Kawasaki disease (KD) is an acute, febrile systemic vasculitis of an unknown etiology. KD has now surpassed rheumatic fever as the leading cause of acquired heart disease in the United States (U.S.) among children younger than 5 years. Although there has been a great deal of research into the cause of KD, there is no known etiology. The lack of a diagnostic test can result in significant delays in treatment, often resulting in cardiovascular sequelae ranging from asymptomatic coronary artery ectasis or aneurysm formation to giant coronary artery aneurysms with thrombosis, myocardial infarction, and sudden death in untreated children. Continued research is integral to the evolution of the management of patients with KD because understanding the mechanism of pathogenesis would allow the development of a diagnostic test and, therefore, more rapid and comprehensive treatment. I review these facts and the coronary affection from the diagnostic and the mechanistic standpoints.
Kawasaki disease, coronary affection, vasculitis, febrile illness, children
INF: Interferon; IgA: Immunoglobulin A; Ig E: Immunoglobulin E; Ig G: Immunoglobulin G; MMR: Measles, Mumps, Rubella; IVGG: Intravenous gammaglobulin; ESR: Erythrocytic sedimentation rate; PO: per oral; Qid: Quarter in die; Qd: Quaque die; Tid; Ter in die.
Kawasaki disease (KD) or Kawasaki syndrome (KS) is an acute, febrile systemic vasculitis of an unknown etiology. KD was first described in Japan in 1967 by Dr. Tomasaku Kawasaki, and since then has been observed in children across the world of every racial group [1,2]. KD has now surpassed rheumatic fever as the leading cause of acquired heart disease in the United States (U.S.) among children younger than 5 years [3]. The onset of the disease is rare after 8 years of age. However, some reports described it in an adult case [4]. Less than 2% of cases has recurrences. There have been great advances in the treatment of KD with the employment of intravenous immunoglobulins [IVIG] and high-dose aspirin [ASA]; [5], however, the lack of a diagnostic test can result in significant delays in treatment, often resulting in cardiovascular sequelae ranging from asymptomatic coronary artery ectasis or aneurysm formation to giant coronary artery aneurysms with thrombosis, myocardial infarction [MI], and sudden death in 20-25% of untreated children [6,7].
Although inflammatory infiltrates have been shown in the myocardium, pancreas, kidney, and biliary tract, no significant sequelae persist in those nonvascular tissues. A report of pulmonary involvement has appeared in the literature [2]. Although there has been a great deal of research into the cause of KD, there is no known etiology. Continued research is integral to the evolution of the management of patients with KD because understanding the mechanism of pathogenesis would allow the development of a diagnostic test and, therefore, more rapid and comprehensive treatment [8].
History of Kawasaki Disease. In 1967, Kawasaki published his first report in Japan describing the cluster of symptoms he observed in 50 patients seen since 1961, which he presented as an emergent disease called "mucocutaneous ocular syndrome" (MCOS) or mucocutaneuos lymph node syndrome [9]. Kawasaki's claim that MCOS was indeed a novel disease was met with a great deal of controversy [6]. A more highly debated topic was the proposed association between the symptoms of MCOS and a series of cardiac complications, which was first recognized by Dr. Noboru Tanaka, a Japanese pathologist, following the autopsy of a child diagnosed with MCOS [6]. The cardiac sequelae were first observed clinically and subsequently presented to the public in a report by Dr. Takajiro Yamamoto [10]. A nationwide epidemiologic survey of MCOS in Japan released in 1970 confirmed the claims of Tanaka and Yamamoto and established the association between MCOS and cardiac complications caused by severe vasculitis [6,11].
Kawasaki published an English translation of his original report of 50 patients in 1974 [12]. However, KD was independently recognized as a distinct disease in Hawaii in the early 1970's by Dr. Marian Melish, a specialist in pediatric infectious disease, and Dr. Raquel Hicks, a pediatric rheumatologist [6]. The pair documented the disease in Hawaiian children, who were mostly Japanese Americans, and in 1973, after seeing photographs of children with KD from Japan and contacting Kawasaki, they concluded the syndrome they had encountered was the same as that recently documented in Japan [6]. In addition, in 1971, Eunice Larson, a pediatric pathologist in Honolulu, observed the cardiac sequelae of a child [later recognized as having KD] and after further investigation made the connection with the Japanese KD [6]. Following this independent discovery of the clinical and pathologic aspects of the disease, Melish, Hicks and Larson collaborated to publish a report in 1976 of their observations of KD in Asian/Pacific children from Hawaii [11]. The history of the emergence of Kawasaki disease in Japan and the United States presents many questions that may have implications on the pathology and origin of the disease. It is matter of some interest whether there were any cases of KD prior to those observed by Kawasaki himself. An answer to this question may provide researchers with a reason for the independent recognition of KD by clinicians and pathologists around the world in the 1960's and 1970's [6].
Burns et al., [6] presents three possible explanations for this phenomenon: [1] KD may have, in fact, been a novel disease that emerged in Japan and was in some way spread to Hawaii through Asian immigration; [2] KD and infantile periarteritis nodosa (IPN) may be on a spectrum of the same disease and, therefore, clinically mild KD may have been systematically misdiagnosed; [3] improvements in medical care, especially the use of antibiotics, may have assuaged the symptoms of KD, such as rash and fever, which allowed the clinical criteria to be independently established. It is possible that with thorough investigation, knowledge of the detailed history of the appearance and apparent dispersal of KD among children throughout the world may add direction to the search for a causative agent.
Although KD has been observed in children of all racial groups, the disease is over expressed among Asian populations, especially the Japanese and those of Japanese descent living outside of the country [13]. The disease is most common among children, with 85% of those diagnosed aged less than 5 years, although the peak incidence in the U.S. occurs in infants aged 1-2 years and it is rarely observed in infants under the age of 1 and adolescents [14]. The annual incidence rate in Japan, as measured in 1998, is 111/100,000 children under the age of 5 [15]. The annual incidence rate in the US, which is measured regionally, is between 4 and 15/100,000 children under the age of 5 [3]. The disease occurs in temporally limited regional epidemics, which are most common in the late winter and spring, and boys are more commonly affected than girls, with a male to female ratio of 1.5:1 [14].
The epidemiology of KD provides insight into the nature of the disease and aids in our understanding of possible causes or risk factors for KD by identifying populations that are more at risk of developing the disease. The fact that some ethnic groups are at higher risk for KD than others suggests that there may be ethnicity-related genetic differences or possibly shared environmental exposures that make some individuals more susceptible to KD [2,16].
In addition, the epidemiologic patterns of KD provide evidence for the role of an infectious agent in its pathogenesis, [8] such as: [1] the seasonal peaks of outbreaks in winter and spring, which are characteristic of infectious diseases; [2] the regional grouping of cases; [3] the rarity of illness in the infant and adolescent, suggesting maternal antibodies acquired transplacentally may protect infants and asymptomatic infection followed by immunity in older children; and [4] the clinical similarities between KD and other infectious diseases, such as scarlet fever. Although there has been no documentation of person-to-person spread of KD [17]. and the etiology remains widely disputed, epidemiologic findings strongly support the hypothesis proposing a causative infectious agent of KD, and serve as the basis of etiological investigations [18].
It is likely that the immunostimulation of an inflammatory response plays a central role in the pathogenesis of KD. This is suggested by, for example, the demonstration of vascular inflammation of the coronary arteries in addition to other smaller blood vessels [1], the increased numbers of circulating activated T-cells and monocytes, and polyclonal B cell activation during the acute phase [1,19], and clinical features associated with immune activation, such as fever and lymphadenopathy [20]. Therefore, etiologic investigations are concentrated on determining a possible trigger of this hyper-reactive immune response [2].
Despite decades of investigation, the etiology of KD remains unknown. The focus of research since the emergence of the disease has shifted from a proposed association between exposure to freshly cleaned carpets and house dust mites to the search for a possible infectious agent responsible for KD pathogenesis [18].
Association between exposures to freshly cleaned carpets and KD
For a period of time in the 1980's and early 1990's, an association between exposure to carpet cleaners and KD was suspected and a number of studies were carried out investigating rug shampoo as a possible causative agent. In 1982, Patriarca et al. [21] published a paper suggesting that an outbreak of KD in Colorado may have been related to the exposure of children to recently applied rug shampoo. The authors suggested that the association might be the result of hypersensitivity to the anionic detergents present in all of the shampoos used, which was unlikely due to the number of household cleaning products also containing such compounds, or due to the inhalation of an infectious agent that undergoes aerosolization during the cleaning process.
Four studies, [22-25] investigating the association between the exposure of children to freshly cleaned carpets and KD in cases from Maryland, New York, Michigan, Colorado and Wisconsin reported no significant associations. However, three of the four studies provided possible reasons for why their analyses could have incorrectly demonstrated negative results. Lin et al. [22] stated that the findings were only conclusive in relation to the Maryland epidemic and did not feel confident that their results negated the possibility of some relationship between rug shampoo and KD. Rogers et al. [23] suggested that their negative result could be due to the fact that the microorganisms that are aerosolized during carpet cleaning also reside in other areas of households, which would reduce the likelihood of recognizing such an association. In addition, Glode et al. [24] suggested that failure to confirm the association between carpet cleaning and KD could be due to differences in endemic [which they studied] and epidemic cases or to the small number of cases analyzed [18 children with KD]. Klein et al. [25] on the other hand, assessed rug shampoo exposure, dust collection from homes, and serum specimen concentration, and concluded that their research was not in support of the hypothesis that there was an association between KD and carpet cleaning. Although drawing into question the relationship between carpet cleaning and KD, the four studies mentioned do not negate the possibility of some association among epidemic cases.
Following the initial proposal of the association between carpet cleaning and KD, there were three studies released confirming the positive results. Rauch et al., [26,27] and Fatica et al. [28] demonstrated this association among children affected by epidemics in Wisconsin, Colorado, Montana, and New York. In Patriarca et al., [21] Fatica et al. [28] and Rauch et al., [27], the intervals between the exposure to freshly cleaned carpets and the onset of illness were generally clustered around 13-30 days. This strengthens the argument for the relationship between carpet cleaning and KD because it suggests that this may be a standard period of incubation or induction for an infectious agent that may have been aerosolized during the process of cleaning [27]. In addition, the report by Fatica et al. [28] included in its study sample a family with two siblings affected, both of whom became sick within 45 days of the application of rug shampoo and one of which had recurrence 2 weeks following a second episode of rug cleaning. The authors indicated that such a strong association demonstrated by the recurrence was unlikely due to chance. There has been a great deal of speculation about the possible mechanisms of this association [18].
Possible role of house dust mites in the pathogenesis of KD
Prior to the publication of Patriarca et al. [21], two Japanese reports were released, Furusho et al., [29] and Hamashima et al. [30] suggesting a house dust mite antigen as a causative agent of KD. Furusho et al. [23] 9 reported increases in the serum levels of anti-mite specific IgG and also IgE during the acute phase of illness and Hamashima et al. [30] found Rickettsia-like bodies, which have been found in biopsy specimens of KD, in the digestive canal of mites in the rooms of KD patients. The studies suggest that the house dust mites may act as an allergen stimulating an allergic reaction or, more likely, that the mites act as vectors carrying a microorganism, possibly rickettsiae, that may be the causative agent of KD. Although Furusho et al. [29] suggested that the increased use of Tatami mats [which provide the moisture, darkness, human skin scales, and yeast that dust mites require] by Japanese people could account for the increasing incidence of KD in Japan, it was Patriarca et al. [21] and a number of subsequent American studies [2,18], that proposed the association between carpet cleaning, the aerosolization and inhalation of house dust mites, and KD. In 1983, Ohga et al., [31] found that within an outbreak in Japan, children with KD were more likely than control children to have rugs, which further supported this theory.
However, following these initial reports, there have been a number of studies refusing the hypothesis that a mite antigen may be involved in the pathogenesis of KD [32,33]. Glode et al. [24], analyzed the house dust mite content, serum anti-mite antibody levels, and exposure to freshly cleaned carpets in cases and controls of a Colorado epidemic and found no significant differences in the prevalence of mites [content was very low in all households], the exposure to freshly cleaned carpets, nor serum antibody concentrations. In addition, Klein et al., [25] found no variation in the density and species-specific prevalence of house dust mites in the home of KD patients.
It is also possible that the association between the agitating of dust mites during carpet cleaning and KD is in some way masked by the properties of mite growth [18]. It is clear that these mites do not reside only in carpets, but also in the dust that collects in heating vents, mattresses and pillows, stuffed toys and furniture [18]. This suggests that although the process of carpet cleaning may provoke the infusion of dust mites into the air, facilitating inhalation, it may not be the only source of mites, and that it is possible, KD patients not exposed to rug shampoo came into contact with mites from another source [34]. In addition, mite prevalence is affected by atmospheric relative humidity and seasonal prevalence; [2], therefore, such factors may affect the validity of studies demonstrating no association between the presence of dust-mites in households of children with KD [18].
Current etiologic and pathogenic hypotheses
Although the etiology of KD remains unknown, the majority of evidence suggests an infectious causative agent, possibly leading to disease in genetically susceptible hosts [35]. The epidemiologic factors suggesting an infectious cause, which were mentioned previously, are supported by the pathology of the disease, which resembles an immune-mediated inflammatory response resulting in endothelial damage. However, after years of searching, all possible known pathogens have been discarded [6].
There have been a number of studies that suggest toxins acting as a superantigen may trigger the immune-mediated response responsible for KD, although this hypothesis has been highly debated. This theory proposes that an infectious agent, possibly a bacterial toxin such as streptococci or staphylococci, produces an antigen that is highly immunostimulant, leading to the activation of large portions [up to 20%] of beta chain T cells and an overreaction of the immune system [36,37]. KD is very similar in its clinical presentation to a number of diseases caused by exotoxins acting as superantigens, such as toxic shock syndrome [TSS] and scarlet fever [34]. In addition Seib, [18]. reported the selective expansion of Vbeta 2+ cells and Vbeta8.1 + T cells in KD patients during the acute phase but not the convalescent phase, which strongly supports the argument for a super antigen-mediated pathogenesis. However, subsequent studies have failed to show such Vbeta T cell expansion [38]. Recently, the two proposed theories of Kawasaki disease etiology, the toxic shock syndrome toxin-1 hypothesis and the coronavirus NL-63 hypothesis, have been studied extensively and have been disproven [8].
Additional hypotheses of the pathogenesis of KD include the role of pro-inflammatory cytokines [39] and anti-endothelial cell antibodies [AECA] [40] in endothelial damage. In addition, the report of IgA plasma cells in the vascular walls of children with KD by Rowley et al. [41] suggests that KD is the result of the entry of an etiologic agent through a respiratory or gastrointestinal portal leading to the stimulation of an antigen-driven immune response with prominent IgA plasma cell involvement.
Recently, Qin etal., [42], described that, surprisingly, IgA plasma cells infiltrate inflamed tissues in acute Kawasaki disease, including the coronary artery, and are oligoclonal, or antigen-driven. Synthetic versions of predominant IgA antibodies in acute Kawasaki disease arterial tissue bind to an antigen present in acute Kawasaki disease ciliated bronchial epithelium and in a subset of macrophages in acute inflamed Kawasaki disease tissues. Light and electron microscopic studies of the antigen in acute Kawasaki disease ciliated bronchial epithelium indicate that the Kawasaki disease-associated antigen localizes to cytoplasmic inclusion bodies that are consistent with aggregates of viral protein and associated nucleic acid. The identification of cytoplasmic inclusion bodies in acute Kawasaki disease ciliated bronchial epithelium has provided direction for future Kawasaki disease etiology studies [8]. Transmission electron microscopic examination of glutaraldehyde-fixed medium-sized bronchi from acute Kawasaki disease fatalities and analysis of the protein and nucleic acid components of the inclusions should provide important information about these inclusion bodies and speed the identification of the specific etiologic agent of Kawasaki disease [8].
The pathophysiology of coronary artery aneurysms
Major pathological changes found in the coronary arteries are in the form of necrotizing arteritis, focal segmental destruction (Figure 1), aneurysms formation, (Figure 2), and thrombosis of a major coronary branch [43].

Figure 1. Histological sample of the coronary artery showing panarteritis in a patient who died 23 days after the onset of Kawasaki disease. Fragmentation of elastic fibres stained black is visible in the internal elastic lamina (arrow) and external elastic lamina (arrow head). Media (bracket) is almost completely destroyed, with severe inflammatory cell infiltration (Elastic van Gieson stain, x20) [44].
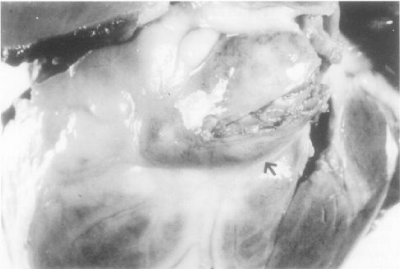
Figure 2. Frontal view of the heart showing a partially opened giant fusiform aneurysm of the left anterior descending coronary artery [arrow] in patient with KD. The lumen is filled with a thrombus [44].
While the idea that matrix metalloproteinases [MMP] may be involved in the pathogenesis of coronary artery vasculitis in Kawasaki disease is relatively new, an increasing number of studies have indicated elevated expression, activity, or protein levels of MMPs in Kawasaki disease [44]. A study conducted by Matsuyama, [45], indicates that plasma levels of MMP-1, -2, -3, and -9, as measured by enzyme linked immunoassay (ELISA), are significantly elevated in the acute stage of Kawasaki disease before treatment with intravenous gammaglobulin (IVGG), compared with levels in age matched controls. Levels of tissue inhibitors of MMPs [TIMP]-1 and -2 were also significantly higher in Kawasaki disease than in controls. Importantly, the data showed that levels of MMP-9 before treatment were significantly higher in Kawasaki disease patients with coronary artery lesions (CALs) than in Kawasaki disease patients without CALs. Levels of MMP-9 decreased after IVGG, regardless of the development of CALs. In contrast, MMP-3 levels of patients with CAL remained elevated after IVGG, and were significantly higher than those of patients without CAL. The absence of differences in TIMP levels between patients with CAL and without CAL is worth noting, given the difference in MMP levels, suggesting that an imbalance between MMPs and TIMPs contributes to CAL formation. The pre-IVGG MMP-9/TIMP-2 ratio and post-IVGG MMP-3/TIMP-1 ratio was both significantly higher in patients who developed CALs than in those without CAL. These results suggest a close association between increased MMP levels with MMP/TIMP imbalance and formation of CALs in Kawasaki disease. Nonetheless, some indirect data suggest that MMPs play a causative role in the development of coronary arterial wall destruction and resultant aneurysm formation (Figure 2), [44]. Findings of such studies may support the use of MMP inhibitors for the prevention of coronary artery complications in patients with this disease [44].
Clinical features
Currently there is no diagnostic test for KD; therefore, diagnosis is based on clinical criteria, which were established by the Centers for Disease Control in 1985 [46] and are listed in Table 1.
Table 1. Diagnostic criteria of Kawasaki disease.
Fever |
Lasting at least 5 days without explanation in addition to
four of the following: |
[1] Non-exudative conjunctival injection |
Bilateral |
[2] Changes of lips or oral mucosa |
Strawberry tongue, injected pharynx, cracked or red lips |
[3] Changes in the peripheral extremities |
Acute phase: erythema and/or indurative oedema of the
palms and soles
Convalescent phase: desquamation from finger tips |
[4] Polymorphous exanthem, primarily on trunk |
Non-vesicles or crust |
[5] Acute cervical lymphadenopathy |
Cervical >1.5 cm |
Key historical clues include the following: Fever: at least 5 days in duration, often abrupt in onset, unresponsive to antibiotic therapy, if given. Irritability - Out of proportion to the degree of fever or other signs.
Physical: The diagnosis of classic Kawasaki disease [KD] requires fever of at least 5 days duration and the presence of 4 of the following (Table 1), [if fever is present with fewer than 4 of the following, the diagnosis is established with ECHO-proven coronary artery disease and exclusion of other diseases with similar clinical features (Table 2), [47].
Table 2. Differential diagnosis of Kawasaki disease: Diseases and disorders with similar clinical findings.
Measeles, Other febrile viral exanthemas, Scarlet fever
Drug reactions,Steven-Johnson syndrome
Bacteremia and sepsis, Meningitis and encephalitis
Rokey mountain spotted fever
Staphylococcal scalded skin syndrome,Toxic shock syndrome
Juvenile rheumatoid arthritis,Leptospirosis
Mercury poisoning |
Changes in extremities [eg, erythema, edema, and desquamation]: This may limit movement and cause the child to refuse to bear weight. Desquamation of the fingers and toes begins in the periungual region, may involve the palms and soles, and is usually observed 1-2 weeks after the onset of fever (Figure 3).
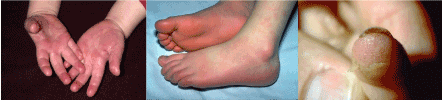
Figure 3 (ABC). Showing cutanous changes in extremities in Kawasaki disease in the form of erythema, edema, desquamation in the palms and soles [A] and [B]. Desquamation of the fingers begins in the periungual region [C], [2].
Bilateral conjunctivitis (not associated with exudates) (Figure 4).
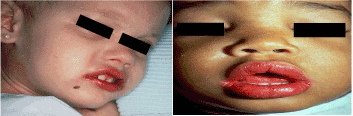
Figure 4 (A B). Showing changes in the conjnictiva and oral cavity in kawasaki disease. Bilateral conjunctivitis, not associated with exudates [A]. Dry/fissured, wollen lips and strawberry tongue [B], [2].
Polymorphous rash [not vesicular]
Cervical lymphadenopathy [usually >1.5 cm and unilateral; the least common of all clinical features, occurring in approximately 40%]
Changes in the lips and oral cavity [eg, pharyngeal erythema, dry/fissured or swollen lips, strawberry tongue) (Figure 4).
If coronary artery aneurysm is present, diagnosis can be based on presence of fever and any three clinical criteria [47].
Clinical findings
- The onset is usually abrupt with a high sustained fever that is unresponsive to antibiotic therapy and lasts for 1 week or longer. In addition, other typical features may be present.
- Lips become erythematous and fissured. Bleeding may be noted.
- The tongue is described as a strawberry tongue because of the diffuse erythema and prominent papillae (Figure 4).
- The neck may be stiff, possibly leading to a workup for meningitis, especially since many such patients are very irritable. Aseptic meningitis may coexist in one half of all patients [2].
Cardiac findings
Cardiovascular manifestations can be prominent in the acute phase of the illness and are the leading cause of long-term morbidity and mortality [11].
In this phase the pericardium, myocardium, endocardium and coronary arteries may all be involved. Pericardial effusion is detected by echocardiography in approximately 30% of patients with Kawasaki disease. It rarely progresses to tamponade and usually resolves spontaneously without specific therapy [11]. Clinically recognizable myocarditis is common. Signs include tachycardia out of proportion to the degree of fever. A gallop rhythm may be heard [47].
Coronary arterial abnormalities develop in approximately 20% of children with untreated Kawasaki disease and are the most common cause of both short- and long- term morbidity and mortality. Aneurysms have been detected within 3 days of onset of illness but more commonly occur after10 days to 3 weeks after the onset of symptoms [48], (Figure 2). The appearance of aneurysms more than 6 weeks after the onset of illness is uncommon. Factors associated withan increased risk of developing coronary arterial aneurysms include male gender; age less than 1 year; other signs or symptoms of pericardial, myocardial, or endocardial involvement, including arrhythmias; prolonged period of inflammation, including fever for more than 10 days; and the recurrence of fever after an afebrile period of at least 48 hours [49].
Myocardial infarction is the principal cause of death in Kawasaki disease. It may occur during the acute phase but happens more commonly within a year and may occur even later in patients who have giant aneurysms. Symptoms of myocardial infarction include inconsolable crying, vomiting, dyspnea, cardiovascular collapse, and shock. Chest pain has been described by children who can communicate the symptom. The majority of documented cases of infarction occur during sleep or while at rest [48,49].
Valvular involvement, primarily mitral regurgitation has been described in about 1% of children with Kawasaki disease [18]. Very rarely, regurgitation is severe enough to require valve replacement [18]. Decreased left ventricular function is present in approximately 50% of all patients with KS. The development of congestive heart failure is observed early in the disease [50].
In patients with coronary arterial abnormalities. aneurysms of other medium –sized muscular arteries including the renal, brachial, and femoral arteries, can occur during the acute illness but more commonly are detected more than a year after the acute illness. Children with giant aneurysms more likely have other arterial involvement [50].
Non-cardiac findings
May include arthritis/arthralgia can occur in the first week of illness and is usually polyarticular, involving knees, ankles, and hands. Commonly a polyarticular arthritis involving the knees, ankles, or hips appears during the second or third week of illness. Arthritis is more common in older girls [51].
Preceding or concurrent respiratory symptoms such as cough rhinorrhea, otitis media, or pulmonary infiltrate are often observed. Other manifestations include, urethritis, abdominal pain, vomiting/diarrhea [one third of patients], sterile pyuria [one third of patients], elevated liver function values-eitheran aspartate or an alanine aminotransferase level of greater than 40 IU per liter, gallbladder distention and aseptic meningitis –a cerebrospinal fluid leukocyte count of greater than 5×105 cells per liter [5 cells per mm3] [47]. Other less common findings include auditory abnormalities, testicular swelling, and peripheral gangrene [52].
Although rare in the United States, reactions of erythema, induration, and ulcerations may occur at the inoculation site of children who have received the BCG vaccine. Clinicians should be aware of this clinical manifestation that could help diagnose atypical or incomplete cases of the disease [53]. Experts now agree that incomplete or atypical cases can and do occur. In this setting, usually in children younger than 6 months of age, fever plus only 3 features establish the diagnosis [54]. The rationale is that treatment is safe and effective and that failure to diagnose KD may have a significant negative impact on outcome [14,55].
Three distinct phases occur, as follows. Some authors add a fourth "chronic" phase [2,56].
- Acute febrile phase [days 1-11]
- The temperature is elevated [>104°F].
- The child is irritable.
- Bilateral conjunctivitis and rash are present.
- The hands and feet develop the erythema and edema that cause the child to refuse to walk.
- The tongue and oral mucosa become red and cracked.
- Hepatic dysfunction may develop.
- Cardiac complications noted in the first stage include myocarditis and pericarditis.
- Sub-acute phase [days 11-21]
- This is characterized by persistent irritability, anorexia, and conjunctival injection.
- The fever usually resolves by this stage. If fever has persisted, the outcome is less favorable because of a greater risk of cardiac complications.
- Thrombocytosis develops, and the platelet count may exceed 1 million/mm3.
- Desquamation of the fingertips and toes begins at this time.
- Aneurysm formation may occur during this stage.
- Children are at greatest risk of sudden death during this phase.
- Convalescent phase [days 21-60]:
- Begins when all signs of illness have disappeared and continues until acute phase reactants [erythrocyte sedimentation rate [ESR], C-reactive protein [CRP] level] have returned to normal.
- The most significant clinical finding that persists through this phase is the presence of coronary artery aneurysms.
- This stage is only of clinical importance in patients who have developed cardiac complications.
- Its duration is of lifetime significance because the aneurysm formed in childhood may rupture in adulthood.
- In some cases of aneurysms rupturing in adult life, careful reviews of past medical histories have revealed febrile childhood illnesses of unknown etiology.
Differential Diagnosis
Because of the principal clinical findings to fulfill the diagnostic criteria are not specific, other diseases with similar clinical features should be excluded (Table 2). Consideration of measles as a possible diagnosis is particularly important because cases have been misdiagnosed as Kawasaki disease and appropriate control measures have not been taken promptly [51].
Laboratory Studies:
- No specific laboratory test exists; however, certain abnormalities coincide with various stages.
- A mild-to-moderate normochromic anemia is observed in the acute stage along with a moderate to alarmingly elevated WBCs count with a left shift.
- Many of the acute phase reactant markers, such as the ESR, CRP level, and serum alpha1-antitrypsin level are elevated. Culture results are all negative [2].
- More recently, 2 urine proteins hold promise as biomarkers of Kawasaki disease: meprin A or filamin C. These 2 biomarkers were diagnostically superior to ESR or CRP [57,58]. Investigators identified more than 190 proteins that were present only in children with Kawasaki disease, including the proteins associated with endothelial and myocardial cell injury [filamin C] and immune regulators [meprin A] [57,58].
- Elevated macrophage migration factor [MIF] and Interleukin-6 [IL-6] may be useful markers in the acute stages of Kawasaki disease. The serum complement level is normal or elevated.
- During the subacute stage, platelet count elevation is the outstanding marker. It begins to rise in the second week and continues to rise during the third week. Levels as high as 2 million have been observed. The acute reactive markers remain elevated [59].
- In the convalescent stage, the levels of platelets and other markers begin to return to values within the reference range. Laboratory values may require 6-8 weeks to normalize [59].
Imaging studies: An echocardiogram [ECHO] is the study of choice to demonstrate coronary artery aneurysms. Coronary artery lesions are frequently detected on the initial echocardiogram of children with Kawasaki syndrome [7]. In order of highest to lowest frequency, the involvement of the coronary arteries is as follows:
- Proximal left anterior descending and right coronary artery
- Left main coronary artery
- Left circumflex artery
- Distal right coronary artery
- Posterior descending artery
- During the acute stage, a baseline ECHO is important. Diffuse dilatation of coronary lumina can be observed in 50% of patients by the 10th day of illness. In children, ensure that pediatric cardiologists perform this study because they are familiar with coronary artery diameters. Coronary artery dimensions must be adjusted for body surface area to accurately identify dilation. A basic rule is that if the internal diameter of a segment is greater than 1.5 times that of an adjacent segment, then dilation probably exists.
- Abnormalities of the coronary arteries include ectasia [coronary size larger than normal for age, [normal coronary artery size ranges from1-2mm in newborns and infants to 4.5-5.0 mm in teenagers) [60,61], or aneurysms that may be fusiform or saccular [near –equal axial and lateral diameters) (Figure 5). Patients with giant aneurysms [internal diameter of at least 8mm] have worst prognosis and are at the greatest risk of developing coronary thrombosis (Figure 6), stenosis, or myocardial infarction. Giant aneurysms generally do not resolve [62]. The ECHO should be repeated in the second or third week and again 1 month after all other laboratory results have normalized. Many centers perform a 1-year ECHO, even when the first ones show no aneurysm, if the ECHO results are abnormal at any point, the child should be referred to a pediatric cardiologist for a complete cardiac workup and follow-up care [63].
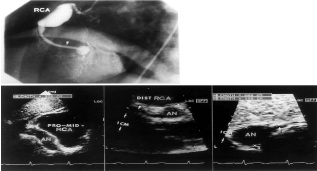
Figure 5. Showing coronary angiogram, and echocardiogram from the same patient with coronary aneurysm.
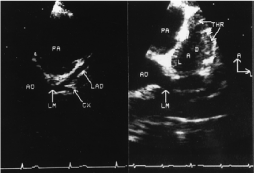
Figure 6. Echocardiographic images from normal subject and from patient with KD.Left, Normal patient: Parasternal short-axis echocardiographic visualization of the left main [LM], left anterior descending [LAD], and circumflex [CX] coronary arteries. AO indicates aorta; PA, pulmonary artery. B, Kawasaki patient: Parasternal short-axis view demonstrating thrombus [THR] in the LAD. Orientation: A, anterior; L, left. [66].
- An ultrasound of the gallbladder may be necessary if any suggestion of liver or gallbladder dysfunction is present [59].
- A chest radiograph should be obtained to assess baseline findings and to confirm clinical suspicion of congestive heart failure [64].
Other Tests: An electrocardiogram [ECG] indicates the presence of various conduction abnormalities, as a result of myocardial inflammation with the occurrence of prolonged PR or QT interval or both. Local areas of ischemia may occur and predispose to atrial or, more commonly, ventricular arrythemias. Additionally, children with the syndrome may suffer acute infarction. One third of children with Kawasaki disease showing decreased R wave voltage, ST segment depression and T wave flattening or inverse [49]. Cardiac enzyme levels [eg, creatine kinase [CK], creatine kinase myocardial band [CK-MB], cardiac troponin, lactate dehydrogenase [LD-1 >LD-2]] are elevated during a myocardial infarction.
- Magnetic resonance imaging [MRI], magnetic resonance angiography [MRA], and ultrafast computed tomography [CT] scanning are other noninvasive tests that can be used to evaluate coronary artery abnormalities. However, larger studies are required to evaluate their reliability.
- Free-breathing 3-dimensional [3D] coronary MRA accurately defines CAA in patients with Kawasaki disease. This technique may provide a noninvasive alternative when the image quality of transthoracic echocardiography is insufficient, thereby reducing the need for serial radiographic coronary angiography in this patient group.
- One study used multislice spiral CT to assess coronary artery abnormalities in 16 adolescents and young adults with KD. Although the numbers were small, CT was 100% sensitive in the detection of coronary artery aneurysms but only 87.5% sensitive for the detection of significant stenosis or occlusion. False-positive results occurred secondary to severe calcification in 5 arteries and cardiac motion artifact in 2. Specificity was therefore 92.5% [50]. It is conceivable that more cases of KD presenting with small pulmonary nodules undetected at plain-film radiography might be seen as more children undergo CT [64,65].
- In another small study, electron beam computed tomography [EBCT] was used to determine if coronary artery calcifications could be used as a marker of future coronary artery events. The authors felt that this study may be useful for risk stratification in long-term management of patients with KD [11].
- Perfusion myocardial scintigraphy, and coronary angiography are covered [11].
- Procedures: A select group with ischemic symptoms may require cardiac catheterization. Stent is needed in children with mild calcification & giant aneurysms [2].
In some cases, patients have many of the typical clinical features of Kawasaki disease but not as many as are required to meet standard diagnostic criteria. Hence, the term "incomplete" rather than "atypical" is used to describe these cases. Incomplete cases usually occur in children younger than 6 months. In this setting, fever plus only 3 features can establish the diagnosis. The rationale is that treatment is safe and effective and that failure to diagnose Kawasaki disease may have a significant negative impact on outcome.
For the diagnosis of incomplete Kawasaki disease, the American Academy of Pediatrics [AAP]/American Heart Association [AHA] recommend that when fever plus 2 or 3 of the typical features are present for 5 days or more and when patient characteristics suggest possible Kawasaki disease, a C-reactive protein [CRP] level and erythrocyte sedimentation rate [ESR] should be obtained [67]. If the CRP level is less than 3 mg/dL and the ESR is more than 40 mm/hr, the child is monitored and actions taken as appropriate.
If the CRP is 3 mg/dL or higher and the ESR is 40 mm/hr or more, the next step is to measure albumin, alanine aminotransferase [ALT], platelets, and WBC count and test the urine for pyuria. Abnormal limits include the following:
- Albumin < 3 g
- Anemia for age
- Elevated ALT level
- Platelets >450,000 [after 7 d]
- WBC count >12,000
- Presence of pyuria
If 3 or more supplemental laboratory criteria are positive, a diagnosis of Kawasaki disease is made. The child should have an echocardiogram and be treated.
If fewer than 3 supplemental laboratory criteria are positive, a cardiac echocardiogram should be performed. If the echocardiogram is negative but fever persists, a repeat echocardiogram may be performed. If the echocardiogram is negative and the fever abates, Kawasaki disease is unlikely. If the echocardiogram is positive, the child is treated for Kawasaki disease.
A French group has suggested adding another diagnostic category, "uncertain Kawasaki disease”, for children with 5 days of fever, fewer than 4 classic signs, normal echocardiographic findings, and an inflammatory syndrome that does not meet AHA criteria. These researchers found that children in this category did well when treated with IVIG and aspirin [68].
Hinze et al., reported a case of Kawasaki disease in a 3-month-old boy manifested by typical signs and CAAs but without fever. They commented on the difficulty in making the diagnosis in young infants [69]. Case reports of other unusual presentations [eg, GI bleeding, lupuslike illness in a recurrent case, arthritis, rhabdomyolysis] have been published. Such presentations appear to be very uncommon [70].
Complications
The development of coronary artery aneurysms with consequences such as thrombosis or rupture determines the degree of disability. Acute myocardial infarction has been reported secondary to true coronary artery obstruction [62]. Dehydration may result from fever and anorexia.Joint inflammation in the acute phase may limit mobility [2].
Treatment
Emergency Department Care:
- Any young child presenting to the emergency department [ED] with symptoms of early or acute stage KS should be evaluated to rule out sepsis or meningitis.
- Intravenous access and cardiac monitoring should be established.
- Depending on the institution, anti-inflammatory therapy may need to begin in the ED.
Medications
Initial therapy for Kawasaki disease is currently directed at reducing inflammation, particularly in the coronary arterial wall and myocardium. Later, therapy is directed toward preventing coronary thrombosis by inhibiting platelet aggregation [49]. Specific therapy awaits discovery of the etiologic agent.
When possible, patients with Kawasaki disease should be treated within the first 10 days of onset of illness with intravenous gamma globulin and high –dose aspirin (Table 3). The beneficial effect of intravenous gamma globulin on coronary abnormalities was first reported in Japan [29]. A multicenter randomized controlled trial in United States demonstrated that defervescence and resolution of inflammation were more pronounced and coronary arterial abnormalities were significantly less frequent in patients treated with intravenous gamma globulin plus aspirin than in patients treated with aspirin alone .A subsequent US trial compared a single 2 g/kg dose of intravenous gamma globulin to the dosage of 400mg/kg of intravenous gamma globulinper day for 4 days; both groups also received 100mg/kg of aspirin per day. Children treated with a single- infusion regimen had significantly fewer coronary abnormalities 2 weeks after enrollment than children treated with the 4 -day infusion. By the seventh week after enrollment, the difference between the two groups was not significant. Children treated with the single-infusion regimen also had more rapid defervescence and return of acute phase reactants to normal [49]. Intravenous gamma globulin has been reported to reduce the likelihood of development of giant coronary artery aneurysms [13,50], and appear to have a direct beneficial effect on abnormalities in cardiac function associated with the acute phase of Kawasaki disease [13].
Table 3. Recommended therapy during the acute stage of the disease.
Intravenousgamma globulin |
2 g/kg as a single infusion over 12 hours [Preferred]. [Or]
400 mg/kg per day; each dose infused over 2 hours [alternate].
[plus]
Aspirin
80-100 mg/kg per day orally in four equally divided doses until patient is afebrile*, Then3-5 mg/kg orally once daily for up to 6-8 weeks** |
Newer studies have shown that high single doses are more effective. In current practice, the dose is 2 g/kg intravenously over 10-12 hours [71,72]. In patients in whom the standard treatment fails [approximately 10-15%] and who continue to have fever 36 hours after the initial dose of IVIG, a second treatment with IVIG at the original dose is recommended [73]. A small subgroup of patients fails to respond to a second dose of IVIG.
A study in an ethnically diverse population in San Diego, California, found that patients with IVIG resistance tended to have higher percent bands; higher concentrations of C-reactive protein, alanine aminotransferase, and gamma-glutamyl transferase; lower platelet counts; and lower age-adjusted hemoglobin concentrations. They were also more likely to have aneurysms. However, a proposed scoring system to predict IVIG resistance proved insufficiently accurate to be clinically useful [74].
*Some clinician recommends high dose aspirin until the 14th day of illness.
**Discontinue aspirin 6-8 weeks after onset of illness if no coronary arterial abnormalities are observed on echocardiogram. Continue indifinetly if there are coronary arterial abnormalities [49].
High dose aspirin alone hastens the resolution of acute manifestations of Kawasaki disease, particularly fever. The combination of aspirin at 80-100mg/kg per day and intravenous gamma globulin has a more rapid anti-inflammatory effect than aspirin alone and appears to decease the rate of development of coronary abnormalities [75].
After the child has become afebrile, aspirin should be reduced to3-5mg/kg given as a single dose for its antithrombotic effect. Low dose aspirin is continued for approximately 6-8 weeks and then discontinued if no coronary arterial abnormalities have been detected by echocardiography [7]. Although data are limited, authors of several case reports have suggested a possible role for thrombolysis in those suffering acute MI as a consequence of thrombus formation in aneurysms. At this time, it seems unlikely that the emergency physician will administer this therapy [75].
The use of dipyridamole to alter platelet activity should be considered during this interval if the patient is at high risk for myocardial infarction, that is, has developed significantcoronary arterial abnormalities (Table 4), [76]. If coronary arterial abnormalities are detected, low dose aspirin therapy should be continued indefinitely and the patient referred to a pediatric cardiologist for long- term follow up [75].
Table 4. Drug Categories used for treatment of Kawasaki disease.
Drug Name |
Gamma globulins , intravenous [Gammagard, Gamimune] |
Mechanism of action |
Neutralizes circulating myelin antibodies through anti-idiotypic antibodies; down-regulates proinflammatory cytokines, including INF-gamma; blocks Fc receptors on macrophages. |
Pediatric Dose |
400 mg/kg/d IV in a single daily infusion for 4 d or single dose of 2 g/kg IV infused over 12 h. |
Contraindications |
Documented hypersensitivity; IgA deficiency; anti-IgE/IgG antibodies; severe thrombocytopenia or coagulation disorders |
Interactions |
May interfere with immune response to live-virus vaccine [MMR],[do not administer within 3 mo of vaccine]. |
Precautions |
Check serum IgA before IVIG, infusions may increase thromboembolic events, may cause elevated antiviral or antibacterial antibody titers, 6-fold increase in ESR. |
Drug Name |
Anti-inflammatory agentsAspirin [Anacin, Ascriptin, Bayer Aspirin, Bayer Buffered Aspirin] |
Mechanism of action |
Inhibits prostaglandin synthesis, which prevents formation of platelet-aggregating thromboxane A2. |
Pediatric Dose |
80-100 mg/kg/d PO divided qid for 2 wk initial; 3-5 mg/kg PO qd for 6-8 wk maintenance. Coronary artery abnormalities: 3-5 mg/kg PO qd long term [with or without dipyramidole]. |
Contraindications |
Documented hypersensitivity; liver damage; hypoprothrombinemia; vitamin K deficiency; bleeding disorders; asthma; use in children [<16 y] with influenza because of association of aspirin with Reye syndrome. |
Interactions |
Effects may decrease with antacids and urinary alkalinizers; corticosteroids decrease salicylate serum levels; additive hypoprothrombinemic effects and increased bleeding time may occur with co administration of anticoagulants, and in patients with asthma. |
Precautions |
Avoid use in patients with severe anemia, with history of blood coagulation defects, or taking anticoagulants. |
Drug Name |
Antiplatelet agents Dipyridamole [Persantine] |
Mechanism of action |
A platelet-adhesion inhibitor, an inhibitor of platelet reactivity. May inhibit phosphodiesterase activity leading to formation of thromboxane A2. |
Pediatric Dose |
Not established; limited data indicate 3-6 mg/kg/d PO divided tid |
Contraindications |
Documented hypersensitivity |
Interactions |
May increase heparin toxicity |
Precautions |
Caution in hypotension; has peripheral vasodilating effects |
Some Japanese and American investigators have attempted to target intravenous gamma globulin therapy only to those patients thought to be at highest risk of developing coronary arterial abnormalities by constructing risk scoring system. None of these scoring system is sensitive or specific enough to enable early prediction of which patients will develop coronary abnormalities [13]. Therefore, the current recommendation is that all chidren diagnosed with Kawasaki disease within 10 days of onset of fever should receive intravenous gamma globulin and high –dose Aspirin as early as possible [13].
Administration of parental live virus vaccines [measles, mumps, and rubella] should be delayed for at least 5 months after intravenous gamma globulin treatment because passively acquired antibodies may interfere with effective immunization [77]. During a measles outbreak, however, it may be prudent to administer measles vaccine earlier to a previously immunized child and repeat the vaccination at a later time. Schedules for administration of other routine childhood vaccinations should not be interrupted.
PO absorption of aspirin may decrease in Kawasaki disease to <50% [compared to typical bioavailability of 85-90%]. This altered bioavailability may explain why higher doses required to achieve a salicylate serum concentration >20 mg/dL, and monitoring serum concentrations can be helpful in apparent non-responders or in certain other circumstances [2], (Table 4). To reduce the risk of Reye's syndrome in patients on long-term aspirin therapy, administration of influenza vaccine is recommended, although the magnitude of just risk is unknown [75]. Also, aspirin therapy should be interrupted if the patient develops varicella or influenza [75]. The dose of aspirin is on the borderline of that causing salicylate toxicity, therefore, monitor for toxicity [ie, vomiting, hyperpnea, lethargy, liver dysfunction]; monitor salicylate level and maintain at 18-28 mg/dL. Avoid using aspirin in patients with severe anemia, with history of blood coagulation defects, or taking anticoagulants; and in patients with asthma [49].
Clopidogrel [Plavix] may be briefly substituted for aspirin in patients who develop influenza or varicella. This agent can also be used in patients allergic to aspirin [72].
Because the mechanism of action of intravenous gamma globulin is unknown, standardization of intravenous gamma globulin preparations is not possible. It is unclear whether all commercially available intravenous gamma globulin preparations or various lots of the same preparation are equally effective. The optimal dose of intravenous gamma globulin for treatment of acute Kawasaki disease remains undetermined. Two US studies using a single dose of 1 g/kg intravenous gamma globulin have been reported. One study was uncontrolled [78,79]. And the other comparing dose of 1 g/kg to 400 mg/kg per day for 4days involved a small number of patients [80]. No trials comparing single doses of 1 g/kg and 2 g/kg have been reported.
Intravenous gamma globulin therapy should be considered for patients in whom the diagnosis of Kawasaki disease is made after the tenth day of illness if they have signs of ongoing inflammation or evolving coronary artery disease. Prolonged fever is associated with increased risk of coronary arterial abnormalities, including giant aneurysms. Some patients who present within 10 days of onset of illness already have coronary arterial abnormalities [50]. These patients should receive aspirin and intravenous gamma globulin [2].
Intravenous gamma globulin therapy may not always result in a prompt anti-inflammatory response. Some patients have persistent fever 24 hours after completion of the infusion. Other patients have an initial defervescence for at least 24 hours and then a recurrence of the fever [81]. In both circumstances, retreatment with intravenous gamma globulin should be considered, although the benefit of retreatment to decrease the risk of coronary arterial sequelae has not been determined [82].
Treatment of atypical or incomplete Kawasaki disease with intravenous gamma globulin is based on clinical judgment [54]. To identify such patients with certainly will not be possible until the etiology of the illness is discovered and a specific diagnostic test developed. Infants particularly those under 6 months of age, frequently lack full diagnostic criteria for Kawasaki disease, and a high index of suspicion is necessary to make the diagnosis in these patients [14]. Because these young patients are at extremely high risk of developing coronary arterial abnormalities, early diagnosis and institution of appropriate therapy is particularly important [54].
Overuse of intravenous gamma globulin should be discouraged because this treatment is expensive and has potential side –effects (Table 4). When possible, patients with questionable diagnosis should be referred to a pediatric facility with established expertise in the diagnosis and management of Kawasaki disease before therapy is initiated [82].
Some have suggested that there is, or may be, a role for corticosteroids as the inclusion of corticosteroids in aspirin-containing regimens for the initial treatment of Kawasaki disease may reduce the incidence of coronary aneurysms [83]. Patients in whom a second dose of IVIG therapy fails can be treated with corticosteroids. Intravenous pulse methylprednisolone may be given at 30 mg/kg for 2-3 hours administered once daily for 1-3 days.
An alternative treatment is infliximab (Remicade) at 5 mg/kg, which is a chimeric mouse-human monoclonal antibody directed against soluble and membrane bound tumor necrosis factor-alpha [72]. Several studies have found infliximab to be useful in treating Kawasaki disease that is refractory to IVIG [84,85]. Burns et al., reported that infliximab was as effective as a second dose of IVIG in patients who did not respond to a first dose of IVIG [85].
Pentoxifylline is methylxanthine that act by inhibiting TNF-mRNA transcription. It can be used with IVGG and aspirin in treatment of KD and is well tolerated [2]. Abciximab is a platelet glycoprotein IIb/IIIa receptor inhibitor used to treat patients with large coronary aneurysms in the acute & subacute KD. It decreases aneurysm diameter by promoting vascular remodeling. It is recommended in large aneurysms [82].
Anticoagulants such as warfarin and low molecular weight heparin are used in patients with large aneurysms in whom the risk of thrombus is high. The goal is to maintain an international normalized ratio [INR] of 2-2.5.
Patients who have thrombosis and acute coronary occlusion should be treated with medical therapy because of the risk of rupture if interventional cardiac catheterization is attempted. In addition to standard treatments and warfarin, thrombolytics are given.
Because the potential exists for allergic complications with the use of streptokinase in patients who have had streptococcal pharyngitis in the last 6 months and because the triggering factor for Kawasaki disease remains uncertain, this medication is best avoided. Other drugs in this category, such as tissue plasminogen activator, tenecteplase-tissue plasminogen activator, and urokinase, may be more appropriate.
Other alternative therapies for resistant cases include cyclophosphamide with and without methotrexate; however, the effectiveness of these latter treatments is still uncertain because they have been used in only a small number of cases [72]. The following are adjunctive therapies for patients who do not respond to conventional therapies.
Ulinastatin is a human trypsin inhibitor purified from human urine. It has been used only in Japan for refractory cases of Kawasaki disease and is believed to function by inhibiting neutrophil elastase and prostaglandin H2 synthase at the mRNA level.
In the future, by identifying a genetic signature for this group, more aggressive therapies, such as anticytokine therapy, plasmapheresis, or cyclosporin A, may be used to reduce the risk of coronary complications [70,86].
Cardiac transplantation has been needed in cases refractory to treatment, in severe myocardial dysfunction, severe ventricular arrhythmias, severe coronary arterial lesions and in failing of other procedures [2].
Coronary artery bypass grafting [CABG] is the standard therapy when myocardial ischemia is detected and Kitamura operation provides good growth potential and long-term graft patency. Also, it is indicated in severe occlusion of the main trunk of the LMCA, severe occlusion of the proximal segment of the LAD and collateral coronary artery in jeopardy [87].
Kawasaki disease is an acute febrile, systemic vasculitic syndrome of an unknown etiology that primarily occurs in children younger than five years of age [88-91]. The principal presentations of Kawasaki disease include fever, bilateral nonexudative conjunctivitis, erythema of the lips and oral mucosa, changes in the extremities, rash, and cervical lymphadenopathy. Coronary artery aneurysms or ectasia develops in 25% of untreated children with the disease, which may later lead to myocardial infarction, sudden death, or ischemic heart disease. Since its first description in Japan in 1967 by Kawasaki, KD has affected children of all racial groups. Due to the absence of a diagnostic test for KD, the disease often masquerades as other exanthems and is misdiagnosed, leading to an unnecessary increase in the incidence of cardiac complications. Although during the 1980's scientists explored an association between KD and carpet cleaners, the numerous contradictory case-control studies published during this period have resulted in no consensus on the matter. It has been demonstrated that a dust mite antigen is not likely to be the causative agent of KD [92-97], but it remains to be determined if mites may act as vectors of the causative agent. Other areas of etiologic and pathogenic research include bacterial toxins producing superantigens, anti-endothelial antibodies, and normal antigen-driven processes, all of which may produce new insight into the nature of the disease. Recently, the identification of cytoplasmic inclusion bodies in acute Kawasaki disease ciliated bronchial epithelium has provided direction for future Kawasaki disease etiology studies and the identification of the specific etiologic agent [98,99]. Management varies from antithrombotic therapy to surgical ligation. Treatment with intravenous gamma globulin [IVIG] is effective, but the mode of action is still unclear. Controlling coronary heart disease risk factors sharply affects the prognosis in patients with CALs. Despite the broad scope of research into this disease, the causative agent of KD remains a mystery [100].
Recommendations
- Clinician should be aware of the clinical manifestations of the disease because early diagnosis and treatment is essential for prevention of cardiac complications.
- Parents should be advised to be conservative in allowing their children to be exposed to freshly cleaned carpets.
- Careful care is necessary in the child who has developed cardiac complications and the pediatrician or cardiologist who provides the long-term care monitors aspirin therapy and decides whether or not to use warfarin or heparin.
- Because of the potential life threats, patients with KD must be admitted to a hospital with a pediatric service and some patients with documented coronary artery aneurysms may need to be transformed to a tertiary pediatric facility.
- Clinician should be aware of the diagnosis of atypical or incomplete cases of KD and should start giving treatment as early as possible.
- Further studies on the effects of MMP inhibition on coronary outcome are needed to define the roles of MMPs and TIMPs in the formation of coronary artery lesions in Kawasaki disease; findings of such studies may support the use of MMP inhibitors for the prevention of coronary artery complications in patients with this disease.
- The development of a diagnostic test, a more specific therapy, and ultimately the prevention of this potentially fatal illness in children are all dependent upon the continued advances in determining the etiopathogenesis of this fascinating disorder.
I thank The National research Centre, Cairo, Egypt, for the continuous support.
- Dahdah N, Fournier A (2013) Natriuretic Peptides in Kawasaki Disease: The Myocardial Perspective. Diagnostics (Basel) 3: 1-12. [crossref]
- Parrlno LS, Parrillo C V, Bowman JG, Windle ML, Young JL, et al. (2007) Pediatrics Kawasaki Disease, Emergency Management Committee. Emer Medi 1607: 686-690.
- Curtis N (1997) Kawasaki disease. BMJ 315: 322-323. [crossref]
- Neches WH (2002) Kawasaki disease in Andersons textbook of pediatric cardiology. 62:1983-99.
- Lang B, Duffy CM (2002) Controversies in the management of Kawasaki disease. Best Pract Res Clin Rheumatol 16: 427-442. [crossref]
- Burns JC, Howard I Kushner, John F Bastian, Hiroko Shike, Chisato Shimizu, et a1. (2000) Kawasaki Disease: A Brief History. Pediatrics 106: 27-35.
- Baer AZ, Rubin LG, Shapiro CA, Sood SK, Rajan S, et al. (2006) Prevalence of coronary artery lesions on the initial echocardiogram in Kawasaki syndrome. Arch Pediatr Adolesc Med 160: 686-690. [crossref].
- Aryeh Z Baer, Lorry G Rubin, Craig A Shapiro, Sunil K Sood, Sujatha Rajan, et al. (2006) Prevalence of coronary artery lesions on the initial echocardiogram in Kawasaki syndrome. Arch Pediatr Adolesc Med 160: 686-690.
- Rowley AH, Shulman ST (2007) New developments in the search for the etiologic agent of Kawasaki disease. Curr Opin Pediatr 19: 71-74. [crossref]
- Kawasaki T (1967) Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation o the fingers and toes in children. Arerugi 16: 178-222. [crossref]
- Yamamoto T, J Kimura (1968) Acute febrile mucocutaneous lymph node syndrome [Kawasaki]: subtype of mucocutaneous ocular syndrome of erythema multiforme complicated with carditis [in Japanese] Jpn J Pediatr 21: 336-339.
- Strizhakov LA, Krivosheev OG, Semenkova EN (2006) Cardiac lesion in systemic vasculites: manifestations, diagnosis, and treatment. Klin Med(Mosk) 84: 8-13. [crossref]
- Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H (1974) A new infantile acute febrile mucocutaneous lymph node syndrome [MLNS] prevailing in Japan. Pediatrics 54: 271-276. [crossref]
- Rowley AH, Shulman ST (2004) Kawasaki Disease. In: Behrman RE, Kliegman RM, Jensen HD, (eds) Nelson Textbook of Pediatrics. 17th (edn). Philadelphia Pa: WB Saunders Co, USA, pp: 823-826.
- Pannaraj PS, Turner CL, Bastian JF, Burns JC (2004) Failure to diagnose Kawasaki disease at the extremes of the pediatric age range. Pediatr Infect Dis J 23: 789-791.
- Yanagawa H, Nakamura Y, Yashiro M, Oki I, Hirata S, et al. (2001) Incidence survey of Kawasaki disease in 1997 and 1998 in Japan. Pediatrics 107: E33. [crossref]
- Gardner-Medwin JM, PavlaDolezalova, Carole Cummins, Taunton R Southwood (2002) incidence of Henoch-Schonlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet 360: 1197-1202.
- Yanagawa H, Yashiro M, Nakamura Y, Hirose K, Kawasaki T (1995) Nationwide surveillance of Kawasaki disease in Japan, 1984 to 1993. Pediatr Infect Dis J 14: 69-71. [crossref].
- Seib C (2003) Kawasaki disease :in depth review of medical literature, project of the Community-Based Leaming Initiative [CBLI] at Princeton University. Pediatr Infect Dis 315: 341-347.
- Leung DY, Cotran RS, Kurt-Jones E, Burns JC, Newburger JW, et al. (1989) Endothelial cell activation and high interleukin-1 secretion in the pathogenesis of acute Kawasaki disease. Lancet 2: 1298-1302. [crossref]
- Leung DY, H CodyMeissner, David R Fulton, FredQuimby, Patrick M Schlievert (1995) Superantigens and Kawasaki Syndrome- Clinical Immunology and Ummunopathology. 77: 119-126.
- Patriarca PA, Rogers MF, Morens DM, Schonberger LB, Kaminski RM, et al. (1982) Kawasaki syndrome: association with the application of rug shampoo. Lancet 2: 578-580. [crossref]
- Lin FY, Bailowitz A, Koslowe P, Israel E, Kaslow RA (1985) Kawasaki syndrome. A case-control study during an outbreak in Maryland. Am J Dis Child 139: 277-279. [crossref]
- Rogers MF, Kochel RL, Hurwitz ES, Jillson CA, Hanrahan JP, et al. (1985) Kawasaki syndrome. Is exposure to rug shampoo important? Am J Dis Child 139: 777-779. [crossref]
- Glode MP, Brogden R, Joffe LS, Adinoff A, Leung DY, et al. (1986) Kawasaki syndrome and house dust mite exposure. Pediatr Infect Dis 5: 644-648. [crossref]
- Klein BS, Rogers MF, Patrican LA, White MC, Burgdorfer W, et al. (1986) Kawasaki syndrome: a controlled study of an outbreak in Wisconsin. Am J Epidemiol 124: 306-316. [crossref]
- Rauch AM (1989) Kawasaki syndrome: issues in etiology and treatment. Adv Pediatr Infect Dis 4: 163-182. [crossref]
- Rauch AM, Eugene S Hurwitz, Lawrence B Schonberger, Mary P Glode, James W Wiggins, et al. (1991) Outbreak of Kawasaki syndrome in Denver, Colorado: association with rug and carpet cleaning. Pediatrics 87: 663-669.
- Fatica NS, Ichida F, Engle MA, Lesser ML (1989) Rug shampoo and Kawasaki disease. Pediatrics 84: 231-234. [crossref]
- Furusho K, Kamiya T, Kaao H, Kiyosawa N, Shinomiya K, et al. (1984) High dose intravenous gamma globulin in Kawasaki disease.Lancet 120 : 1055-1058. [crossref]
- Hamashima Y, Tasaka K, Hoshino T, Nagata N, Furukawa F, et al. (1982) Mite-associated particles in Kawasaki disease. Lancet 2: 266. [crossref]
- Ohga K, Yamanaka R, Kinumaki H, Awa S, Kobayashi N (1983) Kawasaki disease and rug shampoo. Lancet 1: 930. [crossref]
- Jordan SC, Platts-Mills TA, Mason W, Takahashi M, Sakai R, et al. (1983) Lack of evidence for mite-antigen-mediated pathogenesis in Kawasaki disease. Lancet 1: 931. [crossref]
- Tang RB, Hwang BT, Tsai LC, Lin FM, Chang HN (1987) [The significance of mite antigens in Kawasaki disease] Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi 20: 29-36. [crossref]
- Lloyd AJ, Walker C, Wilkinso M (2001) Kawasaki disease: is it caused by an infectious agent? Br J Biomed Sci 58: 122-128. [crossref]
- Cimaz R, F Falcini (2003) An updateo n Kawasaki disease Autoimmunity Reviews. 58: 122-128.
- Fischer P (1996) Kawasaki disease: update on diagnosis treatment, and a still controversial etiology. Pediatr Hematol O ncol 13: 487-501
- Nomura Y, Masuda K, Yoshinaga M, Koichiro Miyata, (2003) Possible relationship between streptococcal pyrogenic exotoxin A and Kawasaki syndrome in patients older than Six months of age. Pediatr Infect Dis J 22:794-798.
- Pietra BA, De Inocencio J, Giannini EH, Hirsch R (1994) TCR V beta family repertoire and T cell activation markers in Kawasaki disease. J Immunol 153: 1881-1888. [crossref]
- Matsubara T, Furukawa S, Yabuta K (1990) Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 56:29-36. [crossref]
- Grunebaum E, Blank M, Cohen S, Afek A, Kopolovic J, et al. (2002) The role of anti-endothelial cell antibodies in Kawasaki disease - in vitro and in vivo studies. Clin Exp Immunol 130: 233-240. [crossref]
- Rowley AH, Eckerley CA, Jäck HM, Shulman ST, Baker SC (1997) IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J Immunol 159: 5946-5955. [crossref]
- Qin LJ, Wang HW, Hu XF (2006) Therapeutic effectiveness of intravenous immunoglobulin at 1 g/kg and 2 g/kg on Kawasaki disease: a comparative and follow-up study. Zhonghua Er Ke Za Zhi 44: 891-895.
- Naoe S, Takahashi K, Masuda H, Tanaka N (1991) Kawasaki disease. With particular emphasis on arterial lesions. Acta Pathol Jpn 41: 785-797. [crossref]
- Senzaki H (2006) The pathophysiology of coronary artery aneurysms in Kawasaki disease: role of matrix metalloproteinases. Arch Dis Child 91: 847-851. [crossref]
- Matsuyama T (1999) Tissue inhibitor of metalloproteinases-l and matrix metalloproteinase 3 in Japanese healthy children and in Kawasaki disease and their clinical usefulness in juvenile rheumatoid arthritis. Pediatr lnt 4l: 23H5.
- Rauch AM, ES Hurwitz (1985) Centers for Disease Control [CDC] case definition for Kawasaki syndrome. Pediatr Infect Dis 4: 702-703.
- Kim HK, Oh J, Hong YM, Sohn S (2011) Parameters to guide retreatment after initial intravenous immunoglobulin therapy in Kawasaki disease. Korean Circ J 41: 379-384.
- Pahlavan PS, Niroomand F (2006) Coronary artery aneurysm: a review. Clin Cardiol 29: 439-443 [crossref]
- Dajani AS, Taubert KA, Gerber MA, Shulman ST, Ferrieri P, et al. (1993) Diagnosis and therapy of Kawasaki disease in children. Circulation 87: 1776-1780. [crossref]
- Babu-Narayan SV, Cannell TM, Mohiaddin RH (2006) Giant aneurysms of the coronary arteries due to Kawasaki disease-regular review withoutradiation using cardiovascularmagnetic resonance. Cardiol Young 16: 511-512. [crossref]
- Kaneko K, Yoshimura K, Ohashi A, Kimata T, Shimo T, et al. (2011) Prediction of the risk of coronary arterial lesions in Kawasaki disease by brain natriuretic peptide. Pediatr Cardiol 32: 1106-1109.
- Freeman AF, Shulman ST (2006) Kawasaki disease: summary of the American Heart Association guidelines. Am Fam Physician 74: 1141-1148. [crossref]
- García Pavón S, Staines Boone T, Hernández Bautista V, Yamazaki Nakashimada MA (2006) [Reactivation of the scar of BCG vaccination in Kawasaki's disease: clinical case and literature review] Rev Alerg Mex 53: 76-78. [crossref]
- Joffe A, Kabani A, Jadavji T (1995) Atypical and complicated Kawasaki disease in infants. Do we need criteria? West J Med 162: 322-327. [crossref]
- Kohut J, Gołba E, Giec-Fuglewicz G, Smoleńska-Petelenz J, Berdej-Szczot E, et al. (2006) [Late recognition of Kawasaki disease--difficulties in diagnosis] Wiad Lek 59: 269-273. [crossref]
- Meissner HC, Leung DY (2003) Kawasaki syndrome: where are the answers? Pediatrics 112: 672-676. [crossref]
- Henderson D (2012) Kawasaki Disease Diagnosed by Urine Proteins? Medscape Medical News.
- Kentsis A, Shulman A, Ahmed S, Brennan E, Monuteaux MC, et al. (2013) Urine proteomics for discovery of improved diagnostic markers of Kawasaki disease. EMBO Mol Med 5: 210-220. [crossref]
- Laupland KB, Dele Davies H (1999) Epidemiology, etiology, and management of Kawasaki disease: state of the art. Pediatr Cardiol 20: 177-183. [crossref]
- Oberhoffer R, Lang D, Feilen K (1989) The diameter of coronary arteries in infants and children without heart disease. Eur J Pediatnl 49: 144-145.
- Arjunan K, Daniels SR, Mayer RA, Schartz DC, Baran H, Kaplan S (1986) Coronary artery caliber in normal children and patients with Kawasaki disease but without aneurysms: An echocardiographic and angiocardiographic study. J Am Coll Cardiol 8: 1119-1124. [crossref]
- Kanamaru H, Sato Y, Takayama T (2005) Assessment of coronary artery abnormalities by multislice spiral computed tomography in adolescents and young adults with Kawasaki disease. Am J Cardiol 95: 522-525.
- Schratz LM, Meyer RA, Schwartz DG (2002) Serial intracoronary ultrasound in children: feasibility, reproducibility, limitations, and safety. J Am Soc Echocardiography 15: 782-790.
- Tremoulet AH, Jain S, Chandrasekar D, Sun X, Sato Y, et al. (2011) Evolution of laboratory values in patients with Kawasaki disease. Pediatr Infect Dis J 30: 1022-1026. [crossref]
- Hiraishi S, Misawa H, Takeda N, Horiguchi Y, Fujino N, et al. (2000) Transthoracic ultrasonic visualisation of coronary aneurysm, stenosis, and occlusion in Kawasaki disease. Heart 83: 400-405.
- Pahl E, Sehgal R, Chrystof D, William HN, Catherine LW, et al. (1995) Feasibility of Exercise Stress Echocardiogaphy for the Follow-up of Children With Coronary Involvement Secondary to Kawasaki Disease. Circulation 91:122-128.
- Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, et al. (2004) Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 110: 2747-2771. [crossref]
- Heuclin T, Dubos F, Hue V, Godart F, Francart C, et al. (2009) Increased detection rate of Kawasaki disease using new diagnostic algorithm, including early use of echocardiography. J Pediatr 155: 695-699.
- Hinze CH, Graham TB, Sutherell JS (2009) Kawasaki disease without fever. Pediatr Infect Dis J 28: 927-928.
- Pinna GS, Kafetzis DA, Tselkas OI, Skevaki CL (2008) Kawasaki disease: an overview. Curr Opin Infect Dis 21: 263-270. [crossref]
- Newberger JW, Takahashi M, Burs JT (1986) The treatment of Kawasaki disease with intravenous gamma globulin N Engl J Med 315: 341-347.
- Uehara R, Yashiro M, Oki I, Nakamura Y, Yanagawa H (2007) Re-treatment regimens for acute stage of Kawasaki disease patients who failed to respond to initial intravenous immunoglobulin therapy: analysis from the 17th nationwide survey. Pediatr Int 49: 427-430.
- Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, et al. (2008) Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr 153: 117-121. [crossref]
- Zulian F, Zanon G, Martini G, Mescoli G, Milanesi O (2006) Efficacy of infliximab in long-lasting refractory Kawasaki disease. Clin Exp Rheumatol 24: 453. [crossref]
- Baumer JH, Love J, Gupta A, Haines LC, Maconochie I, et al. (2006) Salicylate for the treatment of Kawasaki disease in children. Cochrane Database Syst Rev 18: CD004175.
- Feit JR (1995) Kawasaki syndrome: diagnosis and management. R I Med 78: 18-20. [crossref]
- Manson WH, Schneider TL, Tkahashi M (1993) Duration of passively acquired measeles antibody and response to live virus vaccination following gamma globulin therapy for Kawasaki disease. 2: 1055-1058.
- (1989) The use of intravenous immunoglobulins: recommendations of a consensus conference of the working group of immunology of the Italian Society of Pediatrics. Ann Allergy 62: 362-367. [crossref]
- Engle MA, Fatica NS, Bussel JB, O'Loughlin JE, Snyder MS, et al. (1989) Clinical trial of single-dose intravenous gamma globulin in acute Kawasaki disease. Preliminary report. Am J Dis Child 143: 1300-1304. [crossref]
- Suzuki H, Terai M, Hamada H, Honda T, Suenaga T, et al. (2011) Cyclosporin A treatment for Kawasaki disease refractory to initial and additional intravenous immunoglobulin. Pediatr Infect Dis J 30: 871-876.
- (1989) Management o Kawasaki syndrome: a consensus statement prepared by North American participants of the Third international Kawasaki Disease Symposium, Tokyo, Japan, December. Pediatr infect Dis J 8: 663-667.
- Saulsbury FT (2002) Comparison of high-dose and low-dose aspirin plus intravenous immunoglobulin in the treatment of Kawasaki syndrome. Clin Pediatr (Phila) 41: 597-601. [crossref]
- Wooditch A C, Aronoff S C (2005) Effect of Initial Corticosteroid Therapy on Coronary Artery Aneurysm Formation in Kawasaki Disease: A Meta-analysis of 862 Children. pediatrics 116: 989-995.
- Stenbøg EV, Windelborg B, Hørlyck A, Herlin T (2006) The effect of TNFalpha blockade in complicated, refractory Kawasaki disease. Scand J Rheumatol 35: 318-321. [crossref]
- Burns JC, Best BM, Mejias A, Mahony L, Fixler DE, et al. (2008) Infliximab treatment of intravenous immunoglobulin-resistant Kawasaki disease. J Pediatr 153: 833-838. [crossref]
- Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, et al. (2008) ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet 40: 35-42.
- Coskun KO, Coskun ST, El Arousy M, Aminparsa M, Hornik L, et al. (2006) Pediatric patients with Kawasaki disease and a case report of Kitamura operation. ASAIO J 52: e43-47. [crossref]
- Kotby A (2005) Management of Kawasaki disease. The twentyth meeting of Egyptian Society of Pediatric Cardiologists league of ESC.
- Giuda H (2007) Abnormal urine report. The Eighth Annual Congress of the Egyptian Society of Pediatric Nephrology & Transplantation [ESPNT]
- Fheem MS (2007) Albuminuria: risk marker and target for treatment. The Eighth Annual Congress of the Egyptian Society of Pediatric Nephrology & Transplantation [ESPNT]
- Sadeghi E, Amin R, Agami H (2001) Kawasaki, syndrome: The Iranian experience. emhj 7: 16-25.
- Saad E (2003) Profile of cardiovascular manifestation of Kawasaki disease in Makkah area, J Arab Child ,14: 427-435.
- Husain E, Hoque E (2006) Meningoencephalitis as a presentation of Kawasaki disease. J Child Neurol 21: 1080-1081. [crossref]
- Harvey J COHEN, Jane C Burns, John C WHITIN, Xuefeng B LING, James Schilling (2014) Methods for diagnosis of kawasaki disease.
- Norihiro Nishimoto, Tadamitsu Kishimoto, Hideko Nakahara,Chugai Seiya, Kabushiki Kaisha (2004) Treatment of vasculitis with IL-6 antagonist.
- Virginia M. PASCUAL, Zhaohui Xu, Octavio Ramilo, Rolando CIMAZ Protein biomarkers and methods for diagnosing kawasaki disease. [crossref]
- Raymond M Johnson (2014) Material and methods for diagnosing and treating kawasaki disease.
- Jane C. Burns, Protein biomarkers and methods for diagnosing kawasaki disease PCT/US2009/055410, 2010.
- Kenji Yone (1991) Biomarkers for Kawasaki disease.






