization; 4% laser treatment; one study each used alpha‐interferon, photodynamic therapy, or therapeutic vaccination (2% each); while 38% used multiple treatment regimens. Baseline HPV tests were conducted before or at treatment in most studies (up to 96%). The follow-up HPV testing ranged from 1.5 to 80 months after the initial test. HPV persistence tended to decrease with increasing follow-up time, declining from 27% at three months after treatment to 21% at six months, 15% at 12 months, and 10% at 24 months. Post-treatment HPV persistence rates varied widely and were influenced by patient age, HPV-types, detection method, treatment method, and minimum HPV post-treatment testing interval. Even though cryotherapy appeared to be less invasive, and was popular at a time, in this systematic review, both loop excision and conization appeared to outperform cryotherapy in terms of their ability to clear HPV infection.
As a non-invasive tool, focused ultrasound therapy is a new and promising approach in clinical practice [15-17]. While ultrasound beams propagate through human tissue, they can be brought to a focus at a distance from their source. Unlike other ablation techniques (e.g., laser, radiofrequency ablation), focused ultrasound energy produces an ablated area with a sharply demarcated boundary between treated and normal tissue. If the concentrated energy is sufficient, this leads to the thermal destruction of targeted tissue at depth without damage to surrounding or overlying tissues [18]. Previous clinical studies had shown that focused ultrasound therapy was feasible and effective in the treatment of patients with vulvar dystrophy, symptomatic cervical ectopy, and recurrent cervicitis with high-risk HPV (HR-HPV) infection, etc [19-23]. Lin et al. [24] demonstrated that FUS was effective for the treatment of CINI and that the duration of vaginal discharge after treatment and wound healing was short. Our paper reviews and discusses the safety, effectiveness, and feasibility of focused ultrasound therapy as a non-invasive tool for the management of CIN with high-risk HPV (HR-HPV) infection.
The principle of focused ultrasound therapy is based on the ability of ultrasound to penetrate the body’s soft tissues and for multiple ultrasound beams (generated by the extracorporeal electroacoustic transducer) to be focused at a focal point of the body to form a high-energy-density area, generating instantaneous high temperature. The effect of FUS treatment generates heat effects, mechanical effects, and cavitation effects that denature and coagulate the diseased tissues facilitating necrosis, with the necrotic tissue being replaced over time by surrounding normal healthy tissues. It can thus achieve the purpose of targeted destruction of the neoplastic lesions. Importantly, however, the surrounding tissues and tissues along the path coursed by the ultrasonic waves will not be damaged [18], and the structure and function of the surrounding tissues and organs are preserved. Although focused ultrasound belongs to the category of physical ablation therapy in the treatment of cervical diseases, it is different from traditional invasive surgical treatment. First, focused ultrasound has good energy permeability and focusing characteristics. Ultrasound energy can directly focus on the basal layer of the epithelium through the superficial epithelial tissues without damage to these latter tissues. The deeper basal layer cells can then repair from the damage and grow from the inside out to heal the lesion. Because HPV invades the human epithelium, with the viral DNA replicating in the basal layer cells, it is speculated that focused ultrasound may destroy HPV virus particles while simultaneously destroying the basal layer cells. Moreover, focused ultrasound can enhance immune cell infiltration and activation, thereby enhancing the body’s cellular immunity [22,25], and helping to clear the HPV virus and heal the diseased tissue.
The mechanism of healing in cervical lesions following focused ultrasound treatment has been studied by Fu et al. [26] in a mouse CIN model. They found that there was a positive expression of p16, Ki67, and Fas in mouse cervical tissue of the focused ultrasound treatment group, but that p16 and Ki67 expressions were weaker than that of the non treated control group. While the Fas expression was strongly positive, it was higher than that of the control group. Their finding suggested that focused ultrasound treated tissue may reduce the proliferation of neoplastic cells and increase cell apoptosis, thus improving the local microenvironment of the treated cervical tissues and promoting tissue reversion back to normal cervical epithelium. Fu et al. [27] further confirmed their findings in a recent clinical study of 30 patients receiving focused ultrasound surgery (FUS) for CINI, reporting a total curative rate of FUS for CINI treatment of 90.0%. These authors also studied the expression of p16, Ki-67, and Fas in the cervical biopsies in these patients. Here they again found that focused ultrasound therapy reduced the p16 and Ki-67 expression and enhanced Fas expression, consistent with regulation of cell proliferation and apoptosis, and thus accounting for the cervical epithelium being prevented from reverting to CIN lesions.
The focused ultrasound equipment – Seapostar® (Model-CZF 300 ) used for the cervical treatment of Fu et al. [27] was manufactured by Chongqing Haifu Medical Technology Co, Ltd (Chongqing, China). Therapeutic FUS energy was emitted from a 1cm diameter transducer with a short ellipsoidal focal region [28]; the working frequency was 8~12 MHz and impulse frequency of 1000 Hz with the FUS power set at 3.5~4.5 W.
Treatments were performed on days 3 to 10 after menstruation. Neither anesthesia nor analgesia was required. Patients undergoing treatment would lie in a lithotomy position. After a sterile speculum was placed in the vagina to expose the cervix completely, cervical mucus and discharge were removed with a cotton ball. The vagina wall and cervical surface were sterilized with povidone-iodine or chlorhexidine. The surface of the cervix was coated and covered with ultrasound coupling gel before treatment. The treatment probe was applied in close contact over the transition zone (TZ) and CIN lesion of the cervix. It was moved continuously at a speed of about 5~10 mm/second in a circular fashion, against both the diseased area in the transformation zone and an additional 2 mm of healthy tissue around the treated area. The treatment ended when the treated area turned flaky white, and the lesion presented as a depressed area with a modest retraction of the central cervical area (Figure 1). The required treatment time was about 120~180 seconds.
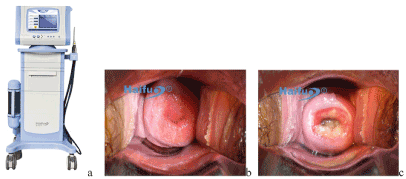
Figure 1. The therapeutic device and methods. (a) The FUS therapeutic device, Seapostar. (b) Before FUS treatment. (c) After FUS treatment, the treated area showed the cervix to have a glossy white appearance but not to have been damaged or denuded, the cervical lesion to have been contracted, and the central cervical area to have been retracted slightly. (Photographs - courtesy of Chongqing Haifu Medical Technology Company)
In recent years, the emerging technology of focused ultrasound has provided a new feasible and effective approach for the treatment of symptomatic chronic cervicitis and early CIN lesions with high-risk HPV infection.
Xiao et al. [29] treated 313 patients with chronic cervical erosion (synonymous with “cervical ectopy”) with a single FUS treatment. They analyzed the therapeutic effect and influencing factors of 300 patients with cervical erosion, including 43 cases of mild erosion, 126 cases of moderate erosion, and 121 cases of severe erosion. The erosion areas healed with success rates from a single FUS treatment for mild, moderate, and severe cervical erosion of 100.0 % (53/53), 95.2% (120/126) and 91.7% (111/121) respectively, with an average success rate of 94.7% (284/300). It was also observed that the treatment effectiveness had an exponential relationship between the diameter of the erosion surface and the sonication time. It was suggested that the sonication time should be adjusted according to the differences of the erosion area and the acoustic energy deposition of the different cervical tissues, leading to improved controllability of the treatment process and success rate of a single treatment.
Chen et al. [21] reported a comparative study of 200 patients randomly allocated to FUS and Nd: YAG laser ablation for symptomatic cervical ectopy. These patients typically suffered from leukorrhea, contact bleeding, recurrent cervicitis, and pelvic pain. The results showed that all patients tolerated these treatments well and had excellent treatment outcomes. The symptom-free rate after treatment was 97.33% in the FUS group, and 98.81% in the laser group (p > 0.05). The ectopy areas healed with a success rate of 95.95% in the FUS group, compared to 96.43% in the laser group. However, the side effects, including vaginal discharge and vaginal bleeding rate in the FUS group, was significantly lower than in the laser group (8.42% vs. 45.56%, p < 0.01). The conclusion was that FUS was effective in treating symptomatic ectopy of the cervix with excellent results and minimal risk. It, therefore, appears to be a promising new treatment method for symptomatic ectopy of the uterine cervix.
In their previous study, Li et al. [22] found that in patients with high-risk HPV infected chronic cervicitis, 75% of the patients with HPV turned negative at six months after focused ultrasound treatment, indicating that focused ultrasound treatment could be useful in removing all or most of the HPV-infected cervical cells and thereby clear the cervix of high-risk HPV infections. In 2013, Li et al. [30] enrolled 4677 patients with symptomatic cervical ectopy for FUS treatment. Three months after treatment, the therapeutic effects and adverse reactions in 4014 cases were evaluated. For the FUS treatment of symptomatic cervical ectopy, 99.8% of cases had symptom relief, and 72.52% were cured. Vaginal bleeding that exceeded normal menstrual volume occurred in 12 cases. According to this study, logistic regression analysis suggested that the extent of the lesions, vaginal hygiene, socio-economic status, age, and history of abortions were influential factors. Therefore FUS appears to be a promising new therapeutic option for the treatment of symptomatic cervical ectopy and potentially for HPV infection.
Fan et al. [31] also studied the effectiveness and safety of FUS in the treatment of persistent high-risk HPV infection of the cervix. They showed that: (1) three months after treatment, the negative conversion rate in the focused ultrasound group was 53.3%, in the interferon group it was 48.3%, and in the control group it was 31.0%; (2) six months after treatment, the negative conversion rates were 86.2%, 79.3%, and 53.6% respectively; and (3) compared to the control group, focused ultrasound and interferon significantly reduced the HPV viral titer of high-risk HPV infected patients. No significant difference, however, was found in the negative conversion rate between the focused ultrasound and the interferon group.
Ren et al. [32] compared the clinical efficacy of focused ultrasound and laser plus electrocoagulation in the treatment of CINI with high-risk HPV infection. Their results confirmed that both treatment methods were effective in curing CINI and high-risk HPV infection. However, there was less vaginal bleeding and discharge after focused ultrasound treatment.
Medical management for cervical HPV infection and related CIN diseases includes local treatment for HPV-infected lesions with immunotherapy, and antiviral treatment for HPV infection. Surgical management includes invasive surgical resection and ablation techniques, such as laser, cryosurgery, and electrocautery, which are standard treatment strategies that focus on CIN lesions. In clinical practice, treatment needs to be individualized according to the degree and extent of the disease and the fertility requirements of patients. However, FUS therapy for CIN and HPV infection, as discussed in this paper, has several major advantages compared with other surgical treatments: (a) it destroys the basal layers which harbor the HPV viruses using the penetrating focused ultrasound energy; (b) all inflammatory cells and diseased tissues are sensitive to ultrasound energy, while normal cervical tissues are unaffected by the FUS therapy; (c) FUS therapy can preserve the integrity of cervical tissue structure, and no scab or necrosis persists after treatment. Furthermore, FUS therapy is simple to operate and can repeat treatment once after 3 months.
Moreover, patients do not need to receive anesthesia. It is also a pollution-free operation that does not generate odors or smoke or involve burning of tissue or emission of radiation. Focused ultrasound treatment not only conforms to the physiological conditions and does not cause hard scarring in the local cervical tissue, but it can promote the reversal of the disease, reduce the persistence of the HPV disease, and prevent its further development into pre-cancerous lesions. Meanwhile, FUS treatment also reduces the psychological and economic burdens of patients typically associated with long-term follow-up.
In China, due to its large expanse and huge population, it is a prominent issue dealing with this problem of persistent HPV infection, low detection rate, and cancer development among infected women. Therefore, the development of this non-invasive focused ultrasound treatment for HPV infection, CIN lesions, and persistent HPV infection represents a suitable alternative treatment to resolve these problems. There is also hope that it will reduce the number of patients with persistent HPV infection associated with asymptomatic transmitters. The use of FUS had followed a standardized clinical practice, strictly following treatment indications, thereby allowing for systematic evaluation of its clinical efficacy and complications in clinical trials. Its well-studied mechanisms of action also provide a scientific and objective basis for the above clinical application. Hopefully, this new non-invasive treatment of FUS will benefit women with CINI, HPV infection, and persistent HPV infection, preventing further spreading during the periods of no intervention and observation.
- Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65: 5-29. [Crossref]
- Lowy D (2008) Human papillomavirus, cervical cancer prevention, and more. Vaccine 26 Suppl 10: 3-4. [Crossref]
- Sasieni P, Castanon A, Cuzick J (2009) Effectiveness of cervical screening with age: population-based case-control study of prospectively recorded data. BMJ 339. [Crossref]
- Trushina O, Novikova E, Sokolov V, Filonenko E, Chissov V, et al. (2008) Photodynamic therapy of virus-associated precancer and early stages cancer of cervix uteri. Photodiagnosis Photodyn Ther 5: 256-259.
- Bosch FX, de Sanjose S, Castellsagué X (2011) Epidemiology of genitoanal HPV infections and associated cancer. Sexually Transmitted Infections and Sexually Transmitted Diseases: Springer; 427-439.
- Graham SV (2017) The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clinical Sci 131: 2201-2221. [Crossref]
- Kajitani N, Satsuka A, Kawate A, Sakai H (2012) Productive lifecycle of human papillomaviruses that depends upon squamous epithelial differentiation. Front Microbiol 3: 152. [Crossref]
- Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, et al. (2013) 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis 17: S1-S27. [Crossref]
- Petry KU (2011) Management options for cervical intraepithelial neoplasia. Best Pract Res Clin Obstet Gynaecol 25: 641-651. [Crossref]
- Istomin Y, Lapzevich T, Chalau V, Shliakhtsin S, Trukhachova T (2010) Photodynamic therapy of cervical intraepithelial neoplasia grades II and III with Photolon®. Photodiagnosis Photodyn Ther 7: 144-151. [Crossref]
- Hillemanns P, Soergel P (2011) HAL/MAL photodynamic therapy for CIN. Photodiagnosis Photodyn Ther 2: 169.
- Nene BM, Hiremath PS, Kane S, Fayette JM, Shastri SS, et al. (2008) Effectiveness, safety, and acceptability of cryotherapy by midwives for cervical intraepithelial neoplasia in Maharashtra, India. Int J Gynecol Obstet 103: 232-236.
- Rema P, Suchetha S, Thara S, Fayette JM, Wesley R, et al. (2008) Effectiveness and safety of loop electrosurgical excision procedure in a low-resource setting. Int J Gynecol Obstet 103: 105-110.
- Hoffman SR, Le T, Lockhart A, Sanusi A, Dal Santo L, et al. (2017) Patterns of persistent HPV infection after treatment for cervical intraepithelial neoplasia (CIN): A systematic review. Int J Cancer 141: 8-23.
- Kennedy JE (2005) High-intensity focused ultrasound in the treatment of solid tumours. Nat Rev Cancer 5: 321-327. [Crossref]
- Chaussy C, Thüroff S, Rebillard X, Gelet A (2005) Technology insight: high-intensity focused ultrasound for urologic cancers. Nat Clin Pract Urol 2: 191-198. [Crossref]
- Wu F (2006) Extracorporeal high intensity focused ultrasound in the treatment of patients with solid malignancy. Minim Invasive Ther Allied Tech 15: 26-35.
- Ter Haar G (2007) Therapeutic applications of ultrasound. Prog Biophys Mol Biol 93: 111-129. [Crossref]
- Li C, Bian D, Chen W, Zhao C, Yin N, et al. (2004) Focused ultrasound therapy of vulvar dystrophies: a feasibility study. Obstet Gynecol 104: 915-921. [Crossref]
- Ye M, Deng X, Mao S, Xue M (2015) High intensity focused ultrasound treatment for non-neoplastic epithelial disorders of the vulva: factors affecting effectiveness and recurrence. Int J Hyperthermia 31: 771-776. [Crossref]
- Chen J, Zhou D, Liu Y, Peng J, Li C, et al. (2008) A comparison between ultrasound therapy and laser therapy for symptomatic cervical ectopy. Ultrasound Med Biol 34: 1770-1774. [Crossref]
- Li CZ, Wang ZB, Yang X, Tang Y, Wang D, et al. (2009) Feasibility of focused ultrasound therapy for recurrent cervicitis with high‐risk human papillomavirus infection. Ultrasound Obstet Gynecol 34: 590-594. [Crossref]
- Zhang L, Zhang W, Orsi F, Chen W, Wang Z (2015) Ultrasound-guided high intensity focused ultrasound for the treatment of gynaecological diseases: a review of safety and efficacy. Int J Hyperthermia 31: 280-284.
- Lin C, Yang J, Li CZ, Liu YM, Zhou DP (2012) Focused ultrasound in treatment of low grade cervical intraepithelial neoplasia. Chinese J Intervent Imag Therap 12.
- van den Bijgaart RJ, Eikelenboom DC, Hoogenboom M, Fütterer JJ, den Brok MH, et al. (2017) Thermal and mechanical high-intensity focused ultrasound: perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol Immunotherap 66: 247-258. [Crossref]
- Fu Z, Yang H, Lu L, Tang H, Li C (2016) Effects of focused ultrasound on grade Ⅰ cervical intraepithelial neoplasia in mice. Tumor 36: 882-887.
- Fu Z, Fan Y, Wu C, Yan P, Ye Y, et al. (2020) Clinical efficacy and mechanism for focused ultrasound (FUS) in the management of cervical intraepithelial neoplasia 1 (CIN1). Int J Hyperthermia 37: 339-345. [Crossref]
- Wang Z, Bai J, Li F, Du Y, Wen S, et al. (2003) Study of a “biological focal region” of high-intensity focused ultrasound. Ultrasound Med Biol 29: 749-754. [Crossref]
- Xiao Y, Sun L (2007) Therapeutic effects of focused ultrasound on chronic cervicitis and the influencing factors. Zhonghua Fu Chan Ke Za Zhi 42: 14-17. [Crossref]
- Li C, Xiong X, Li Y, Li J, Peng B, et al. (2013) Therapeutic effects of focused ultrasound in 4014 patients with symptomatic cervical ectopy. Ultrasound Med Biol 39: 604-610.
- Xiu F, Fang TW, Xiao Y, Dong W, Chengzhi L (2010) Clinical study of Focused ultrasound to treat high risk high risk cervical HPV infection. Sci Technol Guide 28: 79-100.
- Ren Yuxiang WF, Huifen C, Huicheng X. Comparison of therapy efficacy about high-risk subtypes of HPV infection in CIN Ⅰby focused ultrasound and laser coagulation 2012.

