CardioMEMS device late migration from the left to the right pulmonary artery was observed in a patient with heart failure after a chronic obstructive pulmonary disease decompensation episode and brisk coughing spells.
chronic heart failure, pulmonary circulation, device therapy, pressure sensing, complication
The CardioMEMS device, inserted transvenously into the pulmonary artery (PA), allows home monitoring of pulmonary artery pressure (PAP) in patients with heart failure (HF).
It should be implanted in a posterior branch of the left PA with a 7 to 15mm lumen diameter and is expected to endothelize within three months [1]. It is about the size of a paper clip when deployed in the target PA (Figure 1A).
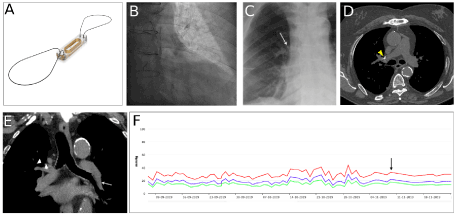
Figure 1. Late CardioMEMS migration. (A) CardioMEMS device. (B) CardioMEMS implantation in the left posterior PA (C) CardioMEMS located in the right anterior hilum after migration (arrow). (D) CT scan images of CardioMEMS in the right PA (arrowhead). (E) CT scan reconstruction of left PA (arrow) showing a rapid loss of caliber that forced a proximal implantation. CardioMEMS in the right PA after migration (arrowhead). (F) Monitorization of the systolic (red), diastolic (green) and mean (blue) PA pressure values. COPD exacerbation (arrow)
The device is externally analyzed through radiofrequency waves by an antenna located on a pillow and the information is transmitted to a web platform that is reviewed by the HF team.
The CHAMPION study (CardioMEMS Heart Sensor Allows Monitoring of Pressure to Improve Outcomes in NYHA Class III Heart Failure Patients) is a randomized clinical trial that included 550 HF patients with New York Heart Association (NYHA) class III, with either reduced or preserved ejection fraction, and showed a significant decrease in PAP, a reduction in hospitalizations for HF, an improvement in quality of life and a trend to lower mortality in the group of patients monitored with the device [2].
PA pressure-guided therapy for HF in routine clinical practice was associated with lower PA pressures, lower rates of HF hospitalization and all-cause hospitalization in the CardioMEMS Post-Approval Study, a multi-center, prospective, observational, single-arm trial of 1200 patients with NYHA class III HF. The results may support a more widespread implementation of this novel approach [3].
CardioMEMS sensor migration is most likely to happen during the implant procedure, especially if implanted in a less than ideal anatomy. However, the mechanisms of late migration are not as clear [4].
We present the case of a 73-year old male with HF with reduced ejection fraction of ischemic etiology, in New York Heart Association functional class III and multiple HF hospitalizations, who was referred for CardioMEMS implantation. He had a history of chronic obstructive pulmonary disease (COPD). The device was implanted in a left posterior PA branch, as recommended, yet in a proximal position because the distal diameter of the left PA measured less than 7 mm (Figures 1B-1E). Both cardioMEMS loops remained correctly anchored.
During the following three months the patient performed daily measurements and HF medication was adjusted based on the recordings (Figure 1F). After this, he presented at the HF Unit with fever, intense coughing spells and shortness of breath, presenting bilateral pulmonary ronchus at physical examination. He was diagnosed with COPD exacerbation. Additionally, since the beginning of the coughing spells he experienced difficulty measuring PA pressures.
Surprisingly, a chest X-ray showed that the device had migrated to the right hilum (Figure 1C) and a thorax CT scan confirmed its presence in the right PA (Figure 1D). After device migration the PAP curves remained reliable with non-significant changes in PAP values. The possibility of retrieving the device was discussed in a multidisciplinary Heart Team meeting. Finally, since the device was performing properly and remained in the same location in multiple chest x-ray during follow-up, the consensus was not to retrieve it.
A late migration of a CardioMEMS device is certainly rare compared to an acute event occurring early after implant. The implantation of CardioMEMS device in a proximal left PA could increase the risk of late migration, specifically in situations of increased intrathoracic pressure. In our case intrathoracic and PAP increase during coughing spells could have triggered device migration.
In case of migration, change of the position on the pillow for the reading to the new location may re-establish a proper connection.
The implantation of CardioMEMS device in a proximal left PA could increase the risk of late migration, specifically in situations of increased intrathoracic pressure. If technically feasible, implantation of CardioMEMS in a proximal location should be avoided. CardioMEMS surveillance should be recommended in patients with underlying respiratory conditions when recordings suddenly become problematic.
Written informed consent was obtained from the patient for publication of this case report.
- Shavelle D, Jermyn R (2016) The CardioMEMS Heart Failure Sensor: A procedural guide for implanting physicians. J Invasive Cardiol 28: 273-279. [Crossref]
- Abraham WT, Adamson PB, Bourge RC (2011) Wireless Pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet 377: 658-666. [Crossref]
- Shavelle DM, Desai AS, Abraham WT (2020) Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure: one-year outcomes from the cardiomems post-approval study. Circ Heart Fail 13: e006863. [Crossref]
- Rali AS, Shah Z, Sauer A, Gupta K (2017) Late Migration of a CardioMEMSTM Wireless Pulmonary Artery Hemodynamic Monitoring Sensor. Circ Heart Fail 10: e003948. [Crossref]

