Mechanical left ventricular assist device implantation (LVAD) has become a well-established option for the treatment of patients with severe congestive heart failure. Infection of the driveline is considered the Achilles’ heel of this procedure and the reported surgical approach, by mean of topic and or driveline relocation, is characterized by a high recurrence rate. An alternative approach consisting in a two-stage surgical procedure with the use of antiseptic bath to eradicate the bacteria from the driveline is proposed. Also, a preventive early driveline replacement is suggested with an original technical solution here described.
L-VAD, driveline infection, pump cable relocation
Mechanical left ventricular assist device (LVAD) implantation has become a well-established option for the treatment of patients with severe congestive heart failure, not only as bridge to heart transplantation but also as destination therapy. Shortage of donors and improvement in the performance of assist devices have made the latter ones more popular, thus increasing the number of patients who will have the device for lifetime. The development of smaller axial flow devices, improved reliability and less invasiveness of the implantation procedure led to the reduction of complications such as bleeding and thromboembolism [1]. According to the Intermacs database, in the last decade the percentage of patients who received LVAD with continuous axial flow pumps as destination therapy increased constantly and reached 73% in 2017 [2]. However, when such devices are employed for longer periods, patients will be exposed to device related complications, including infections and in particularly of the driveline; indeed, the skin at the exit site of the driveline is considered the Achilles’ heel. Freedom from device related infection is approximately 75, 65 and 55% at 12, 24 and 36 months after implantation, respectively, and the majority of LVAD-specific infections are related to the driveline component with over 80% share (for more than 80%) [3]. Considering the risk factors for infection reported in different publications, all patients are actually at risk, regardless of age and gender; moreover, apparently, also the driveline material may play a role [2,4]. With time, the skin in proximity to the driveline exit site loses its characteristics because of foreign body reaction and trauma to the skin margins caused by cable movement and decubitus. Goldstein et al. attributed the high incidence of driveline infection in young patients to their active lifestyle and continuous displacement of the driveline [5]. Moreover, the constant use of disinfectant materials will cause continuous skin irritation and inflammation. As a result, the skin loses its mechanical barrier properties so that bacteria first colonize at the exit site and then with time spread along the driveline, even though antibiotic therapy is administrated. The presence of a foreign body such as the driveline will, most likely, make the attempt to eradicate bacteria with antibiotic therapy alone a mission impossible.
The different proposed surgical treatments and strategies, including driveline relocation with the use of omentum flap, often is not enough and is characterized by poor results. We propose a novel treatment consisting of a two-stage driveline relocation strategy with the attempt to eradicate the colonized bacteria from the driveline by means of an antiseptic solution “bath”.
A 56-year-old man, with non-ischemic dilated cardiomyopathy in advanced heart failure, underwent LVAD implantation (HeartMate 3, Thoratec Corp, Pleasanton, CA), as destination therapy in March 2017. The postoperative course was uneventful, so he was discharged and periodically followed-up at the outpatient clinic. An infection at the exit site of the driveline, presenting with skin inflammation and pus secretion, was detected for the first time 18 months after implantation (Figure 1A). Based on colture-derived antibiograms, specific antibiotic therapy was administrated, and the driveline exit site was treated in a conservative manner with antiseptic solutions. During a 20-month period, repeated culture samples were taken showing changes in the bacterial flora, first Klebsiella and then Pseudomonas aeruginosa requiring antibiotic therapy adjustments. Despite antibiotic therapy and topical management, the signs of infection got worse and fistula formation was seen along the driveline course (Figures 1B-1D). Considering the high recurrence rate of infection reported in the literature, we opted for an alternative surgical approach consisting in two stage driveline relocation.
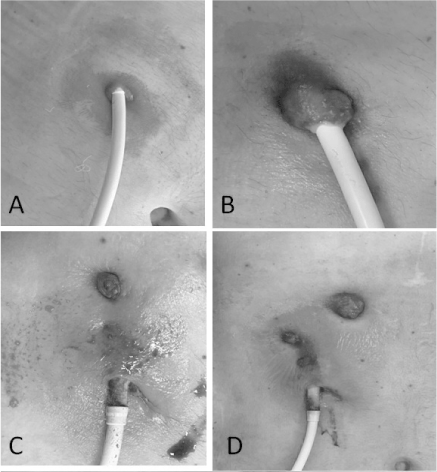
Figure 1. Images A-D showing infection spread along the driveline and fistula formation during 18, 23, 30 and 36 months, respectively, after device implantation. In images C and D the polyester cover can be seen because skin retraction
Forty-eight hours prior to surgery warfarin administration was interrupted and replaced with subcutaneous low molecular heparin. Monitoring included invasive arterial blood pressure measurement, EKG and peripheral oximeter and central vein catheter positioning for IV infusion and under general anesthesia, the power unit of the LVAD was disconnected temporally several times for short periods up to 3 minutes, to evaluate patient's hemodynamic conditions. After skin disinfection and drape positioning, an incision was made above the driveline course, away from the area of the fistula and where skin and subcutaneous tissue seemed to be intact and free from infection. The subcutaneous pump cable, just below the diaphragm was then exposed, disconnected from the console cable, disinfected with povidone iodine and pulled out, being careful to avoid damage to the cable as well as to the connector. The total exposed pump cable was inserted into a tube retrieved from the extracorporeal circuit, (a segment of the venous line with the largest diameter), in order to create a “bath”. The power unit was then reconnected, and the ends of the tube were sealed tightly with tape. Infusion lines were inserted in the proximal and distal ends for inflow irrigation and passive drainage respectively (Figure 2). The first surgical stage was completed by surgical debridement in order to eliminate the fistula and part of the driveline tunnel. To reduce tension, the subcutaneous layer was mobilized and sutured in away to ensure healing by primary intention. The exit site of the tunnel was not left open to allow residual drainage. The “bath” was filled with Povidone iodine 10% and continuous irrigation/washout was maintained with flow velocity of 1 L/24 h. The “bath" was kept horizontally so the cable was completely submerged. Two days later, the second surgical procedure was performed. A new tunnel was created with a diameter strictly matching the size of the driveline. Blood levels of leucocits, fibrinogen, C-reactive protein were constantly assessed, showing gradual improvements up to complete normalization. Antibiotic therapy continued for 6 weeks after surgery. No signs of infection were detected in both the old and new driveline exit sites at two moth’s follow-ups.
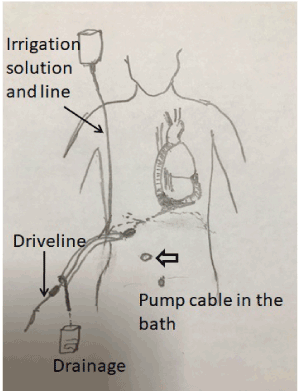
Figure 2. The design illustrates the irrigation drainage system, where the subcutaneous exposed pump cable segment, is immersed in PVC tube, "the bath". * indicate the previous driveline exit site
Bauer et al. in a systematic review, emphasised the difficulty in managing device related infections and in particular the lack of standardized surgical treatment of wound infections related to the driveline [3]. Performing surgery in a contaminated field, while maintaining also the same driveline, is challenging and resulting in a high recurrence rate.
The driveline consists of two separate cables: the pump cable, extending from the assist device and designed to be positioned in part in the subcutaneous layer, and the modular cable which connects via plug the pump cable to the system controller. To promote tissue growth, the pump cable is covered in part with woven polyester. Over time, tissue will bond to the textured material and anchor the driveline to the surrounding tissue; the purpose of the special cover is conceived to promote in growth tissue but when infection is occurred, most likely it will be difficult to eradicate the colonized bacteria from the woven polyester material, resulting in recurrence. There are different modalities to manage LVAD-specific infections, from conventional cloture-guided antibiotic therapy alone or associated with minor to radical treatment including driveline relocation and even pump replacement. Zierer et al. reported poor results following aggressive surgical treatment aimed at eradicating driveline infection, characterized by repeated revision procedures in the majority of the patients [6]. It should be emphasized that the success of procedures aiming at eradicating infection, which is impossible, since the contaminated pump cable cannot be replaced; such a condition could explain the high failure rates.
In this report we proposed to immerse completely, for a period of time, the pump cable in a “bath”, created from a PVC tube sealed in both ends. Continuous washout with aggressive antiseptic solutions might help to eradicate the bacteria deep-rooted in the woven material employed to cover the device. The principle of continuous irrigation with different solutions including povidone iodine, saline or antibiotics was applied to treat postoperative mediastinitis and was abandoned because of some limitations including large mediastinum space and the related risk of iodine intoxication and other approaches have been suggested [7,8]. Differently from the mediastinum irrigation, with the proposed “bath” the exposed cable is totally immersed and continuously washed. Since it is an extracorporeal treatment, there is no risk for intoxication; moreover, any antibacterial solution can be used. The high recurrence rate of infection following driveline relocation might negatively affect the process of decision making either of the physicians making it difficult to suggest surgical treatment, or of the patient himself, who could refuse the procedure based on information given. As in the presented case, radical surgery was performed with a great delay, 20 months after the first signs of infection were detected, and infection was spread along the driveline, putting pocket and device at risk.
It will be logical to add another connector to be located in the abdominal subcutaneous layer. When indicated, the subcutaneous connector can be exposed surgically, and the pump cable can be disconnected and pulled out, eliminating the contaminated driveline; a new sterile cable can be then inserted in a new clean tunnel, reducing the risk of recurrence. Considering the high incidence of infection in relation to the time since implantation, the question is more likely to be not if infection will occur but when. Early replacement and repositioning of a new cable as soon as infection signs occur can be done safely with low risk of infection. An earlier cable replacement will also reduce the risk of pocket and device infection. Finally, although rare, Stulak et al. reported repair of damaged cable, and Schima et al. also reported successful repair of a damaged outer sheath by means of expandable latex tubing [9]. Rather than artisanal repair, new pump cable segment and connector, will simplify the procedure, and eventually will guaranty long lasting restoration. Nonetheless, the proposed solution might reduce hospital readmissions, long lasting antibiotic therapy, improve the patients’ psychological state and economic costs.
While survival rates of end-stage heart failure patients have improved with the implantation of ventricular assist devices,LVAD-related infections are important cause of morbidity, mortality, and of hospital readmissions. The treatment of driveline infections remains a serious challenge, therefore it is of high priority to look for alternative solutions.
- Kirklin JK, Pagani FD, Goldstein DJ (2020) America association for thoracic surgery international society for heart and lung transplantation guidelines on selected topics in mechanical circulatory support. Heart Lung Transplant 39: 187-121. [Crossref]
- Kormos RL, Cowger J, Pagani ED (2019) The society of thoracic surgeons intermacs database annual report: Evolving indications, outcomes, and scientific partnerships. J. Heart Lung Trans 38: 114-126. [Crossref]
- 3 Bauer TM, Choi JH, Luc JGY (2019) Device exchange versus non-exchange modalities in left ventricular assist device-specific infections: A systematic review and meta-analysis. J. Artif. Organs 43: 448-457.
- Imamura T, Murasawa T, Kawasaki H (2017) Correlation between driveline features and driveline infection in left ventricular assist device selection. J. Artif. Organs 20: 34-41. [Crossref]
- Goldstein DJ, Naftel D, Holman W (2012) Continuous-flow devices and percutaneous site infections: Clinical outcomes. J. Heart Lung Trans 31: 1151-1157.
- Zierer A, melby SJ, Voeller RK (2007) Late-onset driveline infections the Achilles’ heel of prolonged left ventricular assist device support. Ann Thorac Surg 84: 515-520. [Crossref]
- Zeitani J, Pompeo E, Nardi P (2013) Early and long-term results of pectoralis muscle flap reconstruction versus sternal rewiring following failed sternal closure. European Journal of Cardio-Thoracic Surgery 43: 144-150. [Crossref]
- Stulak JM, Schettle S, Haglund N (2017) Percutaneous driveline fracture after implantation of the heartmate ii left ventricular assist device. ASAIO J 63: 542-545. [Crossref]
- Schima H, Stoiber M, Schloglhofer T (2013) Repair of left ventricular assist device driveline damage directly at the transcutaneous exit site. Artif Organs 38: 422-425. [Crossref]


