Abstract
The mechanisms underlying the rapid-acting antidepressant effects of ketamine have not been fully identified. In current study, we observed that a single subanesthesia dose of ketamine strongly decreased the immobility time of Sprague–Dawley rats in forced swim test. Ketamine injection significantly increased GluR1 expression and GluR1 Ser845 phosphorylation in prefrontal cortex. Intracerebroventricular injection of PKA inhibitor H89 completely eliminated the fast antidepressant-like responses of ketamine in force swim test. Our results suggest that activation of PKA and phosphorylation of GluR1 Ser845 are critical for fast antidepressant action of ketamine.
Key words
ketamine, antidepressant, AMPA receptor, GluR1, forced swim test
Introduction
Major depressive disorder (MDD) is a serious public health problem with a lifetime prevalence of 17% in the United States [1]. Despite the high incidence of MDD and its socioeconomic impact, the etiology of MDD remains largely unknown. Existing treatments for MDD usually take weeks to months to achieve their antidepressant effects, and a significant number of patients do not have adequate improvement even after months of treatment. In addition, increased risk of suicide is a major public health concern during the first month of standard antidepressant therapy. Thus, improved therapeutics that exert antidepressant effects within hours or several days of administration are urgently needed.
Recent clinical trials show that a single subanesthesia dose (0.5-20 mg/kg) of ketamine, a noncompetitive ionotropic glutamatergic NMDA receptor antagonist, produces rapid antidepressant responses in patients suffering from MDD [2]. Treatment-resistant, depressed patients reported alleviation of core symptoms of major depression within hours of a single dose of intravenously infused ketamine, with effects lasting up to 2 weeks [3]. Subsequent studies reported significant efficacy of ketamine in reducing suicidal ideation in individuals exhibiting treatment-resistant depression [4].
The discovery of ketamine’s rapid-acting antidepressant effect has been exciting. However, the psychotomimetic properties and abuse potential of ketamine necessitate caution in promoting this compound as a general treatment for depression. Understanding the underlying mechanism of action of ketamine linked to behavioral improvement is of significant importance for developing novel, safe and fast-acting antidepressants.
Materials and methods
Animals
Male and female Sprague–Dawley rats weighing 200–350 g were used for all behavioral experiments (purchased from Guangdong Animal Experiment Center, Guangzhou, Guangdong, China). Animals were maintained on a 12-h light/dark cycle in a temperature- and humidity-controlled facility with ad libitum access to food and water. All experiments were in accordance with US National Institutes of Health guidelines and approved by the Guangzhou Medical University Institutional Animal Care and Use Committee.
Forced swim test
In the pretest session, rats were placed into a Plexiglas cylinder (65-cm-height, 30-cm-diameter) filled to a height of 45 cm water (22°C to 24 °C) for 15 min. Water was changed between subjects and rats were dried by paper towel after swim. After ketamine or saline injection for different period of time, the test session were performed twenty four hours later. The test session was 15 min for each subject and was record ed by a video camera positioned on the side of the cylinder. However, only the last 5 min of test session was analyzed and scored by an observer blind to group assignment. A decrease in immobility time is suggestive of an antidepressant-like response.
Acute prefrontal brain slices preparation
These methods are described in detail in our previous publications [5,6]. Rats were killed by decapitation after sedation with isoflurane. Prefrontal cortices were isolated and sectioned into 400-μm-thick slices in ice-cold ACSF ( in mM: 124 NaCl, 3 KCl, 1.25 NaH2PO4, 1.5 MgCl2, 2.5 CaCl2, 26 NaHCO3, and 10 glucose, bubbled with 95% O2/5% CO2) using a Leica VT 1200S vibratome (Leica Microsystems Inc., Bannockburn, IL, USA). The slices were maintained at room temperature for over 1 hour in an interface holding chamber in a humidified atmosphere saturated with 95% O2/5% CO2. The slices were then transferred to submersion-type bottles containing 10 ml ASCF and bubbled with 95% O2/5% CO2.
Intracerebroventricular injection
Rats were deeply anesthetized with isoflurane and heads were mounted in a stereotaxic instrument. Double cannulae (26 gauge; Plastics One, Roanoke, VA, USA) were inserted bilaterally into left and right lateral ventricles (coordinates relative to bregma: −0.9 mm anterior/posterior (AP), ±1.5 mm medial/lateral (ML), −3.3 mm dorsal/ventral (DV) from dura). Postoperative care consisted in administration of carprofen (5 mg/kg) and topical triple antibiotic for 3 days. After a 7-day recovery period, rats were injected with 50 nM H89 (in 10 µl ACSF) or same amount of DMSO (in 10 µl ACSF) into the lateral ventricles at the rate of 0.25 µl/min with an injection cannula (26GA) protruding 0.5 mm beyond the guide cannula 1 hour before ketamine injections (i.p.). The injection cannula stayed in the guide cannula for 5 min after infusions.
Western blotting
After drug treatment, prefrontal homogenates were lysed using buffer containing 50 mM Tris-Cl, 150 mM NaCl, 0.02% NaN3, 1% Nonidet P-40, 0.1% SDS, 0.5% sodium deoxycholate, 5 μg/ml leupeptin and 1 μg/ml aprotinin. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked at room temperature (22°C to 24°C) for 1 h in a solution containing 130 mM NaCl, 2.5 mM KCl, 10 mM Na2HPO4, 1.5 mM KH2PO4, 0.1% Tween 20 and 5% BSA (pH 7.4). The membranes were treated with the primary antibodies against Ser845-phosphorylated GluR1 (1:1000; Millipore AB5849). After rinses in TBS-Tween, the membrane was incubated for 1 hr at room temperature in horseradish peroxidase-conjugated goat anti-rabbit IgG (1:1000, Cell Signaling Technology #7074). The immunoblot was developed with enhanced chemiluminescence (Amersham). Membranes were then stripped, blocked, and reprobed with antibodies against GluR1 (0.5 μg/ml; Thermo Scientific PA1-37776) or β-actin (1:2000; Cell Signaling Technology #4967). Levels of phosphorylation, expressed as the ratio of phospho-specific intensity divided by total protein intensity and computed with ImageJ, were used for statistical analysis. For display purposes, blots were cropped and brightness and contrast were adjusted globally using Photoshop.
Statistical analysis
Data are presented as mean ± SE. Significance of the difference between two groups was assessed with Student’s unpaired two-tailed t-test or one-way ANOVA test. A p value less than 0.05 is considered statistically significant.
Results
Ketamine reduced immobility of animal in forced swim test
We examine the antidepressant effect of ketamine and detected ketamine's acute effect in Sprague–Dawley rats for significant behavioral responses in forced swim test, an antidepressant (AD)-predictive task. Compared to saline injection group, 1 h, 3 h and 6 h ketamine injection (10 mg/kg, i.p.) significantly reduced immobility time of animals during forced swim test (Figure 1).
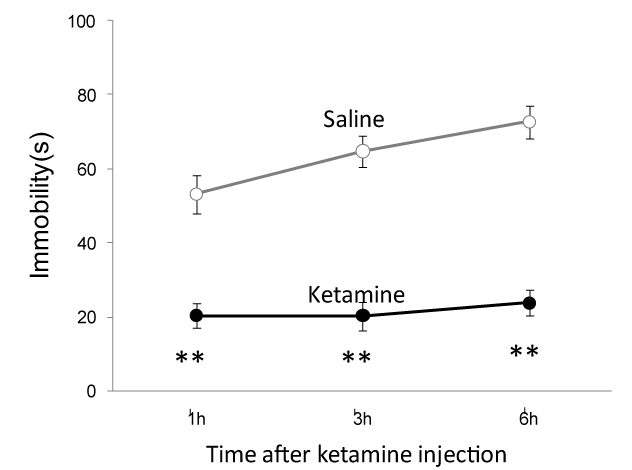
Figure 1. Ketamine decreases the immobility time in forced swim test. Compared to the saline group, 1 h, 3 h and 6 h ketamine injection (10 mg/kg, i.p.) strongly reduced immobility time of Sprague–Dawley rats in forced swim test after different period of ketamine injection ( Saline vs ketamine (in second): 50.3 ± 5.2 (n=6 rats) vs. 20.3 ± 3.4 (n=7 rats) at 1 h; 64.8 ± 4.2 (n=6 rats) vs. 20.2 ± 3.8 (n=7 rats) at 3 h and 72.7 ± 4.5 (n=x rats) vs. 23.7 ± 3.6 (n=7 rats) at 6 h). **, p<0.01 compared with the saline group.
Ketamine enhanced GluR1 Ser845 phosphorylation
Previous studies show that AMPA receptor activation is necessary for ketamine’s antidepressant-like effect [7,8]. While, AMPA receptor phosphorylation is critical for the activation of the receptor. Among the multiple phosphorylation sites of AMPA receptor, the phosphorylation of GluR1 Ser845 site is important for AMPA receptor translocation from cytosol onto postsynaptic membrane. We therefore examine the impact of ketamine on GluR1 Ser845 phosphorylation in prefrontal cortex, a brain region that is heavily involved in the etiology of depression. After ketamine injection, prefrontal cortical brain slices were prepared as described in the Methods. Using the acutely prepared prefrontal cortical slices, western blot experiments detected that ketamine application time-dependently increased GluR1 Ser845 phosphorylation and total GluR1 expression (Figure 2 A-C).
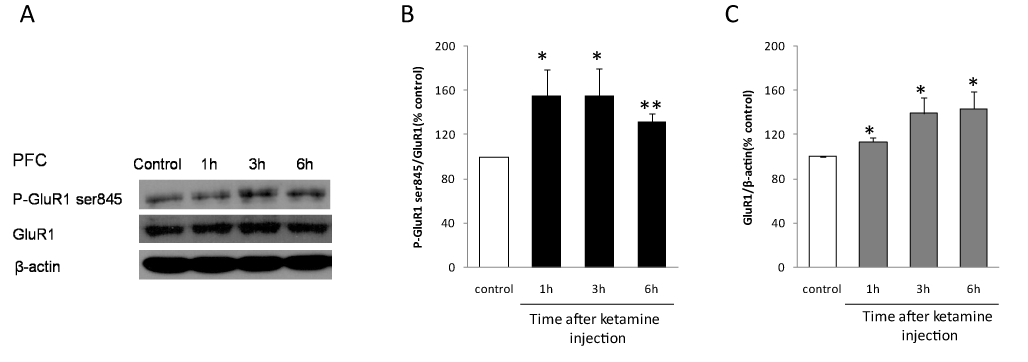
Figure 2. Ketamine increased GluR1 Ser845 phosphorylation and GluR1 expression in prefrontal cortex. A. Using acutely prepared prefrontal cortical slices, the representative western blot shows that 1 h, 3 h and 6 h ketamine injection enhanced GluR1 ser845 phosphorylation and GluR1 expression. B and C, data summary of ketamine’s effect on GluR1 ser845 phosphorylation and GluR1 expression from 5 independent western blot experiments. *, p<0.05, **, p<0.01, compared with the control group with one-way ANOVA test, n= 5 animal in control group and each time point after ketamine injection.
Activation of PKA is necessary for the fast acting antidepressant response of ketamine.
GluR1 Ser845 is phosphorylated by PKA. To test whether GluR1 Ser845 phosphorylation is necessary for ketamine’s antidepressant effect, the PKA inhibitor H89 (50 nM, in 2 µl ACSF) was injected intracerebroventricularly (i.c.v.) 1 hour before ketamine injection (i.p.). H89 administration alone increased immobility time of animal in forced swim test. However, H89 totally blocked ketamine-induced reduction of immobility (Figure 3).
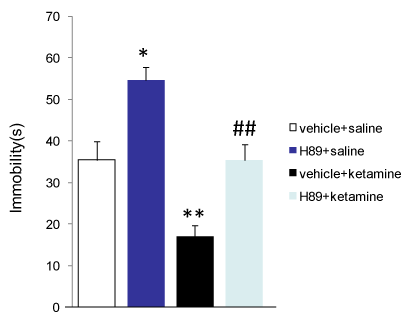
Figure 3. H89 blocks the antidepressant-like responses of ketamine in forced swim test. Intracerebroventricular injection of H89 (50 nM), a PKA inhibitor, significantly increased the immobility time of SD rats in forced swim test (vehicle (DMSO) + saline group: 35.4 ± 3.1 s, n=7 rats; H89 + saline group: 54.6 ± 3.1 s, n= 7 rats). H89 completely blocked ketamine-induced reduction of immobility of animals ( vehicle + ketamine group: : 17.1 ± 2.6 s, n=7 rats; H89 + ketamine group: 35.4 ± 3.7 s, n= 7 rats), suggesting that PKA activation and GluR1 Ser845 phosphorylation is necessary for the antidepressant-like responses of ketamine in forced swim test. *, p<0.05, **, p<0.01 compared with vehicle + saline group; ##, p < 0.01 compared with vehicle + ketamine group with one-way ANOVA test.
Discussion
Previous studies show that AMPA receptor activation and synaptic protein synthesis is necessary for ketamine-induced antidepressant-like responses [7,8]. It has been speculated that inhibition of tonically-active GABAergic interneurons and activation of voltage-gated calcium channels underlie ketamine-induced synaptogenesis [7,9]. However, blocking GABAergic transmission by picrotoxin, a GABAA receptor antagonist, does not affect depressive behaviors [8], indicating that blocking GABAergic transmission-induced disinhibition of principle neurons is not sufficient to generate the rapid antidepressant response of ketamine. Monteggia et al. [10,11] demonstrated that ketamine deactivates eukaryotic elongation factor 2 (eEF2) kinase (also called CaMKIII), resulting in reduced eEF2 phosphorylation and de-suppression of BDNF translation as well as increases surface expression of AMPA receptors. However, other researchers observed that relative to CaMKII, CaMKIII exhibits approximately two orders of magnitude greater affinity for calmodulin and is sensitive to > 5-fold lower calcium concentrations. They also demonstrated that CaMKIII activity and eEF2 phosphorylation are suppressed by NMDA antagonist only in the condition when synaptic transmission is blocked by TTX [12]. Furthermore, a recent clinical research study observed that the expression of eEF2 (p-eEF2) increases in the blood plasma of depressed patients treated with ketamine [13]. These results suggest that suppression of eEF2 phosphorylation probably is not an upstream signaling of ketamine-induced increase of BDNF release and AMPA receptor expression, especially in vivo where TTX is absent.
Phosphorylation of AMPA receptors has been show to play an important role in expression of synaptic plasticity at excitatory synapses. There are a least ten phosphorylation sites have been identified on the C-terminal domains of AMPA receptor GluR1-GluR4 subunits [14-16]. Among these phosphorylation sites, GluR1 Ser845 is phosphorylated by PKA[17-19]. Phosphorylation of GluR1 Ser845 increases the open-channel probability of AMPA receptors and therefore enhances the function of AMPA receptor [19,20]. Furthermore, GluR1 Ser845 phosphorylation is suggested to be important for rapid synaptic insertion of GluR1 subunit-containing receptors [21]. Our result show that ketamine strongly enhanced GluR1 Ser845 phosphorylation, suggesting that ketamine may enhances the function of AMPA receptor through phosphorylation of the receptor, which may underlie the rapid antidepressant effect of ketamine. Indeed, H89 totally inhibited the antidepressant-like effect of ketamine in forced swim test, indicating that PKA activation and GluR1 Ser845 phosphorylation are necessary for the rapid antidepressant action of ketamine.
Competing interests
The authors declare no competing interests
References
- Kessler D, Sharp D, Lewis G (2005) Screening for depression in primary care. Br J Gen Pract 55: 659-660. [Crossref]
- Mathew SJ, Shah A, Lapidus K, Clark C, Jarun N, et al. (2012) Ketamine for treatment-resistant unipolar depression: current evidence. CNS Drugs 26: 189-204. [Crossref]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, et al. (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47: 351-354. [Crossref]
- aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, et al. (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67: 139-145. [Crossref]
- Cai X, Kallarackal AJ, Kvarta MD, Goluskin S, Gaylor K, et al. (2013) Local potentiation of excitatory synapses by serotonin and its alteration in rodent models of depression. Nat Neurosci 16: 464-472. [Crossref]
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, et al. (2004) Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron 44: 351-364. [Crossref]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, et al. (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329: 959-964. [Crossref]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, et al. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475: 91-95. [Crossref]
- Duman RS, Aghajanian GK (2012) Synaptic dysfunction in depression: potential therapeutic targets. Science 338: 68-72. [Crossref]
- Kavalali ET, Monteggia LM (2012) Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry 169: 1150-1156. [Crossref]
- Monteggia LM, Gideons E, Kavalali ET (2013) The role of eukaryotic elongation factor 2 kinase in rapid antidepressant action of ketamine. Biol Psychiatry 73: 1199-1203. [Crossref]
- Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM (2007) Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron 55: 648-66. [Crossref]
- Yang C, Zhou ZQ, Gao ZQ, Shi JY, Yang JJ (2013) Acute increases in plasma mammalian target of rapamycin, glycogen synthase kinase-3beta, and eukaryotic elongation factor 2 phosphorylation after ketamine treatment in three depressed patients. Biol Psych 73: e35-36. [Crossref]
- Carvalho AL, Kameyama K, Huganir RL (1999) Characterization of phosphorylation sites on the glutamate receptor 4 subunit of the AMPA receptors. J Neurosci 19: 4748-4754. [Crossref]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL (2000) Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci 20: 7258-7267. [Crossref]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL (2000) Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405: 955-959. [Crossref]
- Barria A, Derkach V, Soderling T (1997) Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J Biol Chem 272: 32727-32730. [Crossref]
- Mammen AL, Kameyama K, Roche KW, Huganir RL(1997) Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem 272: 32528-32533. [Crossref]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL (1996) Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16: 1179-1188. [Crossref]
- Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, et al. (2000) Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci 20: 89-102. [Crossref]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, et al. (2003) PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6: 136-143. [Crossref]



