The field of ligand-targeted liposomes (LTLs) has expanded rapidly and many experiments have shed light on some of the factors involved in the successes and failures of ligand-targeted liposomes. Ligand-targeted liposomes have the potential to revolutionize the treatment of cancer. However, these highly engineered liposomes generate new problems, such as accelerated clearance from circulation, compromised targeting owing to non-specific serum protein binding, and hindered tumor penetration.
The search for the 'magic bullet in anti-cancer therapy, as proposed by Ehrlich in the early 1900's continues. This hypothetical agent has selective toxicity towards a target population of malignant cells, but not 'normal' cells. Unfortunately, there are a limited number of features that distinguish malignant from non-malignant cells and that can be exploited to increase the selectivity of chemotherapy. However, the differential over-expression of cell surface antigens and/or receptors opens up the possibility for antibody-directed therapy. Since the advent, by Kohler and Milstein, of methods to produce large-scale quantities of monoclonal antibodies (mAb's), there has been an increasing trend towards the use of antibodies to target therapeutic agents. Included in the growing list of agents that have been conjugated to mAb are toxins and liposomes. Antibodies were the first ligands that were coupled to liposomes and the resulting formulations were named immunoLiposornes. More recently, other ligands, such as peptides that can bind to tumor specific antigens have also been used. Collectively, liposomes that have had a ligand coupled to their surface are referred to as 'ligand targeted liposomes', or abbreviated to 'targeted-liposomes'. The development of these formulations has focused on the achievement of certain characteristics that constitute the 'ideal targeted-liposome'. The first requirement is a rapid and simple method to couple ligands to the liposome surface. In this regard, it is important to consider the labile nature of some ligands, particularly antibodies, and potentially some liposome components. This means that they will not endure lengthy, multi-step protocols. Secondly, the chosen coupling method needs to be efficient to minimize wastage of ligand and coupling lipid. In many cases the ligand is either expensive, or difficult to prepare (in the case of mAb), or both. In addition, because the coupling lipid introduces functional groups (vide infra) that may be reactive in vivo if left exposed (i.e. not conjugated to antibody); the minimum amount should be incorporated into the liposomes to avoid possible toxicities and potential for opsonization. Third, the antibody density should be optimized to achieve specific targeting and reasonable circulation times. In this regard, the pharmacokinetics of immunoliposomes have been well studied. Not surprisingly, the attachment of antibodies to liposomes accelerates the rate of liposome clearance from circulation. It is likely that the IgG acts as an opsonin, similar to its normal immunological function. Fortunately, the antibody density can be decreased to a level where reasonable circulation times and effective targeting cm be mutually achieved. For whole antibodies (MW=150 000 Da) the optimal density is generally between 30-60 µg/µmol liposome PL. However, the optimal ligand density will be affected by several ligand-dependent factors including its targeting effectiveness, and immunogenicity. Fourth, the ligand must retain the ability to bind to its target. An important consideration here is the preservation of the Ligand structure. AIso, the ligand must be accessible for target recognition. Fifth, the coupling method and the attachment of the chosen ligand should not affect the drug loading and retention characteristics of the resulting liposomes in a negative way (Figure 1) [1].
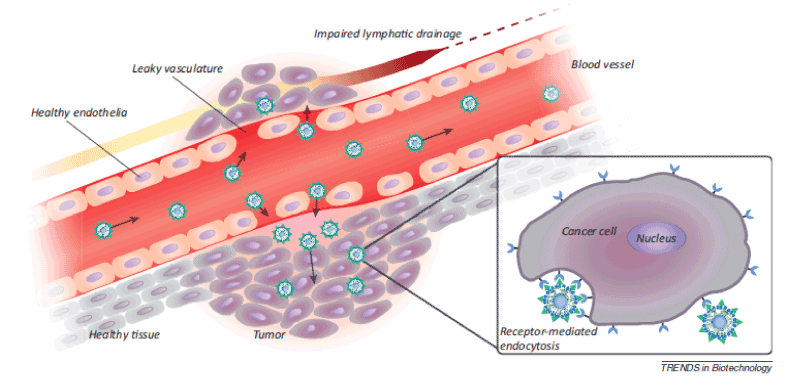
Figure 1. Passive targeting of nanoparticles to tumors through the enhanced permeability and retention (EPR) effect. Angiogenic vessels in rapidly growing tumors are abnormally constructed with large vascular fenestrae and impaired lymphatic drainage. As a result, particles <200 nm preferentially accumulate in the tumor interstitium. Post-extravasation, intracellular uptake of ligand-targeted liposomes is facilitated by receptor-mediated endocytosis [2].
The inherent target selectivity of Stealth liposomes, based on the preferential accumulation and leaking into the tumor vascular bed, can be dramatically enhanced by chemically coupling tumor-specific monoclonal antibodies or antibody fragments (stabilized immunoliposomes, SIL) or another targeting moiety (peptides, carbohydrates, glycopropteins, receptor ligands), i.e. essentially any molecule that selectively recognizes and binds to target antigens or receptors over-expressed or selectively expressed on cancer cells. The ability to selectively target liposomal anticancer drugs via specific ligands against antigens expressed on malignant cells could improve the antitumor activity of the liposomal formulations. Targeted liposomal delivery to cancer cells aims to increase the therapeutic efficacy at the target and to minimize nonspecific toxicities. In fact, the greater advantages of targeted liposomes encapsulating cytotoxic drugs over free drugs has been unquestionably demonstrated in a number of experimental models of malignancy, including neuroblastoma and melanoma [3].
Early in the history of liposomes, it was recognized that a means of increasing the selectivity of the interaction of liposomes with diseased cells was desirable. If this interaction triggered receptor mediated endocytosis of the liposome and its cargo into the desired cellular target, then so much the better. Antibodies were used in early experiments to mediate their specific attachment target cells, and receptor-mediated endocytosis of liposomes was demonstrated. At the same time, new methodologies were being developed for attaching antibodies to liposomes. Soon it was shown that antibody-targeted liposomes could improve the selective toxicity of liposomal anticancer agents to cultured cells. However, antibody-targeted liposomes were rapidly cleared from circulation, limiting their in vivo distribution to non-MPS tissues. Nevertheless, some in vivo uptake of liposomes could be demonstrated if the target cells were rapidly accessible from the circulation. After the development of long-circulating (PEG ylated) liposomes, it became apparent that, when antibodies were attached at the liposome surface, their antigen binding was masked by the presence of PEG in the same liposomes, particularly longer chain PEG molecules, although some accumulation of these liposomes could be demonstrated at target sites easily accessible from the circulation. Newer coupling methods were developed that involved the attachment of antibodies, their fragments, and other ligands to the terminus of PEG molecules engrafted to the liposome surface. In one early example, this resulted in improved in vivo survival in animal lung tumor model relative to non-targeted liposomal drugs. Overall, the methods for producing ligand-targeted liposomes are tedious, difficult to control, and lead to poorly defined systems that are often rapidly cleared from the circulation. The ‘post-insertion’ technique was developed to address these concerns. In this technique, micelles formed from PEG-linked ligands are incubated with pre-formed, drug-loaded, non-targeted liposomes (including commercial preparations) to convert them into ligand-targeted liposomes. The technique is proving to have wide applicability for introducing a variety of substances onto the liposome surface [4].
Cells communicate with other cells via a number of different cell-interactive molecules, such as cell surface receptors, cell adhesive molecules including those of the extracellular matrix, and small extracellular ligands. The specific interactions mediated by cell-interactive molecules, such as carbohydrates, have been adapted for more efficient liposomal delivery. Direct administration of carbohydrate-conjugated liposomes or ligand-conjugated liposomes targeted to carbohydrates expressed on cell surfaces face a number of unavoidable obstacles during blood circulation. Among these, contact with the RES and cellular internalization as was described in the section on immunoliposomes (Figure 1D) are critical concerns. The antitumor effects of 1-aminolactase-coupled liposomes containing Adriamycin were evaluated on AH66 rat hepatomas transplanted in nude mice. The uptake of these liposomes by the tumor cells was higher than that of liposomes that did not contain 1-aminolactose. Coupling of 1-aminolactose coupling to the liposomal surface resulted in increased the in vivo antitumor efficacy due to active targeting to the tumor cells mediated by the ligand. As described earlier, melanoma cells aberrantly express GM3 ganglioside and gastrointestinal cancer cells express LeX antigens on the cell surface. Immunoliposomes formulated with anti-GM3 antibodies were effectively targeted to the melanoma cells and anti- LeX immunoliposomes were also successfully targeted to human colonic adenocarcinoma cells in vivo. In a typical example of the application of carbohydrate–carbohydrate interaction to targeting, LeX-containing liposomes preferentially interacted with LeX antigens expressed on the surface of embryonal carcinoma cells. In addition, GM3-containing liposomes were found to be able to bind to Gg3-ceramide and Lactosyl-ceramide which are normally expressed in vascular endothelial cells. It was mentioned earlier that over-expressed folate receptors are appropriate targets for tumor-specific targeting. To date, folate-coupled liposomes have been successfully delivered to folate receptor-over-expressing cancer cells in vitro as well as in vivo. Transferrin has also been employed as a targeting ligand to direct liposomal drugs to various types of cancer cells in vivo. PEG-immunoliposomes with anti-transferrin antibodies coupled to the distal ends of the PEG preferentially associated with C6 glioma cells in vitro. When compared to other liposomal formulations, significantly increased gliomal doxorubicin uptake was observed after treatment with the tumor-specific long-circulating liposomes containing doxorubicin (Figure 2) [5].
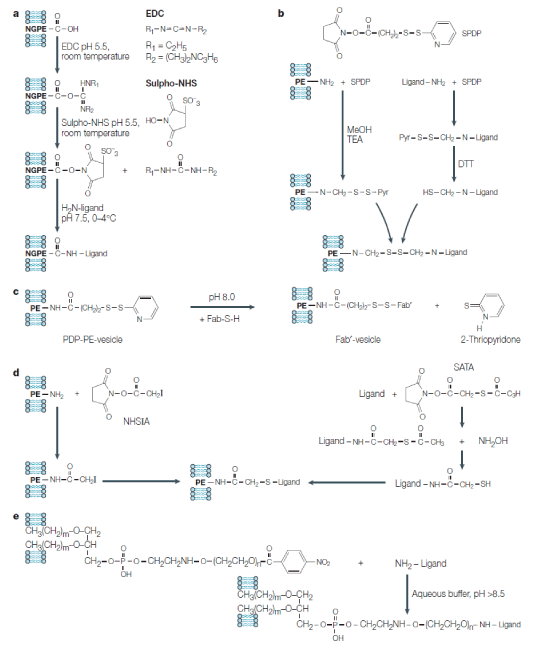
Figure 2. Chemical reactions to attach various ligands (antibodies) to the liposome surface (all these reactions can be used to directly attach ligands to the liposome surface or to attach ligands to liposomes via the PEG spacer):
A) Attachment of amino-group-containing ligands to liposomes via the liposome-incorporated reactive phospholipid N-glutarylphosphatidylethanolamine (NGPE). Water-soluble 1-ethyl-3(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysulphosuccinimide (sulpho-NHS) are used to activate NGPE for the interaction with amino-group of the ligand. B) Attachment of amino-group-containing ligands to amino-group-containing phospholipid (such as phosphatidylethanolamine (PE)) incorporated into the liposomal membrane using a heterobifunctional reagent, N-succinimidyl pyridyl dithio propionate (SPDP). Both amino-containing liposomal phospholipid and ligand are activated with SPDP, producing pyridyl dithio propionate (PDP) derivatives; the PDP group in the ligand is reduced with dithiothreitol (DTT) into the HS-group. Because of the interaction of activated liposome with activated ligand aromatic pyridyl sulphydryl (-S-Pyr) group of PDP-lipid is displaced with the aliphatic -SH group yielding a disulphide bridge between the liposome and the ligand. C) In a particular case of SPDP-mediated coupling, SH-group-containing Fab fragment (Fab-SH) of immunoglobulin G antibody can be directly attached to a PDP-containing liposome with the formation of a disulphide bridge. D) Attachment of thioacetate-activated ligand to the iodoacetate-activated liposome. The thioacetate group can be introduced into the amino-group-containing ligand (protein) by activation with succinimidyl-S-acetyl thioacetate (SATA) and converted into a free sulphydryl group by the subsequent treatment with hydroxylamine. The iodoacetate group can be introduced onto the liposome surface by the activation of amino-group-containing phospholipid with N-hydroxysuccinimido-iodoacetate (NHSIA). SH-containing ligand then interacts with iodoacetate-liposome. E) Attachment of amino-group-containing ligands to PEGylated liposomes via p-nitrophenylcarbonyl (p-NP) group on the distant terminus of the lipid-conjugated polyethylene glycol (PEG) chain. Direct interaction of the ligand amino-group with the liposomal pNP group yields carbamate (urethan) bond [6].
Naturally, occurring ligands to cell-surface receptors such as vitamins, hormones and growth factors may also be used as immuno-tolerant homing devices. They offer some potential advantages over antibodies, such as lack of immunogenicity and low preparation costs. It was shown that liposomes to which the epidermal growth factor (EGF) was covalently coupled could be targeted to hepatocytes expressing the EGF-receptor. As many carcinoma cells show a relatively high expression of the EGF-receptor, EGF-targeted liposomes may be useful for tumor targeting. Folate has also been studied as targeting ligand. The folate receptor has been identified as a surface marker in ovarian carcinomas. Receptor overexpression is found in many other types of cancer as well. Folate-targeted liposomes that specifically bind to cells that overexpress the folate receptor have been designed. When folate is directly attached to the phospholipid head groups or when a short spacer is used to couple folate with the bilayer, the folate-exposing liposomes are not recognized by the folate receptor. A long PEG spacer (MW 3,350) between the folate and the liposome surface is required for receptor recognition. Such folate-targeted liposomes are extensively internalized by receptor-mediated endocytosis after target cell binding. Incorporation of DOX resulted in 86-fold higher cytotoxicity to folate receptor-expressing tumor cells compared to non-targeted liposomes and 2.7-fold higher cytotoxicity relative to free DOX [7].
Inge V.R et.al they showed the liposomes composed of Egg phosphatidylcholine (EPC), cholesterol, egg phosphotidylglycerol (EPG), and Maleimidophenyl butyryl phosphatidylethanolamine (MPB-PE), the RI7217 Transferrin Receptor anti-TfR antibody is a suitable ligand for targeting to the brain in vivo. Uptake in the brain capillaries was up to 10 times higher compared to untargeted liposomes, and uptake in the brain parenchyma was up to 4.3 times higher. These results show the importance of liposomes and the transferrin receptor for targeting to the brain in vivo. Potential CNS drugs with poor Blood-brain barrier (BBB) penetration can be encapsulated inside the liposomes. In this way, RI7217-coupled liposomes could increase uptake of CNS drugs into the brain [8].
Effective targeted liposomal delivery systems require that both targeting specificity and liposome longevity be addressed. Our data indicate that peptides are capable of directing liposomes to receptors expressed on pathologically stimulated vascular cells such as platelets. Liposomes bearing an RGD (arginine–glycine–aspartic acid) peptide bound to activated platelets, while apparently avoiding platelet aggregation due to modest affinity of the peptide. With respect to oligosaccharide surface modifications, liposome clearance rates were sensitive to changes in vesicle diameter caused by incorporation of surfactants into the lipid bilayer. Therefore, in the design of liposomal surface modifications meant to alter vesicle circulation lifetimes, molecular geometry must be carefully controlled to provide sufficient surface coverage without disrupting vesicle structure [9].
Ligand-targeted therapeutics (LTTs) are a successful means of improving the selective toxicity of anticancer therapeutics. A radio-immuno-therapy, an immune-toxin and an immune-conjugate have received clinical approval and over 100 ligand-targeted therapeutics are currently in clinical trials. Recent advances in antibody engineering have allowed humanized or fully human antibody fragments to be made, which will reduce problems with immune responses against mouse antibodies. Phage-display techniques allow the selection of new targeting moieties that have high affinity for the selected target. The choice of targeting ligand can be crucial to the success of targeting applications. Variables that must be considered include the degree of receptor expression; whether the ligand is internalized or not; choice of antibody, antibody fragments or non-antibody ligands; and binding affinity of the ligand. New approaches to LTTs include the use of cross-linked antibody fragments, bispecific antibodies and fusion proteins that carry both the targeting moiety and the therapeutic moiety in the same molecule. The principles of LTTs can also be applied to micro-reservoir systems such as liposomes and polymers. Targeting of micro-reservoir systems can significantly increase the number of therapeutic molecules that can be delivered per targeting molecule and can allow sustained release of the therapy over time. More basic research needs to be done to understand how to optimize factors such as drug-release rates and pharmacokinetics and biodistribution, and also to understand the mechanisms behind some of the side effects that are caused by some classes of LTTs. Important issues that need to be addressed include what are the best ways to test LTTs in the clinic, given that they might have their best responses in an adjuvant setting, and how to resolve non-clinical considerations that surround the complex intellectual-property rights in this field. The principles of LTTs can also be applied to the targeted delivery of gene medicines such as antisense oligonucleotides [10].
- Debbie LI (2002) A novel method to prepare ligand-targeted liposomal drugs for clinical applications. spring, Canada pp.18-20.
- Gavin TN, Jared FS, Jonathan DA, Tanyel K, Basar B (2014) Ligand-targeted liposome design: challenges and fundamental considerations. Trends Biotechnol 32: 32-45.
- Daniela DP, Fabio P, Chiara B, Danilo M, Monica L, et al. (2008) Drug delivery systems: application of liposomal anti-tumor agents to neuroectodermal cancer treatment. Tumori 94: 246-253.
- Theresa MA, Pieter RC (2013) Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev 65: 36-38.
- Yong SP (2002) Tumor-directed Targeting of Liposomes. Biosci Rep 22: 267-281.
- Vladimir PT (2005) Recent Advances with Liposomes as Pharmaceutical Carriers. Nat Rev Drug Discov 4: 145-160.
- Enrico M (2001) Targeted Liposomes for Cytosolic Drug Delivery to Tumor Cells, FEBODRUK BV Enschede the Netherlands p. 43.
- Inge VR, Enrico M, Gert S, Wim EH, Raymond MS (2011) Targeted liposomes for drug delivery across the blood-brain barrier. Ipskamp Drukkers BV (Ed). The Netherlands pp: 74-75.
- Brian JL, Sharon MS, Zhong X, Matthew SS, Nancy JR, et al. (2002) Surface modification of liposomes for selective cell targeting in cardiovascular drug delivery. J Control Release 78: 235-247.
- Theresa MA (2002) Ligand-Targeted Therapeutics in Anticancer Therapy. Nat Rev Cancer 2: 750-763.


