Left ventricular non-compaction is a form of cardiomyopathy defined by a spongy left ventricular myocardium. Despite the condition being the leading reason for referral for patients with depressed left ventricular ejection fraction, current classification systems categorize it as an unclassified cardiomyopathy. The lack of recognition as a distinct form of cardiomyopathy has undermined research and expert consensus on diagnosis and specific clinical management guidelines. In addition, current research on epidemiology, diagnosis and clinical management are small scale-studies with inconsistent findings. The purpose of the present review is to combine research findings on pathogenesis, prognosis, clinical manifestation, diagnosis and clinical management methods. The intention is to advance knowledge and understanding of the clinical status and management of left ventricular non-compaction.
left ventricular non-compaction, cardiomyopathy, trabeculated left ventricular (LV), non-compacted endocardial layer, diagnosis, clinical management
Left Ventricular Non-Compaction (LVNC) is a recently described anatomic variant of the left ventricular (LV) myocardial structure. However, controversy arises as to whether it is a distinct form of cardiomyopathy or a morphological trait shared by other forms of cardiomyopathies, more specifically, dilated cardiomyopathy (DCM). Both the World Health Organization (WHO)/ the International Society and Federation of Cardiology (ISFC) Task Force on the Definition and Classification of Cardiomyopathies, and the European Society of Cardiology (ESC) Working Group on Myocardial and Pericardial Diseases categorize LVNC as unclassified cardiomyopathy. However, the recognition of LVNC as a distinct clinical entity could have important clinical implications in facilitating diagnosis and treatment, and more importantly, in unmasking its true incidence in the general population to attract much-needed funding for both specific LVNC research and clinical management. This review collates available research and clinical evidence on LVNC with an emphasis on its clinical description, epidemiology, pathogenesis, prognosis, clinical presentation, diagnosis and clinical management.
Historical aspect
The initial description of the entity of spongy myocardium in medical literature goes back to 1926 [1] but its recognition as a disease entity, LVNC, is as recent as 1990 [2-4] (Table 1).
Table 1. Historical description of spongy myocardium (LV Non-Compaction).
Year |
1st Author [Ref #] |
Description |
1926 |
Grant [1] |
He was the first to describe a spongy myocardium as a
congenital cardiac malformation in children. |
1932 |
Bellet & Gouley [2] |
Observed abnormally “spongy” myocardial
walls linked to aortic atresia and coronary-ventricular fistula
in an autopsy of a newborn with congenital heart disease |
1975 |
Dusek et al. [3] |
Described a persistent of spongy myocardium
with embryonic blood supply in children. |
1984 |
Engberding et al. [4] |
Provided the first clinical description of
spongy myocardium based on echocardiographic findings in an adult. |
1990 |
Chin et al. [5] |
Proposed the term LVNC following diagnosis in 8 cases in
the absence of congenital cardiac abnormalities. |
LVNC: Left Ventricular Non-Compaction
In 1926, Grant [1] made the seminal mention of a spongy myocardium in medical literature when he described an unusual anomaly of the coronary vessel in a malformed heart of a child. Six years later (1932), Bellet and Gouley [2] reported spongy myocardial walls associated with antemortem atresia and coronary-ventricular fistula in a newborn with congenital heart disease (CHD). However, it is Dusek and associates [3], in 1975, who get the credit for bringing the entity of spongy myocardium into clinical focus. They described a histologically persistent form of spongy myocardium with embryonic blood supply. Until then, spongy myocardium was associated with co-occurring congenital cardiac malformations in children. Later in 1984, Engberding and Bender [4] published the first clinical description of spongy myocardium in an adult following antemortem diagnosis using two-dimensional (2D) echocardiography (Echo). The diagnosis demonstrated a spongy myocardium having prominent sinusoid (inter-trabecular recesses) attributed to an abnormal lack of sinusoidal regression during cardiac embryogenesis.
LVNC terminology
Despite earlier studies mentioning a spongy myocardium, it was the 1990 landmark study by Chin et al. [5], which reported eight cases of spongy myocardium in patients aged 11 months to 22.5 years in the absence of demonstrable congenital abnormalities and termed the condition “LV non-compaction”. Until then, LVNC lacked a unified terminology, variously termed as spongious myocardium, myocardial dysplasia, myocardial dysgenesis, persistence of myocardial sinusoids, or hypertrabeculation syndrome [6]. Later in 1997, Ritter et al. [7] published a ten-year experience with LVNC in adults, reporting 17 cases in a total of 37,555 transthoracic echocardiographic studies. Currently, LVNC is a rare myocardial disorder listed as unclassified cardiomyopathy by both the WHO/ISFC Task Force on the Definition and Classification of Cardiomyopathies [8] and the European Society of Cardiologists (ESC) Working Group on Myocardial and Pericardial Diseases [9]. However, the American Heart Association (AHA) recognizes LVNC as a distinct clinical entity and classifies the myocardial condition as a genetic form of cardiomyopathy [10].
Clinical definition
The 2008 position statement for ESC Working Group on Myocardial and Pericardial Diseases defines LVNC as a myocardial disorder “characterized by prominent left ventricular trabeculae and deep inter-trabecular recesses” [9]. The American Heart Association (AHA) [10] defines LVNC as “a congenital cardiomyopathy characterized by distinctive (spongy) morphological appearance of the LV myocardium. Non-compaction involves predominantly the distal (apical) portion of the LV chamber with deep inter-trabecular recesses (sinusoids) in communication with the ventricular cavity, resulting from an arrest in the normal embryogenesis”.
General population
The precise prevalence of LVNC in the general population remains incompletely described in medical literature. Currently, there are no epidemiologic studies on LVNC. Further, physicians may miss diagnosis because LVNC is a rare condition in adult populations [11] or because of indirect effect of the condition not recognized as distinct form of cardiomyopathy by the WHO and the ESC classification on cardiomyopathies [12].
Pediatric population
The reported prevalence of LVNC is higher in children when compared to other forms of primary cardiomyopathies or to adult populations. In a recent Australian epidemiological study of primary cardiomyopathies in children, LVNC accounted for 9.2% of all reported cardiomyopathy cases, translating into the third most prevalent phenotype of cardiomyopathy in children after dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM) [13]. In five-year experience at Texas Children’s Hospital, Pignatelli and associates [14] reported similar prevalence of LVNC, which accounted for 9.5% of all primary forms of cardiomyopathies in pediatric population.
Adult population
The true prevalence of LVNC in adult population remains unclear. However, two observational studies with long-term follow-up of adults with depressed ejection fraction (< 45%) and symptoms of heart failure (HF) referred for echocardiographic assessment reported a prevalence of between 0.01% [15] and 0.26% [16]. Three retrospective studies [6,17,18] also reported a prevalence of between 4.5 and 2.6 in 10,000 adults sent for echocardiographic assessment. However, because of biased selection based on symptomatic patients, a precise understanding of the true prevalence of LVNC in the general adult population remains tenuous.
Pathogenesis
It is well documented that the disturbance of embryogenesis is the key pathogenic feature in the development of LVNC [6,11,19,20,21]. Thus, understanding the origination and development of the myocardium during the embryonic period is critical to appreciate structural variations in the LVNC myocardium. Two landmark studies, Virtove et al. [22] and Freedom et al. [23], demonstrated that human myocardial development and maturation go through four distinct phases: (a) early heart tube; (b) the emergence of trabeculations; (c) trabecular remodeling; and (d) development of the multi-layered spiral system. Of these four, the emergence of trabeculations and trabecular remodeling are the two key phases that may help explain the appearance of the LVNC myocardium. (Figure 1)
The emergence of trabeculations
During the normal embryonic development, endomyocardial trabeculations emerge from the apical region of the primitive ventricles (heart tube) at the end of the fourth week of gestation [23,24]. These early trabeculations increase in surface area and consequently increase myocardial mass before the epicardial coronary vessels develops. At this stage, the heart consists of a spongy network of muscle fibers and trabeculations separated by recesses (Figure 1, Panel A). The inter-trabecular recesses (also known as sinusoids) communicate with the ventricular cavum to receive blood supply. The trabeculations pattern is ventricle specific – thicker in the left ventricle with larger inter-trabecular spaces. When this myocardial pattern persists post-natal, structural appearance of the heart is spongy [22].
Trabecular remodeling or compaction
Trabecular remodeling (or compaction process) begins at the eight (8) week of the human gestation following the completion of ventricular septation – partitioning of the primitive heart into the left and right chambers by the septum (Figure 1, Panel B and C). Increased ventricular volume compresses the trabeculations accompanied by an increase in the thickness of the compacted myocardium. In the human heart, some luminal trabeculations coalesce to form the anterior and posterior papillary muscles of the mitral valve while apical trabeculations form the inner ventricular surface [23]. Trabecular remodeling coincides with the development of epicardial coronary vasculature, which transforms the larger inter-trabecular spaces into capillaries from epicardium towards the endocardium, from the base of the heart to the apex, and from the septum to the LV free wall during week 12 and 18 of gestation [23,25]. These changes are more pronounced in the LV than in the right ventricle (RV) [23,24]. For undetermined reasons, in LVNC patients, the compaction process does not occur, resulting into the development of thickened non-compacted endocardial layer having prominent diffuse trabeculations in the LV cavity. These trabeculations lack communication with epicardial circulation and has deep recesses and thin compacted epicardial layer [26,27]. Towbin and Bowles [28] described two forms of LVNC: (a) isolated LVNC and (b) non-isolated LVNC associated with congenital heart disease (CHD). The non-isolated LVNC may be detected in combination with septal defects, pulmonary stenosis or hypoplastic LV. Although both forms occur in all age groups, infantile presentation is the most common [28].
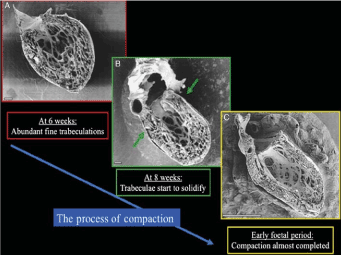
Figure 1. The Process of Normal Trabecular Compaction [19]
Parietal view of human embryonic left ventricles showing the process of trabecular compaction. (A) At week six, abundant fine trabeculations; (B) compaction of trabeculae starting (thickening) at the base of the heart; (C) completion of trabeculae compaction forms most of the myocardium mass. Scale bars 100 mm (A and B), 1 mm.
Congenital or Acquired
Although it is well documented that the key pathogenic mechanism of LVNC is congenital through a disturbed embryogenic process [3,5,12,22,29], controversy persists of whether LVNC could be strictly congenital or could also develop postnatal (acquired) [30]. This controversy originates from observations made in some echocardiographic studies that reported cases where LVNC was absent in initial studies but became evident in subsequent examinations raising a valid question of whether LVNC could develop postnatal [19]. Strong support for this hypothesis has emerged from an observation that morphological features of both dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM) may be absent at birth but develop during life. Since DCM and HCM share common genetic mutations in the sarcomere protein genes with LVNC, there may be a strong possibility of an acquired form of LVNC [19]. Growing research evidence supporting the hypothesis of acquired LVNC are also emerging, which strongly suggest that pathogenetic mechanisms resulting into non-compaction or increased trabeculation could also occur in adult life. In young athletes, Gati et al. [31] observed increased LV trabeculation might represent cardiac remodeling, where trabeculations become more prominent with well-preserved compaction layer. The study also reported electrocardiography and echocardiography assessment of highly trained young athletes reveals 0.9% have concomitant deep T-wave inversion and reduced baseline indices of systolic dysfunction (Figure 2), which is a characteristic diagnosis of LVNC.
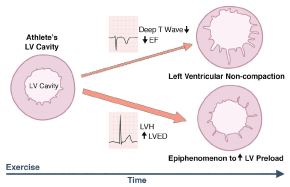
Figure 2. Significantly Increased Left Ventricular Trabeculations in Young Athletes
Increased LV trabeculation in athletes may suggest physiological myocardial remodeling with some expressing a triad of depressed left ventricular systolic dysfunction, repolarization changes suggesting left ventricular non-compaction. EF: Ejection fraction; LV: Left Ventricular; LVED: Left ventricular end diastolic diameter; LVH: left ventricular hypertrophy.
In a related study recruiting pregnant women, Gati et al. [32] observed de-novo LV trabeculations in > 25% of the patients suggesting LV trabeculations could occur due to increased loading conditions or other physiological adaptation mechanisms associated with pregnancy. In addition, increased trabeculation in patients with sickle-cell anemia could suggest exaggerated myocardial response that may elevate cardiac response. However, although acquired LVNC may be observed during pregnancy, and in patients with sickle-cell anemia and athletes [33], there is need for additional large-scale clinical trials to confirm whether LVNC can develop postnatal.
Current understanding of disease course and prognostication of LVNC is limited or at most inconclusive. Some studies report an ominous prognosis while others a less ominous prognosis, more so in patients with non-symptomatic diagnosis [34].
Ominous prognosis
Observations made on long-term follow-up period (44 months) on a small group of LVNC patients (34 adults) referred to tertiary care centers suggest that generally LVNC patients have an ominous prognosis [17]. The study associates LVNC with serious cardiac complications including heart failure (53%), ventricular tachycardia (41%), thromboembolic events (24%) and all-cause deaths (35%). Patients with symptomatic heart failure, New York Heat Association (NYHA) class II-IV, history of sustained ventricular tachycardia (VT) or enlarged atrium (predictors of cardiac death or heart transplantation) often always have unstable course and an ominous prognosis [32]. Initial reports from case series indicate mortality rates of between 35% and 47% between 42 and 72 months follow-up since diagnosis [7,15]. Other related studies report mortality or indication for cardiac transplantation of 36% in a follow-up of 2.9 years [34] and 34% cardiac death in 54 months follow-up period [11].
Favorable prognosis
Despite increasing reports of an ominous prognosis, some case series report a more favorable prognosis. In a recent report of a larger cohort (45 patients), consecutively identified at a referral center for cardiomyopathy over a 10-year period, Murphy et al. [20] find a less ominous prognosis than previously reported. At 46 months, survival from death or cardiac transplantation was 97%, thromboembolic events at 4%, and family screening uncovered 25% of symptomatic patients having impaired systolic function and LV hypertrophy but without LVNC. In a related study, Lofiego et al. [35] followed 68 LVNC patients for a median period of 46 months and reported a more favorable prognosis in asymptomatic patients (26%) or in patients with incidental or familial discovery with no major cardiovascular events. The variable findings on prognostication of LVNC warrants large scale prospective multi-center trials or epidemiologic studies to determine whether LVNC conveys an ominous or a favorable prognosis.
Clinical presentation
LVNC patients have variable phenotypic expression of cardiomyopathy with clinical features ranging from asymptomatic to symptomatic. The classical triad of (a) symptomatic HF, (b) ventricular arrhythmias and (c) systemic embolic events are the main clinical manifestation and the most frequent reasons for referral for patients with reduced ejection fraction [11,36]. These clinical manifestations are comparable in pediatric and adult populations [5]. For ventricular arrhythmias, Ikeda et al. [11] observe various patterns ranging from atrial fibrillation to persistent ventricular tachycardia.
Symptomatic heart failure
Symptoms of heart failure represent a serious complication and the most frequently encountered reason for referral of LVNC patients [11,36]. The other is inconclusive echocardiographic findings [36]. Symptomatic patients have significantly higher incidence of LV systolic dysfunction (61%) compared to asymptomatic patients (2%) [37]. Conventional therapy of heart failure is usually recommended for patients with LVNC and HF [38,39]. Patients with LVNC and HF refractory to optimal medical therapy should be considered for device therapy, while heart transplantation is indicated to LVNC patient’s refractory to both medical and device therapy [40].
Sudden cardiac death
Sudden cardiac death (SCD) is a fatal complication of LVNC requiring close monitoring of LVNC patients for ventricular arrhythmias [36]. The incidence of ventricular arrhythmias in LVNC patients ranges between 2% and 62% [35,41]. Ventricular arrhythmias, whether sustained or non-sustained) affects 27% of LVNC patients on a Holter monitor [42]. Device therapy (Implantable Cardioverter Defibrillator [ICD]) or radiofrequency ablation may be considered for LVNC patients with sustained ventricular arrhythmias or those who have survived an episode of cardiac arrest [40,43].
Systemic embolic events
Systemic thromboembolic events are the third serious complication for LVNC patients and one of the main reasons for referral at presentation. The incidence of thromboembolic events in LVNC patients ranges between 5% and 38% [7,35]. The risk of thromboembolic events in LVNC patients increases significantly in the setting of atrial fibrillation and LV systolic dysfunction [30]. In some LVNC patients, prominent myocardial trabeculations and deep inter-trabecular recesses could stagnate circulation predisposing them to formation of blood clots (thrombus) in the non-compacted (trabeculated) myocardial layers [44-46]. However, Stollberger et al. [30] report no significant differences on the rates of embolic events between LVNC patients and matched controls with LV systolic dysfunction. Asymptomatic patients with preserved systolic function however have not demonstrated thromboembolic events at short-term follow-up [17,35].
Diagnosis of LVNC lacks specific practice guidelines. However, a position statement from the ESC on myocardial and pericardial diseases propose a general framework for diagnosing cardiomyopathies. The guidelines propose a multimodal cardiac imaging using echocardiography and/or cardiac MRI complemented by electrocardiography and family screening. Although a multimodal diagnostic approach is recommended [21], echocardiography imaging remains the reference diagnostic standard due to its availability, cost-effectiveness and reproducibility [21,36].
Echocardiography
The hallmark of LVNC diagnosis is the characterization of the normal myocardial structure relative to pathological (trabeculated) LVNC myocardium [19]. This is supported by three echocardiographic criteria for the diagnosis of LVNC developed from findings of three single-center retrospective studies (Table 2).
Table 2. Echocardiographic diagnostic criteria for LVNC.
1st Author [Ref #] |
Diagnostic Criteria |
|
Description |
Ratio |
Imaging |
Chin et al. [5] |
2-layered myocardial structure (compacted epicardial/non-compacted endocardial).
X is the distance from the epicardial surface to the through of the trabecular recess,
and Y is the distance from the epicardial surface to peak of trabeculation. |
Ratio of X/Y ≤ 0.5 |
Parasternal short-axis view, measurements
of the X/Y ratio at end-diastole |
Jenni et al. [12] (Zurich Group) |
Thickened myocardium with 2-layered structure made
of thin compacted epicardial layer (C)
and thicker non-compacted endocardial layer (N) or
trabecular meshwork with deep endomyocardial spaces.
Color Doppler evidence of deep inter-trabecular
recesses supplied by intraventricular blood.
Absence of coexisting cardiac abnormalities |
Ration of N/C ≥ 2.0. |
Short-axis views, measurements of the
N/C ratio at end-systole |
Stollberger et al. [30,47] (Vienna Group) |
More than (>) 3 trabeculations protruding from LV wall,
apically to papillary muscles visible in one imaging plane.
Revised to include 2 layer myocardial structure (non-compacted [NC] and compacted [N]) |
Ratio of NC/C ≥ 2.0 |
Apical four chamber view measurements of the
N/C ratio at end-systole |
Diagnostic criteria
Chin et al. [5] proposed the initial echocardiographic diagnostic criteria for LVNC in 1990, which brought LVNC into clinical focus. The diagnostic criteria quantify the depth of penetration of inter-trabecular recesses in relation to the posterior wall thickness at the end of diastole then calculates the X-to-Y ratio, where X is the distance from the epicardial surface to the through of the trabecular recess while Y is the distance from the epicardial surface to peak of trabeculation. Chin et al. [5] validated this method against eight subjects referred for echocardiography with normal echocardiography studies, which did not indicate progressive decrease of X-to-Y ratio from mitral valve to apex. Jenni et al. [12] defined LVNC echocardiographic diagnostic criteria and validated them using pathoanatomical heart preparations (Figure 3).
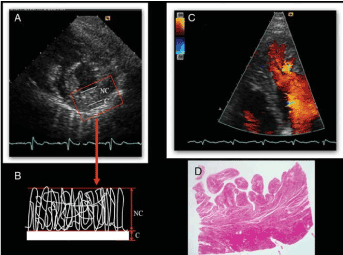
Figure 3. Validation of Jenni et al. Diagnostic Criteria [12]
Panel 3(A) shows parasternal short axis view of LVNC patient. The end-systolic frame reveals 2-layered structure of a thickened myocardium (labelled NC and C). Panel 3(B) Quantifies the thickness of NC at its maximal thickness and determines NC/C ratio. Panel 3(C) Apical 4-chamber view at end-diastolic revealing inter-trabecular recesses filled with intraventricular blood.3(D) Transmural, histological using hematoxylin and eosin stain.
Stollberger et al. [30,47] diagnostic criteria departed from that of Chin et al. [5] and Jenni et al. [12] on imaging and quantifying the two distinct myocardial layers. Stollberger et al. [30] termed the condition LV hyper-trabeculation instead of non-compaction. As such, their original criteria was > 3 trabeculation protruding from LV wall apically to the papillary muscles. However, later they added non-compaction to their description, and revised their diagnostic criteria to include two myocardial layers [47] as earlier suggested by Chin et al. [5] and Jenni et al. [12].
Criteria limitations
Concerns have been raised about the accuracy of the three diagnostic criteria (Table 2). Kohli et al. [48] applied all the three criteria in 199 patients with LV systolic dysfunction referred to a HF clinic and 60 prospectively evaluated normal controls. The study reported poor correlation between the three criteria. The study found 30% of the patients fulfilled all the three criteria while 24% fulfilled one or two and concluded that the poor correlation could be due to differences in morphological descriptions of normal trabeculations in echo planes and in cardiac cycle in which the authors described the morphology [5,12,47,48]. Kohli et al. [48] also found 8% of the controls satisfied one or mode diagnostic criteria suggesting the criteria is too sensitive. It is also unclear at what stage does the fine trabeculations move from normal variant to pathological [48]. To address these concerns, several recent studies report diagnostic value of novel echocardiographic techniques such as Tissue Doppler imaging (TDI) strain rate imaging (SRI) and Speckle Tracking Imaging (STI) in providing a more objective assessment and quantification of LVNC myocardium [49-54].
Cardiac magnetic resonance
Diagnostic criteria: Cardiac magnetic resonance (CMR) imaging complement echocardiography findings. The modality adds anatomic details and functional information on kinesis about the compacted and non-compacted myocardial layers [33]. It is considered when echocardiography image quality is poor [19,32]. Currently, two sets of CMR imaging criteria developed by Petersen et al. [55] and Jacquier et al. [56] are in use (Table 3).
Table 3. CMR imaging diagnostic criteria.
1st Author [Ref #] |
Ratio |
Measurement |
Petersen et al. [55] |
Non-compacted to Compacted myocardial layers > 2.3 |
At end-diastole |
Jacquier et al. [56] |
Trabeculated left ventricular mass > 20% of the global/compacted left ventricular mass |
At end-diastole |
Petersen et al. [55] proposed the first CMR imaging criteria by comparing the ability of CMR to distinguish between pathological LVNC and lesser degrees of trabeculation observed in normal hearts. The study reports non-compacted endocardial layers were more frequent in LVNC patients in apical and lateral segments than in basal and septal as observed in echocardiographic studies. The NC-to-C ratio was > 2.3 in diastole described pathological LVNC with specificity (99%), sensitivity (86%), positive prediction (75%), and negative prediction (99%). Jacquier et al. [56] criteria took a different approach. They characterized LV myocardium by quantifying and comparing global LV mass and trabeculated LV mass in LVNC, DCM, HCM patients and normal controls (Figure 4).
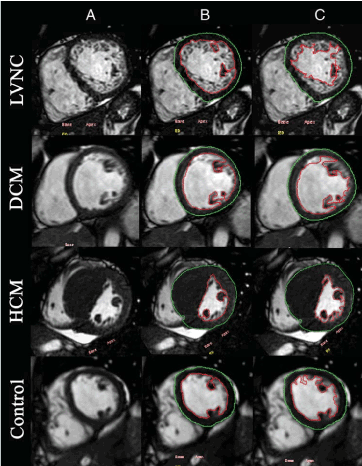
Figure 4. Measuring Global and Trabeculated LV Mass in LVNC, DCM and HCM [56]
Figure 4(A) shows short-axis at end-diastole image for measuring; 4(B): inclusion of papillary muscles and exclusion of LV trabeculation to measure compacted mass; and 4(C): inclusion of papillary muscles and trabeculations to measure global LV mass.
Based on the system of measuring global and trabeculated LV mass, Jacquier et al. [56] report the percentage of trabeculated LV mass in LVNC patients was thrice higher than in DCM and HCM patients but the percentage of LV compacted mass was the same in LVNC, DCM and healthy controls. In LVNC patients, trabeculated LV mass was 20% greater than compacted LV mass. In addition to quantifying LV mass, CMR imaging is able to characterize myocardial fibrosis in patients with LV systolic dysfunction, which is an important prognostic predictor in DCM and HCM phenotypes [57-59]. Using the Peterson et al. [55] CMR diagnostic criteria, Nucifora et al. [60] identified 42 LVNC patients, 55% had myocardial fibrosis identified with late gadolinium enhancement (LGE) CMR. However, the value of LGE in the diagnosis of LVNC remains unclear.
Criteria limitations: A persisting question is, at which cardiac cycle should LV mass be measured to improve the accuracy of CMR diagnosis of LVNC: is it at end-diastole or at end-systole? Stacey et al. [61] compared diastolic and systolic CMR criteria for diagnosing LVNC in a retrospective cohort of 122 patients and report end-systolic ratio > 2.0 was a stronger predictor of systolic dysfunction and existing HF. However, the study’s limitations – retrospective analysis, small sample size and insufficient patient history with normal and impaired systolic function – warrants additional comparative studies to elucidate the superiority of end-systolic to end-diastolic LV measurement in CMR diagnosis of LVNC.
Computed tomography
Diagnostic application: Multi-detector computed tomography angiography (CTA) imaging could also be used to assess LV myocardium in LVNC patients [62]. Coronary CTA provides excellent spatial and contract resolution to assess morphology of LV myocardium as well as provides additional information about coronary arteries and intra-thoracic vasculature. Coronary CTA might be useful in cases where CMR imaging is contradicted or poor echocardiography image quality has been obtained [63].
Diagnostic limitations: Coronary CTA has no diagnostic criteria for LVNC because current criteria recommends evaluation of LV mass either at end-diastole or at end-systole. These criteria would require CTA at 40% or 0-90% of the R-R interval at end-systole or end-diastole respectively. These phases are not routinely available for low-dose electrocardiographic-gated coronary CTA [32].
Family Screening
LVNC associated genes: Several genes associated with LVNC have been identified (Table 4), suggesting the value of family screening in identifying at risk individuals or in complementing imaging findings to confirm the diagnosis. Nearly all the genes associated with LVNC have been linked with the development of dilated cardiomyopathy (DCM) or congenital heart defects (CHD) (Table 4).
Table 4. Genes associated with LVNC.
Location |
Gene/Locus |
Phenotype |
Additional Phenotype |
Inheritance |
Ref [#] |
1p36.32 |
PRDM16 |
LVNC 8 |
DCM |
AD |
66 |
1q32.1 |
TNNT2 |
LVNC 6 |
DCM |
AD |
67 |
10q23.2 |
LDB3 |
LVNC 3 |
DCM |
AD |
68 |
11p15 |
None |
LVNC 2 |
None |
AD |
69 |
11p11.2 |
MYBPC3 |
LVNC 10 |
DCM |
AD |
70 |
14q11.2 |
MYH7 |
LVNC 5 |
DCM |
AD |
71 |
15q14 |
ACTC1 |
LVNC 4 |
DCM |
AD |
72 |
15q22.2 |
TPM1 |
LVNC 9 |
DCM |
_ |
70 |
18q11.2 |
MIB1 |
LVNC 7 |
None |
AD |
73 |
18q12.1 |
DTNA |
LVNC 1 |
With/without CHD |
AD |
74 |
Xq28 |
G4.5/TAZ |
Barth Syndrome |
Failure to thrive/grow |
X-linked Recessive |
75 |
AD: Autosomal Dominant; CHD: Congenital Heart Defect; DCM: Dilated Cardiomyopathy; LVNC: Left ventricular non-compaction.
The location of the genetic mutation, gene locus and phenotype may vary but the dominant phenotype associated with the development of left ventricular non-compaction remains DCM. Other phenotypes are due to the presence or absence of congenital heart disease and the failure to thrive or grow in children.
There is already research evidence on the clinical value of family screening supporting LVNC diagnosis. Habib et al. [64] identified 105 LVNC patients using echocardiography, where family screening was the referral reason for 8% of the patients.
Family screening challenges: Patients with LVNC often present with pleiotropic phenotypes (a single gene controlling more than one trait), which imposes challenges in conducting genome-wide association studies. Such studies would have improved understanding on the genetic basis of LVNC and family screening for at risk individuals [65].
Proposed diagnostic algorithm
Current clinical management approaches for LVNC only identify multimodal imaging using echocardiography and cardiac MRI to characterize the normal myocardial structure relative to pathological (trabeculated) LVNC myocardium [19]. However, there are no suggestions on diagnostic algorithm for diagnosing LVNC. Thavendiranathan and associates [66] reviewed published diagnostic criteria and developed a diagnostic algorithm. Figure 5 provides a schematic illustration of the proposed LVNC diagnostic algorithm [67-77].
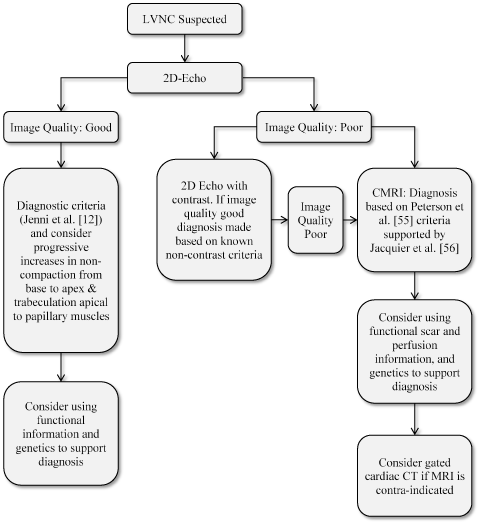
Figure 5. Proposed Diagnostic Algorithm for LVNC [66]
The diagnosis of suspected LVNC depends on initial findings of two-dimensional echocardiography findings. Diagnosis for good image quality relies on Chin et al. [5] and Jenni et al [12] diagnostic criteria while poor (inconclusive) image quality requires two-dimensional echo with contrast to cardiac magnetic resonance based on Peterson et al. [55] and Jacquier proposed criteria
The algorithm proposes that diagnosis of LVNC begins with echocardiography imaging as defined by Jenni et al [12]. If the image is good and characterization of normal to trabeculated myocardium is demonstrable, diagnosis is made, which could be supported by functional information and genetic analysis. If the echocardiography image is poor, 2D echocardiography with contrast could be considered or cardiac MRI based on Peterson et al. [55] criteria supported by Jacquier et al. [56] criteria. In the case cardiac MRI is contra-indicated, gated cardiac CT should be considered.
Meta-analysis of diagnosis criteria
At present, there is no consensus on practice guidelines or expert recommendations on diagnostic criteria for LVNC. The current criteria is based on findings from small single-center retrospective studies. The aim of this systematic review and meta-analysis is to compare current diagnostic criteria reported in peer-reviewed publications.
Search criteria
The search for primary references and reviews was conducted in the electronic databases PubMed and EMBASE as well as a review of published bibliographies. Search terms used were (diagnosis OR echocardiography OR cardiac magnetic resonance) AND (left ventricular non-compaction OR isolated ventricular non-compaction OR non-compaction cardiomyopathy OR hypertrabeculation syndrome OR spongy myocardium). The search was restricted to published peer-reviewed articles between 1990 (when Chin et al. [5] developed the initial diagnosis criteria) and 2017. There was no restriction on language or age of the patients (the search included both adults and pediatrics).
Study selection
Studies were selected if they utilized echocardiography or cardiac magnetic resonance imaging to assess patients suspected with LVNC and more importantly, reported original diagnostic criteria. Studies were included if they provided information on the following diagnostic features and outcomes: (a) selection criteria; (b) diagnostic criteria; (c) cardiac phase of myocardial description; and (d) ratio of non-compacted endocardial layers and compacted epicardial layers. Duplicate studies (that assessed existing criteria), and those available only in abstract form (without a published manuscript) or data was not readily extractable were excluded. Data extracted included first author, year of study, number of patients, percentage of male patients, mean years, diagnostic method. Selection criteria. Asymptomatic patients, diagnostic criteria. Cardiac phase of imaging and ratio of non-compacted to compacted myocardial layers (Table 5).
Table 5. Summary of published diagnostics criteria for LVNC.
1st Author [Ref. #] |
Year |
No. of Patients |
No. of Male |
Mean Age (yrs.) |
Diagnostic Method |
Selection Criteria |
Asymptomatic patients |
Diagnostic Criteria |
Cardiac Phase |
Ratio |
Chin et al. [5] |
1990 |
8 |
5 |
8.9 |
Echo |
Referred for echo |
2 |
See Table 2 |
End-diastole |
X/Y ≤ 0.5 |
Jenni et al. [12] |
2001 |
7 |
5 |
39.1 |
Echo |
Referred for echo |
NR |
See Table 2 |
End Systole |
NC/C ≥ 2 |
Stollberger et al. [47] |
2002 |
62 |
49 |
- |
Echo |
Referred for echo > 3 trabeculations distal to papillary |
7 |
See Table 2 |
End Systole |
NC/C ≥ 2 |
Petersen et al. [55] |
2005 |
7 |
5 |
29.3 |
CMR |
Echo/CMR demonstration of 2-layered trabeculated and compacted myocardium |
4 |
2-layered structure: compacted epicardial and non-compacted endocardial |
End Systole |
NC/C ≥ 2.3 |
Jacquier et al. [56] |
2010 |
16 |
10 |
48.0 |
CMR |
Based on Jenni et al [12] criteria |
NR |
Ratio of total LV trabeculated mass to global myocardial mass |
End-diastole |
LV trabeculated mass > 20% |
Grothoff et al. [77] |
2012 |
12 |
3 |
39.2 |
CMR |
Based on Jenni et al [12] criteria plus familial screening,
neuromuscular disorders & LVNC complications (embolization,
VT, regional wall motion abnormalities) |
1 |
2-layered structure: compacted epicardial and non-compacted endocardial |
End Systole |
NC/C ≥ 2.3 |
Captur et al. [78] |
2013 |
30 |
16 |
41.0 |
CMR |
Based on Jenni et al [12] criteria plus family
history and LVNC-complications
(HF, VT, thromboembolism) |
NR |
Fractal analysis: Fractal dimension (FD) = Global LV trabecular complexity |
NA |
FD ≥ 1.30 |
*NR: Not Reported; Echo: Echocardiography; CMR: Cardiac Magnetic Resonance; NC: Non-Compacted; C: Compacted; NA: Not Applicable
Study characteristics
The search on online databases as well as a review of bibliographies yielded 244 studies that used cardiac imaging modalities to characterize the morphology of the LV myocardium. After a detailed review, only seven (7) studies using echocardiography or cardiac magnetic resonance (CMR) imaging to diagnose LVNC and reported original diagnostic criteria were included in this systematic analysis. The publication year for the studies ranged between 1990 and 2013. All the seven studies were small-scale retrospective single center clinical trials. The combined patient population in the seven studies was 142, male to female ratio 93:49, mean age 34.3 years (range 8.9 to 48.0 years). Three studies [5,12,47] developed original LVNC diagnostic criteria based on findings from echocardiography imaging while the remaining four studies [55,56,77,78] developed their criteria based on findings from CMR characterization of the LV myocardium.
Echocardiography-defined LV myocardium is the common imaging modality and criteria for diagnosing LVNC. In all the three echocardiographic studies [5,12,47], the principle diagnostic feature is a two-layered myocardial structure consisting of compacted (non-trabeculated) thin epicardial layer and a much thicker non-compacted endocardial layer consisting of trabecular meshwork and deep inter-trabecular recesses. Although the basis of diagnosis is the ratio between these two layers as measured by echocardiography, the three studies differed on the way they measured the ratio. Chin et al. [5] used the ratio of global LV mass (compacted and non-compacted: X) to non-compacted mass (Y) defined as X:Y ≤ 0.5 measured at parasternal short-axis view while Jenni et al. [12] measured the ratio of non-compacted endocardial layer (NC) to compacted epicardial layer, defined as NC:C ≥ 2.0 at short axis (Figure 6).
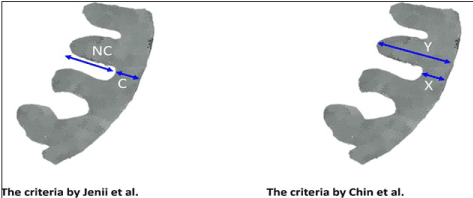
Figure 6. Comparison of Chin et al. and Jenni et al. Criteria [11]
Chin et al. [5] used the ratio of global LV mass (compacted and non-compacted: X) to non-compacted mass (Y) defined as X:Y ≤ 0.5 measured at parasternal short-axis view while Jenni et al. [12] measured the ratio of non-compacted endocardial layer (NC) to compacted epicardial layer, defined as NC:C ≥ 2.0 at short axis.
In addition, Chin et al. [5] and Jenni et al. [12] diagnostic criteria differed in the cardiac cycle used to describe the 2-layer myocardium: Chin et al. [5] measured at the end-diastole while Jenni et al. [12] at end-systole. Jenni et al. [12] also added two criteria: (a) color Doppler evidence of deep inter-trabecular recesses supplied by intraventricular blood; and (b) the absence of coexisting cardiac abnormalities. The additional criteria were validate using valvular heart disease, hypertrophic or dilated cardiomyopathy. The validation report very few other cardiac diseases (5%) meet all the three LVNC echocardiographic criteria.
In a departure from Chin et al. [5] and Jenni et al [12] criteria, originally, Stollberger et al. [47] defined LVNC as more than three (> 3) trabeculations protruding from LV wall, apically to papillary muscles visible in one imaging plane. However, this definition was later revised to include a 2-layered myocardial structure defined as NC:C ≥ 2.0, same to Jenni et al. [12] description but measured from apical four-chamber view. The three studies also differed at the cardiac phase the measurement was taken. While Chin et al. [5] measured at end-diastole Jenni et al. [12] and Stollberger et al. [47] measured at end-systole.
The criteria for LVNC diagnosis using cardiac magnetic resonance (CMR) imaging was developed much later and patient selection was based mainly on Jenni et al. [12] echocardiography criteria. Petersen et al. [55] described the initial criteria in 2005 followed by Jacquier et al. [56], Grothoff et al. [77] and Captur et al. [78] in 2010, 2012 and 2013 respectively. Same to echocardiography, in two studies, the basis of CMR diagnosis of LVNC is the ratio of NC to C myocardial layers: NC:C > 2.3 with measurement taken at end systole [55,77]. On the other hand, Jacquier et al. [56] measured the ratio of total LV trabeculated mass to global myocardial mass and defined LVNC as LV trabeculated mass > 20% at end-diastole while Captur et al. [78] used fractional analysis and defined LVNC as fractional dimension ≥ 1.3. Same to echocardiographic criteria, CMR-based LVNC diagnosis also differ at the time the image is taken: either at end-diastole or at end-systole, and ratio of myocardial layers used to detect LVNC.
Unlike other frequently encountered forms of primary cardiomyopathies, such as dilated, hypertrophic and arrhythmogenic right ventricular dysplasia that have a well-defined diagnosis criterion, the diagnosis of LVNC lacks specific practice guidelines. Traditionally, LVNC diagnosis depended on echocardiography imaging modality. All the three reviewed criteria derive their diagnosis on morphological features of the LV myocardium. Further, diagnosis requires the presence of prominent trabeculations with deep intra-trabecular recesses and a two-layered appearance of the LV myocardium consisting of compacted epicardial (first layer) and non-compacted endocardial layer (second layer).
Despite these similarities, important differences in the three echocardiography-based criteria exist based on the ratio of compacted to non-compacted LV myocardium. The seminal diagnostic criteria by Chin et al. [5] compared the distance between epicardial surface and trough of intra-trabecular recess (X) with the distance between epicardial surface and peak of trabeculations in end-diastole. Diagnosis was based on progressive decrease of the X/Y ratio an increase of total LV wall thickness (Y) from base to apex was observed in LVNC patients and not in healthy controls. The X/Y ratio of 0.5 found in chin et al. [5] was confirmed by a subsequent study from short axis view [19]. However, Chin et al. [5] diagnostic criteria was based on a small sample of eight patients and eight controls, and in addition, failed to describe the specific location of trabeculations and the minimum number of trabeculations.
A subsequent chronological study by Jenni et al. [12] defined a 2-layered LV myocardium with trabecular spaces filled with blood from ventricular cavity and imaged by Doppler Echo. An NC:C ratio > 2.0 imaged at end-diastole differentiated LVNC from hypertrophic and dilated cardiomyopathy. Different from Chin et al. [5], Jenni et al. [12] specified that the most involved location for imaging is the mid-lateral and inferior walls, and the apex but the study did not provide specificity or sensitivity of data. The third echocardiographic criteria by Stollberger et al. [47] based on a post-mortem study originally defined LVNC as greater than (<) 3 trabeculations located in the apical region to the insertion of papillary muscles imaged on the apical plane. However, subsequent publications recognized the presence of a two-layered LV myocardium and the ratio of NC to C > 2.0 as the basis of diagnosis.
In the diagnosis of LVNC, Cardiac magnetic resonance (CMR) imaging is a recent modality. Currently, three criteria have been published, and same to echocardiography, use the ratio of a two-layered myocardium (compacted and non-compacted) for diagnosis [55,56,77]. However, CMR has superior contrast-to-noise ratio (CNR) and signal-to-noise ratio (SNR) and clinically useful when echocardiography image is not definitive. Peterson et al. [55] and Grothoff et al. [77] report NC/C ratio ≥ 2.3 measured at end-systole while Jacquier et al. [56] and Captur et al. [78] used fractional analysis for diagnosis of LVNC. In most cases, diagnosis was based on ratio of total LV trabeculated mass to global myocardial mass > 20%. Compared to Jenni et al. [12] criteria, in CMR, the location of non-compaction is not useful in LVNC diagnosis. When Petersen et al. [55] CMR criteria was applied in a multi-ethnic study of atherosclerosis involving 323 patients, the NC:C > 2.3 ratio was detected in only one myocardial region in 43% of the patients and in 2% of patients in at least 2 regions. The findings suggest that CMR morphological criteria has reduced specificity in patients with low pretest probability of LVNC [66]. In addition to a 2-layered morphology, CMR provides additional diagnostic morphological features for consideration such as thinned compacted epicardial layer at sites of non-compaction [66]. CMR also provides more comprehensive myocardial characterization. Late gadolinium enhanced (LGE) CMR can also be used on detecting thrombus on LVNC patients.
Whereas echocardiography criteria is the frequently used for diagnosis, the three criteria are non-specific and overlapping. Kohli et al. [48] applied all the three criteria on 199 patients with LV systolic dysfunction and 60 prospectively selected normal controls. The study found 79% fulfilled Chin et al. [5] criteria, 30 (64%) fulfilled Jenni et al. [12] criteria while 53% fulfilled Stollberger et al. [47] criteria, with 47% percent fulfilling one or more criteria. [61]. The overlapping nature of the three criteria affects the determination of prevalence, which would range between 12% and 19% if only one criteria is used. Stacey et al. [61] examined diagnostic accuracy of measurement taken at end-diastole and end-systole using CMR. The study finds end-systole has higher odds ration than end-diastole for combined cardiac events – CHF and systolic dysfunction.
In sum, multi-modal imaging is the accepted standard of diagnosing LVNC. Echocardiographic criteria depends on quantifying LV mass and calculating the ratio of non-compacted to compacted myocardial layer. On the other hand, CMR not only depends on the ratio of non-compacted to competed myocardial layer but also on the ratio of LV trabeculated mass and LV mass as well as fractional dimension of LV mass complexity. Beyond myocardial structure, it is important to include a functional component of LVNC diagnosis. Assessing the distribution of hypokinetic segments especially at the apex, regional dysfunction, and myocardial strain values may present important indices for evaluating LVNC functional dysfunction. However, functional analysis is an ongoing research area.
Unlike other primary forms of cardiomyopathies such as DCM and HCM, leading national heart and cardiology associations such as AHA in the U.S. and ESC in Europe have not provided specific practice guidelines nor algorithm for clinical management of LVNC. In an attempt to fill this gap, Thavendiranathan and associates [66] conducted a systematic literature review of small-scale retrospective clinical trials on clinical management of LVNC searched and appraised from online database MEDLINE between 1970 and 2012, and proposed an algorithm for clinical management of LVNC.
Proposed algorithm
Current clinical management of LVNC relies on the presence or absence of cardiac dysfunction or arrhythmias [33]. The clinical management algorithm proposed by Thavendiranathan et al. [66] depends primarily on the presence or absence of LV dysfunction and/or symptoms of heart failure (Figure 7).
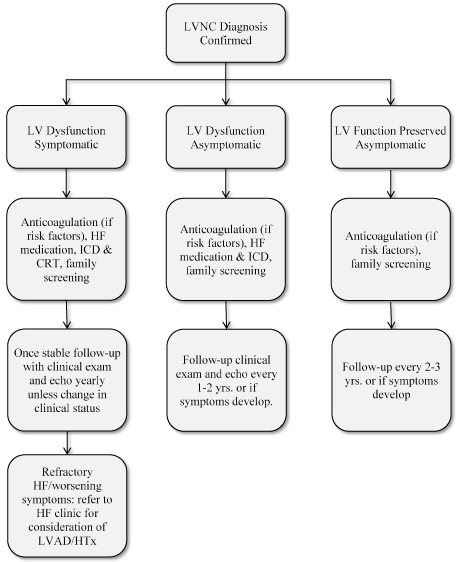
Figure 7. Proposed Clinical Management Algorithm for LVNC [66]
Once the diagnosis of LVNC has been confirmed, clinical management depends on the extent of left ventricular dysfunction: preserved, asymptomatic or symptomatic. Based on the findings, treatment includes anticoagulation, heart failure treatment, family screening and regular clinical follow-up. LV: Left Ventricular; LVAD: Left Ventricular Assist Device; HF: Heart Failure HTx: Heart Transplantation.
According to the proposed algorithm, clinical management of LVNC should begin with classifying patients into those with preserved (normal) LV function and those with LV dysfunction. Then those with LV dysfunction are further classified into symptomatic and asymptomatic sub-groups. For each of these groups specific clinical management approaches are identified as discussed in the subsequent sections.
Patients with preserved ventricular function
The primary clinical management method for patients suspected with LVNC but with preserved LV systolic function and LV mass is continuous clinical evaluation and monitoring of LV function [66]. Monitoring should include clinical history, physical examination, cardiac imaging, Holter monitoring, assessment of troponin levels, and family screening every three years especially for those identified with genetic mutation [33]. The aim of continuous monitoring is to assess the presence of risk factors and symptoms to inform the necessary prophylactic therapy. Further, anticoagulation therapy is suggested for patients with identified risk factors, who also require continuous family screening to assess for the development of symptoms [66].
Patients with LV dysfunction
Clinical management for patients with LV dysfunction depends on the presence or on the absence of symptoms. For symptomatic LVNC patients presenting with LV systolic dysfunction or increased LV mass, clinical management is similar to that of other cardiomyopathies: (a) treatment for heart failure; (b) management of arrhythmias; and (c) prevention of thromboembolic events [36]. Deep inter-trabecular recesses slow circulation in cardiac chambers increasing the risk of thrombus formation. Thus, anticoagulation therapy may be considered in LVNC patients for the prevention of thromboembolic events especially for patients with depressed LV ejection fraction but only after a careful assessment of benefit and risks [15]. For LVNC patients presenting with syncope, symptomatic ventricular arrhythmias, or with severely depressed LV ejection fraction (LVEF < 35%), implantable cardioverter defibrillator (ICD) or bi-ventricular pacing may be considered. However, they are no large-scale studies indicating the value or efficacy of ICD in patients with LVNC and HF [15,36].
Meta-analysis of clinical management, characteristics and outcomes
There is no specific clinical management approach for patients with LVNC. Current management approaches largely utilize the traditional heart failure treatment based on LV function or dysfunction and the presence or absence of HF symptoms. This systematic review and meta-analysis aims to examine clinical outcomes and clinical strategies used in the management of LVNC.
Search criteria
Three electronic databases PubMed, EMBASE and Google Scholar were searched for studies examining clinical characteristics and outcomes in patients with LVNC. A combination of search terms used were (clinical characteristics OR clinical outcomes OR clinical management) AND (left ventricular non-compaction OR isolated ventricular non-compaction OR non-compaction cardiomyopathy OR hypertrabeculation syndrome OR spongy myocardium). Additional studies were retrieved from bibliographies of the selected studies. The search was restricted to studies investigating humans only and thus, excluded studies based on animal models. However, there was no restriction on the publication language used or the age of the patients: studies including either or both pediatric and adult LVNC patients were eligible for inclusion.
Study selection
A hierarchical approach (title, abstract and manuscript) was utilized to screen all the retrieved citations for inclusion. Case series were included if they provided data on more than one clinical characteristic or outcome in at least ten LVNC patients. For studies that used a duplicate cohort of LVNC patients, the latest study was included. Studies were excluded if (a) they included non-compaction associated with congenital heart disease or did not report data on LVNC patient; (b) they were available only in abstract form with insufficient data provided for retrieval; or (c) data was not readily extractable. Bibliographies of all selected studies and relevant reviews were screened to identify studies missed by the electronic search. The extracted data included author, publication year, sample, female (%), number of asymptomatic patients, follow-up period, number of events (atrial fibrillation (AF), ventricular tachycardia (VT), congestive heart failure (CHF), thrombotic events (TE) and sudden cardiac death (SCD), and treatment options ICD and heart transplantation (HTx) (Table 6).
Table 6. Summary of clinical characteristics and outcomes in LVNC patients.
1st Author [Ref. #] |
Year |
Sample |
Female (%) |
Asymptomatic |
Follow-up (Months) |
AF |
VT |
CHF |
TE |
ICD |
HTx |
SCD |
Pignatelli et al. [14] |
2003 |
36 |
44 |
8 |
38 |
2 |
1 |
14 |
3 |
1 |
0 |
5 |
Oechslin et al. [15] |
2000 |
34 |
26 |
NR |
44 |
NR |
14 |
18 |
8 |
12 |
4 |
6 |
Stollberger et al. [16] |
2007 |
86 |
24 |
30 |
51 |
NR |
NR |
NR |
0 |
1 |
1 |
4 |
Aras et al. [17] |
2006 |
67 |
34 |
12 |
30 |
NR |
33 |
34 |
9 |
0 |
0 |
10 |
Murphy et al. [20] |
2004 |
45 |
38 |
17 |
46 |
3 |
9 |
0 |
3 |
2 |
0 |
2 |
Lofiego et al. [35] |
2007 |
65 |
63 |
26 |
46 |
6 |
4 |
22 |
3 |
11 |
9 |
6 |
Stanton et al. [42] |
2009 |
30 |
40 |
NR |
30 |
2 |
4 |
NR |
NR |
11 |
NR |
3 |
Habib et al. [64] |
2011 |
105 |
34 |
17 |
28 |
7 |
7 |
33 |
11 |
29 |
9 |
12 |
Total/ Average |
|
468 |
37.9 |
20.7 |
39.1 |
18 |
57 |
33 |
11 |
67 |
9 |
48 |
AF: Atrial Fibrillation; HTx: Heart Transplantation; SCD: Sudden Cardiac Death; TE: Thromboembolism; VT: ventricular Tachycardia
Study characteristics
Eight (8) studies conducted between 2000 and 2011 meeting the inclusion criteria were included in this meta-analysis. The studies reported data on common LVNC complications: AF [14,20,35,42,64]; ventricular tachycardia [14,15,17,20,35,42,64]; CHF [14,15,17,20,35,64], thromboembolic events [14-17,20,35,64], and sudden cardiac death [14-17,20,35,42,64], as well as traditional HF treatment strategies such as ICD/biventricular pacemaker [14-17, 20,35,42,64] and heart transplantation [14-17,20,35,64]. The combined population was 468 patients diagnosed with LVNC using published echocardiography or CMR imaging criteria. The mean age was 37.9 years, range 24years [16] to 63 years [35]. About a third of the patients were female (37.9%) and 20.7% were asymptomatic at presentation. The median follow-up period since diagnosis was 39.1 months, range 28 months [64] to 51 months [16].
Clinical outcomes
The most frequently reported clinical complications (events) of LVNC were VT (57), SCD (48), CHF (33), AT (18) and TE (11). Further assessment of LVNC event rate reported the two leading event rate were CHF(39.0%: range 33.6-44.6%) and VT(25.3%: range 20.3-31.3%). These were followed by SCD(11.1%: range 8.5-14.5%); TE(11.1%: range 8.1-14.9%); and AF(7.2%: range 4.7-10.9%). All the event rates were significant (p<0.05). In the eight studies, the combined event rate for the five events was 16.6% range 12.7% to 21.4% (p<0.05) (Figure 8). In all the studies, the five events predicted a general ominous prognosis for LVNC patients. The initial LVNC treatment was the traditional HF medical therapy using ACE-inhibitors/Angiotensin Receptor Blocker (ARB) and beta-blockers. Device therapy (ICD ± biventricular pacemaker) and heart transplantation (HTx) were also common in patients who were refractory to traditional HF medical therapy. Device therapy was used on 7.2% of the patients and HTx in 7.8% of the patients. Since no study assessed treatment efficacy of ICD or HTx, their contribution to the reported cardiac death of 7.6% of the 468 patients could not be determined.
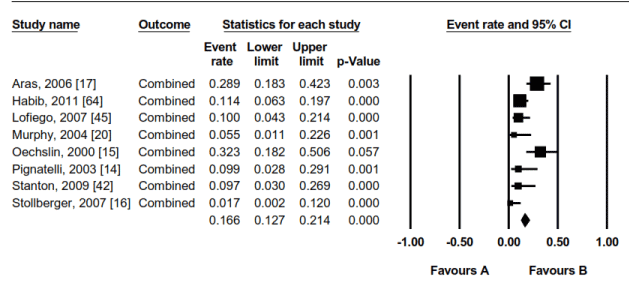
Figure 8. Combined Event Rates in LVNC Patients in the 8 Studies
Effect size outcomes for combines events rates for ventricular tachycardia, sudden cardiac death, congestive hea4rt failure, atrial fibrillation and thromboembolic events.. Significant values p < 0.05.
Current management of LVNC is comparable to those of other primary cardiomyopathies, which relies on the treatment for heart failure (HF), the management of arrhythmias and the prevention of thromboembolic events [36]. However, due to the lack of specific treatment guidelines, management of LVNC uses the traditional HF guidelines for medication, device therapy and heart transplantation (HTx). The principal target for clinical management of LVNC is the prevention of adverse cardiac events [36]. Similar to clinical manifestation of symptomatic HF, this meta-analysis finds VT, SCD, CHF, AT and TE as frequently encountered adverse events. These adverse events also support the current clinical management approaches using anti-coagulation to treat thromboembolic events [15] and device therapy to manage HF symptoms [15,36].
The present findings also support Thavendiranathan et al. [66] algorithm for clinical management that proposes LVNC treatment should depend on the presence or absence of LV dysfunction and symptomatic HF – treatment of symptomatic HF, managing arrhythmias and the prevention of thromboembolic events [36]. Although Thavendiranathan et al. [66] algorithm suggests treatment depends on the presence or absence of symptoms, this meta-analysis did not discriminate symptomatic and asymptomatic patients. However, the findings emphasizes on the need for continuous monitoring for LV dysfunction or symptomatic HF to inform the most appropriate treatment course. Consistent with these findings, classical HF medication such as ACE-inhibitors/ARB recommended for the maintenance of LV function and anticoagulation therapy for the prevention of thromboembolic events are applicable to LVNC patients[33,66].
Although the use of HF treatment on LVNC patients has been effective in improving survival free of adverse cardiac events, there is need to identify patients who will benefit most from HF medication and those requiring chronic therapies such as device therapy or HTx at subclinical stage of LVNC. At present, clinical value of ICD and HTx in conveying a protective effect against cardiac death in LVNC patients remains unclear. In addition, with a high event rate of CHF and VT in LVNC patients, additional studies are warranted to determine clinical efficacy of anticoagulation and HF medication or identification of risk factors such as family screening for identification of LVNC patients at risk of CHF and VT [66].
Left ventricular non-compaction (LVNC) is a cardiac condition characterized by prominent left ventricular trabeculae and deep inter-trabecular recesses resulting principally from an arrest in the normal compaction process of the developing myocardium. Emerging evidence in athletes and pregnant women also suggests LVNC may develop postnatal. The exact epidemiology of LVNC is not clear but small-scale studies suggests LVNC is predominant in pediatric populations. The presence of HF symptoms, NYHA class II-IV and a history of ventricular tachycardia (VT) predict an ominous prognosis. Frequent clinical manifestation of LVNC is the triad of symptomatic HF, systemic thromboembolic events (TE) and sudden cardiac death (SCD). A multimodal imaging approach using echocardiography and/or cardiac magnetic resonance (CMR) imaging is recommended for the diagnosis of LVNC but echocardiography remains the reference imaging standard. Diagnosis requires the presence of a two-layered myocardium: a compacted (C) endocardial layer and a non-compacted (NC) epicardial layer consisting of trabeculated meshwork and deep intra-trabecular spaces. Diagnosis is mainly confirmed based on NC to C ratio ≥ 2.3 for echocardiography and ≥ 2.3 for CMR image taken at end-systole or the ratio of total trabeculated mass > 20% of the compacted LV mass at diastole for CMR. Clinical management of LVNC draws from the traditional heart failure treatment, aimed to prevent or treat symptoms of heart failure, fatal arrhythmias and thromboembolic events, which are the frequent adverse events in LVNC patients. However, treatment methods differ based on the presence of symptomatic or asymptomatic LV dysfunction or preserved LV function. Treatment mainly includes heart failure medical therapy: ACE-inhibitors/ARB and beta-blockers, and for patient’s refractory to medical therapy, device therapy and heart transplantation are considered.
- Grant RT (1926) An unusual anomaly of the coronary vessels in the malformed heart of a child. Heart, 13, 273-283.
- Bellet S, Gouley BA (1932) Congenital heart disease with multiple cardiac anomalies. Am J Med Sci, 183: 458-464.
- Dusek J, Ostadal B, Duskova M (1975). Postnatal persistence of spongy myocardium with embryonic blood supply. Arch Pathol, 99: 312-317. [Crossref]
- Engberding R, Bender F (1984). Identification of a rare congenital anomaly of the myocardium by two-dimensional echocardiography: persistence of isolated myocardial sinusoids. Am J Cardiol, 53: 1733-1734. [Crossref]
- Chin TK, Perloff JK, Williams RG, Jue K, Mohrmann R (1990). Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation, 82: 507-513. [Crossref]
- Val-Bernal JF, Garijo MF, Rodriguez-Villar D, Val D (2010). Non-compaction of the ventricular myocardium: A cardiomyopathy in search of a pathoanatomical definition. Histol Histopathol, 25: 495-503. [Crossref]
- Ritter M, Oechslin E, Sütsch G, Attenhofer C, Schneider J, et al. (1997) Isolated noncompaction of the myocardium in adults. Mayo Clin Proc 72: 26-31. [Crossref]
- Richardson, P., McKenna, W., Bristow, M., Maisch, B., & Mautner, B. (1996). o’connell J, olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health organization/International Society and Federation of cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation, 93, 841-842.
- Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, et al. (2008). Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J., 29: 270-276. [Crossref]
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, et al. (2006) Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113: 1807-1816. [Crossref]
- Ikeda U, Minamisawa M, Koyama J (2015) Isolated left ventricular non-compaction cardiomyopathy in adults. J Cardiol 65: 91-97. [Crossref]
- Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA (2001). Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart, 86: 666-671. [Crossref]
- Nugent AW, Daubeney PE, Chondros P, Carlin JB, Cheung M, et al. (2003) The epidemiology of childhood cardiomyopathy in Australia. N Engl J Med 348: 1639-1646. [Crossref]
- Pignatelli RH, McMahon CJ, Dreyer WJ, Denfield SW, Price J, et al. (2003). Clinical characterization of left ventricular noncompaction in children. Circulation, 108: 2672-2678. [Crossref]
- Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R (2000). Long-term follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol, 36: 493-500. [Crossref]
- Stöllberger C, Winkler-Dworak M, Blazek G, Finsterer J (2007). Prognosis of left ventricular hypertrabeculation/noncompaction is dependent on cardiac and neuromuscular comorbidity. Int J Cardiol., 121: 189-193. [Crossref]
- Aras D1, Tufekcioglu O, Ergun K, Ozeke O, Yildiz A, et al. (2006). Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. J Card Fail, 12: 726-733. [Crossref]
- Sandhu R, Finkelhor RS, Gunawardena DR, Bahler RC (2008). Prevalence and characteristics of left ventricular noncompaction in a community hospital cohort of patients with systolic dysfunction. Echocardiography, 25: 8-12. [Crossref]
- Oechslin E, Jenni R (2011). Left ventricular non-compaction revisited: A distinct phenotype with genetic heterogeneity? Eur Heart J, 32: 1446-1456. [Crossref]
- Murphy RT, Thaman R, Blanes JG, Ward D, Sevdalis E, et al. (2004). Natural history and familial characteristics of isolated left ventricular non-compaction. Eur Heart J, 26: 187-192. [Crossref]
- Virtová R, Kubánek M, Šramko M, Voska L, Kautznerová D, et al. (2013). Isolated non-compaction cardiomyopathy: A review. cor et vasa, 55: e236-e241.
- Freedom RM, Yoo SJ, Perrin D, Taylor G, Petersen S (2005). The morphological spectrum of ventricular noncompaction. Cardiology in the Young, 15: 345-364. [Crossref]
- Sedmera D, Pexieder T, Vuillemin M, Thompson RP, Anderson RH (2000) Developmental patterning of the myocardium. Anat Rec 258: 319-337. [Crossref]
- Collins P (1995). Embryology: development of the heart. In: Williams P.L., (Ed.). Gray’s Anatomy (38th Ed.). London: Churchill Livingstone.
- Hieokawa K (1972). A quantitative study on pre-and postnatal growth of human heart. Acta Pathol Jpn., 22(4), 613-624. [Crossref]
- Borges AC, Kivelitz D, Baumann G (2003). Isolated left ventricular non-compaction: cardiomyopathy with homogeneous transmural and heterogeneous segmental perfusion. Heart, 89: e21-e21. [Crossref]
- Shemisa K, Li J, Tam M, Barcena J (2013). Left ventricular noncompaction cardiomyopathy. Cardiovasc Diagn Ther, 3: 170-175. [Crossref]
- Towbin JA, Bowles NE (2002) The failing heart. Nature 415: 227-233. [Crossref]
- Angelini A, Melacini P, Barbero F, Thiene G (1999) Evolutionary persistence of spongy myocardium in humans. Circulation 99: 2475. [Crossref]
- Stöllberger C, Finsterer J (2004). Left ventricular hypertrabeculation/noncompaction. J Am Soc Echocardiogr., 17: 91-100. [Crossref]
- Gati S, Chandra N, Bennett RL, Reed M, Kervio G, et al. (2013). Increased left ventricular trabeculation in highly trained athletes: do we need more stringent criteria for the diagnosis of left ventricular non-compaction in athletes?. Heart, 99:401-408. [Crossref]
- Gati S, Rajani R, Carr-White GS, Chambers JB (2014). Adult left ventricular noncompaction: Reappraisal of current diagnostic imaging modalities. JACC Cardiovasc Imaging, 7: 1266-1275. [Crossref]
- Arbustini E, Weidemann F, Hall JL (2014). Left ventricular noncompaction: A distinct cardiomyopathy or a trait shared by different cardiac diseases? J Am Coll Cardiol, 64: 1840-1850. [Crossref]
- Tian T, Liu Y, Gao L, Wang J, Sun K, et al. (2014). Isolated left ventricular noncompaction: clinical profile and prognosis in 106 adult patients. Heart Vessels, 29: 645-652. [Crossref]
- Lofiego C, Biagini E, Pasquale F, Ferlito M, Rocchi G, et al. (2007) Wide spectrum of presentation and variable outcomes of isolated left ventricular non-compaction. Heart 93: 65-71. [Crossref]
- Jenni R, Oechslin EN, van der Loo B (2007) Isolated ventricular non-compaction of the myocardium in adults. Heart 93: 11-15. [Crossref]
- Fazio G, Sutera L, Corrado G, Novo S (2007) The chronic heart failure is not so frequent in non-compaction. Eur Heart J 28: 1269. [Crossref]
- Engberding R, Yelbuz TM, Breithardt G (2007). Isolated noncompaction of the left ventricular myocardium. Clin Res Cardiol., 96: 481-488.
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, et al. (2009). 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol., 53: e1-e90. [Crossref]
- Epstein AE, DiMarco JP, Ellenbogen KA, Estes NM, Freedman RA, et al. (2008). ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) Developed in Collaboration With the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Heart rhythm, 5: e1-e62.
- Kovacevic-Preradovic T, Jenni R, Oechslin EN, Noll G, Seifert B, et al. (2009). Isolated left ventricular noncompaction as a cause for heart failure and heart transplantation: a single center experience. Cardiology, 112: 158-164. [Crossref]
- Stanton C, Bruce C, Connolly H, Brady P, Syed I, et al. (2009). Isolated left ventricular noncompaction syndrome. Am J Cardiol., 104: 1135-1138. [Crossref]
- Chinushi M, Iijima K, Furushima H, Izumi D, Sato A, et al. (2013). Suppression of storms of ventricular tachycardia by epicardial ablation of isolated delayed potential in noncompaction cardiomyopathy. Pacing Clin Electrophysiol, 36(4): e115-9. [Crossref]
- Petersen SE, Timperley J, Neubauer S (2005) Left ventricular thrombi in a patient with left ventricular non-compaction in visualisation of the rationale for anticoagulation. Heart 91: e4. [Crossref]
- Vatta M, Mohapatra B, Jimenez S, Sanchez X, Faulkner G, et al. (2003). Mutations in Cypher/ZASPin patients with dilated cardiomyopathy and left ventricular non-compaction. J Am Coll Cardiol., 42: 2014-2027. [Crossref]
- Finsterer J (2009) Cardiogenetics, neurogenetics, and pathogenetics of left ventricular hypertrabeculation/noncompaction. Pediatr Cardiol., 30: 659-681. [Crossref]
- Stollberger C, Finsterer J, Blazek G (2002). Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am J Cardiol, 90: 899-902. [Crossref]
- Kohli SK, Pantazis AA, Shah JS, Adeyemi B, Jackson G, et al. (2007). Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: Time for a reappraisal of diagnostic criteria? Eur Heart J, 29: 89-95. [Crossref]
- Bellavia D, Michelena HI, Martinez M, Pellikka PA, Bruce CJ, et al. (2010). Speckle myocardial imaging modalities for early detection of myocardial impairment in isolated left ventricular non-compaction. Heart, 96: 440-447. [Crossref]
- van Dalen BM, Caliskan K, Soliman OI, Nemes A, Vletter WB, et al. (2008). Left ventricular solid body rotation in non-compaction cardiomyopathy: A potential new objective and quantitative functional diagnostic criterion? Eur J Heart Fail., 10: 1088-1093. [Crossref]
- van Dalen BM, Caliskan K, Soliman OI, Kauer F, van der Zwaan HB, et al. (2011). Diagnostic value of rigid body rotation in noncompaction cardiomyopathy. J Am Soc Echocardiogr., 24: 548-555. [Crossref]
- Pacileo G, Baldini L, Limongelli G, Di Salvo G, Iacomino M, et al. (2011). Prolonged left ventricular twist in cardiomyopathies: a potential link between systolic and diastolic dysfunction. Eur J Echocardiogr., 12: 841-849. [Crossref]
- Caselli S, Autore C, Serdoz A, Santini D, Musumeci MB, et al. (2012). Three-dimensional echocardiographic characterization of patients with left ventricular noncompaction. J Am Soc Echocardiogr, 25: 203-209. [Crossref]
- de Groot-de Laat LE, Krenning BJ, ten Cate FJ, Roelandt JR (2005). Usefulness of contrast echocardiography for diagnosis of left ventricular noncompaction. Am J Cardiol., 95: 1131-1134. [Crossref]
- Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, et al. (2005). Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol., 46: 101-105. [Crossref]
- Jacquier A, Thuny F, Jop B, Giorgi R, Cohen F, et al. (2010). Measurement of trabeculated left ventricular mass using cardiac magnetic resonance imaging in the diagnosis of left ventricular non-compaction. Eur Heart J., 31: 1098-1104. [Crossref]
- Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, et al. (2006). Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol., 48: 1977-1985. [Crossref]
- Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, et al. (2010). Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol, 56: 875-887. [Crossref]
- O'Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, et al. (2010). Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. Journal of the American College of Cardiology, 56: 867-874. [Crossref]
- Nucifora G, Aquaro GD, Pingitore A, Masci PG, Lombardi M (2011). Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur J Heart Fail, 13: 170-176. [Crossref]
- Stacey RB, Andersen MM, St Clair M, Hundley WG, Thohan V (2013) Comparison of systolic and diastolic criteria for isolated LV noncompaction in CMR. JACC Cardiovasc Imaging 6: 931-940. [Crossref]
- Sidhu MS, Uthamalingam S, Ahmed W, Engel LC, Vorasettakarnkij Y, et al. (2014). Defining left ventricular noncompaction using cardiac computed tomography. J Thorac Imaging, 29: 60-66. [Crossref]
- Grillone S, Nucifora G, Piccoli G, Gianfagna P, Hysko F, et al. (2013). Biventricular non-compaction demonstrated on multi-slice computed tomography with echocardiographic correlation. J Cardiovasc Med (Hagerstown), 14: 677-680. [Crossref]
- Lu JT, Campeau PM, Lee BH (2014). Genotype-phenotype correlation: promiscuity in the era of next-generation sequencing. Obstetrical & Gynecological Survey, N Engl J Med 48:1977-1985. [Crossref]
- Habib G, Charron P, Eicher JC, Giorgi R, Donal E, et al. (2011). Isolated left ventricular non-compaction in adults: clinical and echocardiographic features in 105 patients. Results from a French registry. Eur J Heart Fail, 13: 177-185. [Crossref]
- Thavendiranathan P, Dahiya A, Phelan D, Desai MY, Tang WH (2013). Isolated left ventricular non-compaction controversies in diagnostic criteria, adverse outcomes and management. Heart, 99: 681-689. [Crossref]
- Ichida F, Tsubata S, Bowles KR, Haneda N, Uese K, et al. (2001). Novel gene mutations in patients with left ventricular noncompaction or Barth syndrome. Circulation, 103: 1256-1263. [Crossref]
- Arndt AK, Schafer S, Drenckhahn JD, Sabeh MK, Plovie ER, et al. (2013). Fine mapping of the 1p36 deletion syndrome identifies mutation of PRDM16 as a cause of cardiomyopathy. Am J Hum Genet., 93: 67-77. [Crossref]
- Luedde M, Ehlermann P, Weichenhan D, Will R, Zeller R, et al. (2010). Severe familial left ventricular non-compaction cardiomyopathy due to a novel troponin T (TNNT2) mutation. Cardiovasc Res, 86: 452-460. [Crossref]
- Vatta M, Mohapatra B, Jimenez S, Sanchez X, Faulkner G, et al. (2003). Mutations in Cypher/ZASPin patients with dilated cardiomyopathy and left ventricular non-compaction. J Am Coll Cardiol, 42: 2014-2027. [Crossref]
- Sasse-Klaassen S, Gerull B, Oechslin E, Jenni R, Thierfelder L (2003). Isolated noncompaction of the left ventricular myocardium in the adult is an autosomal dominant disorder in the majority of patients. Am J Med Genet A, 119: 162-167. [Crossref]
- Probst S, Oechslin E, Schuler P, Greutmann M, Boyé P, et al. (2011). Sarcomere gene mutations in isolated left ventricular noncompaction cardiomyopathy do not predict clinical phenotype. Circ Cardiovasc Genet 4:367-374. [Crossref]
- Klaassen S, Probst S, Oechslin E, Gerull B, Krings G, et al. (2008). Mutations in sarcomere protein genes in left ventricular noncompaction. Circulation, 117: 2893-2901. [Crossref]
- Luxán G, Casanova JC, Martínez-Poveda B, Prados B, D'Amato G, et al. (2013). Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat Med, 19: 193-201. [Crossref]
- Monserrat L, Hermida-Prieto M, Fernandez X, Rodríguez I, Dumont C, et al. (2007). Mutation in the alpha-cardiac actin gene associated with apical hypertrophic cardiomyopathy, left ventricular non-compaction, and septal defects. Eur Heart J., 28: 1953-1961. [Crossref]
- Barth PG, Valianpour F, Bowen VM, Lam J, Duran M, et al. (2004). X-linked cardioskeletal myopathy and neutropenia (Barth syndrome): An update. Am J Med Genet A., 126: 349-354. [Crossref]
- Grothoff M, Pachowsky M, Hoffmann J, Posch M, Klaassen S, et al. (2012). Value of cardiovascular MR in diagnosing left ventricular non-compaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur Radiol., 22: 2699-2709. [Crossref]
- Captur G, Muthurangu V, Cook C, Flett AS, Wilson R, et al. (2013). Quantification of left ventricular trabeculae using fractal analysis. J Cardiovasc Magn Reson.15: 36.








