In the case of lesion of the peripheral nervous system, the treatment of choice is a microsurgical suture of the nervous stumps or, in case of a large nervous gap, an autologous nervous graft. Nowadays bioengineering is searching for new solutions for promoting axonal growth. A particularly interesting aspect is the role of tensile forces in the regeneration of the nerve fiber. In the seventies and eighties, Bray demonstrated that the application of mechanical tension, through glass microneedles applied on the axonal growth cone, promoted axonal elongation. Recently, Riggio et al. have proposed a new technique to create a tensive force on the axon: the dual-use of magnetic nanoparticles (MNPs) and an external magnetic field. This study represents the first animal experimentation of this innovative strategy. The lesion of the median nerve in the rat was used as an experimental model. This article presents the results of the preliminary phase of the study.
nervous regeneration, magnetic nanoparticles, micro/nanotechnologies
Neuronal regeneration represents one of the most actual scientific research topic [1], as peripheral nervous system lesions have a high incidence, are often disabling and represent an important social cost.
Nowadays the gold standard treatment for losses of nervous substance is still the autologous nervous graft. The success of this treatment is due to the presence of Schwann cells, who can recall the growing neuron and to create a microhabitat ideal for neuronal regeneration [2]. Anyway, nervous grafts have some limits: donor site damage, the use of small sensitive nerves to repair bigger and more complex nerves, the need for suturing the stumps of the graft that causes a negative fibroblast growth [3]. At the moment there are some alternatives to autologous nervous grafts, like tubulization using an autologous artery or vein or muscle in a vein or even homologous grafts harvested form cadavers [3-8].
Bioengineering research has focused on neuronal guides as an alternative to biological grafts. Neuronal guides work as stiff support imitating the structure of extracellular matrix (ECM) [9,10] and limiting the loss of growth factors and the local infiltration of scarring tissue [11].
Axonal elongation is an interesting mechanical phenomenon, which depends on the dynamic movements of the cytoskeleton and the development of tensile forces inside the nervous fibre [12].
In 1979, Bray indirectly demonstrated the existence of a tensive force in the elongating neuron [13] and 1984 demonstrated that the application of mechanical tension, utilizing glass microneedles applied on the axonal growth cone, promoted axonal elongation without reducing the diameter [14].
Recently, Riggio et al. [15] have proposed a new technique to create a tensive force on the axon: the dual-use of magnetic nanoparticles (MNPs) and an external magnetic field.
The current study has demonstrated that MNPs, after neuronal internalisation and under an external magnetic field, can develop a minimal tensile force (in the range of pN) that is easily controllable and is able not only to favor axonal growth but also to direct it in the same direction of the magnetic field.
The final aim of our experimental study is to demonstrate in vivo the capacity of MNPs and the magnetic force of stimulating and orientating axonal growth, using an experimental model the lesion of the rat median nerve.
This article reports the results of the preliminary phase of the study, demonstrating that MNPs are internalized both in Schwann cells and axons and do not interfere with natural nerve regeneration.
Animals
The Local Ethical Committee approved the experimental protocol according to the European Council Directive 86/609/EEC of 24 November 1986. Ten adult female Sprague Dawley rats, weighing between 320 and 450 g were included in this study. Animal housing was standardized such that animals were kept at controlled temperature and humidity with food and water ad libitum and with light /dark cycles of 12 hours/12 hours.
MNPs
Magnetic nanoparticles of iron oxide (Fe3O4) were synthesized by a modification of the oxidative hydrolysis method [16]. In particular, in situ polymer coating was achieved by adding polyethyleneimine (PEI, 25 kDa) during the reaction, as described in a previous work [17].
Surgical Procedure
The left arm of each animal was used as an experimental model, while the right one was used as a negative control. Rats underwent general anesthesia with an intraperitoneal injection of Zoletin (Tiletamina and Zolazepam) 40 mg/kg and Xilor (Xilazina) 7,5 mg/kg. All surgical procedure was performed using an operating microscope with a magnification of 40X (OPTIKA SZM-B) and microsurgical instruments. Before surgical incision, a topic anesthetic (EMLA cream) was used on the surgical site. After skin incision and exposition of the median nerve, a small amount of local anesthetic (Mepivacaine 2%) was injected in the nerve proximal to the level of neurotomy. Afterward, the nerve was divided with straight microsurgical scissors and a silicone tube (external diameter 2 mm, luminal diameter 1 mm and length 5 mm) was sutured to the proximal and distal stumps using a U-stitch with 9-0 nylon (Sharponit Black monofilament) on each side. Subsequently, the surgeon checked surgical hemostasis and the wound was sutured with simple interrupted stitches using 4-0 Vycril. The same procedure was performed in the left and the right arm, but in the left one, the solution containing MNPs with a concentration of 1,1 µg/ml was injected in the silicone tube (Figure 1).
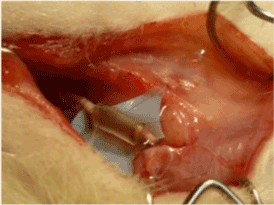
Figure 1. the solution containing MNPs with a concentration of 1,1 µg/ml was injected in the silicone tube
Then, animals were daily monitored to prevent possible skin ulcerations, wound diastasis or auto-mutilations. To study the different phases or nervous regeneration rats were divided into three experimental groups: in group 1 the removal of the silicone tube with the nervous stumps was performed a week after the implantation, in the group 2 two weeks after the implantation and group 3 four weeks after implantation.
At the moment of the removal, each rat was sacrificed, then on each side, the median nerve was exposed and divided proximal and distal to the silicon tube, so that the tube was removed with the two nervous stumps.
Histology
Explanted nerves were fixed in formalin for one hour and then dehydrated, cleared with xylene and embedded in paraffin. Sections of 14 µm were obtained employing a microtome. Some sections were stained with Prussian blue, following producer (Sigma-Aldrich, St. Louis, MO, USA) instructions for MNPs blue marking; the other sections were dipped for 5 minutes in toluidine blue 0,1% w/v in borate 0,1 % w/v, washed with water and mounted with Eukitt.
Prussian blue (also known as Berlin blue) is a blue pigment, light stable, produced by oxidation of ferrous ferrocyanide salts. Thanks to its affinity for iron, it is used to selectively stain in blue MNPs (that are made of iron).
All the animals survived and maintained good conditions until the end of the experiment. During the daily monitoring in the postoperative period, no altered behaviors or auto-mutilations or wound diastasis or skin ulcerations were observed. The results of the specimens removed after a week are reported below.
Under the optic microscope, it was evident that the nervous gap inside the tube had been filled with cells and biological matter after only seven days. Histologic sections were stained with Prussian blue, a pigment with high affinity for iron so that MNPs were stained in blue. Most of MNPs were found inside round cells placed around the nervous fiber. These cells presented histological features typical of Schwann cells (Figure 2a). MNPs were found also inside nervous fibre (Figure 2b). The histological results suggested that Schwann cells had migrated inside the conduit: in Figure 2c it was visible the front of the cell migration flow (evidenced by a yellow dashed line). At the proximal stump, it was evident the production of collagen fibers (Figure 2c, square 2). The MNPs that were not engulfed, had been moved away from the migration front (Figure 2c, yellow arrow). Some sections were stained with toluidine blue, a pigment that colors in dark blue the cell nucleus and in light blue the cytoplasm. It is especially useful for staining nervous tissue.
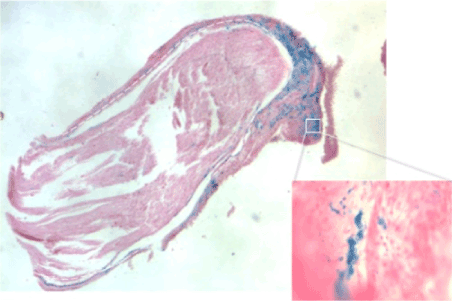
Figure 2a. histological features typical of Schwann cells
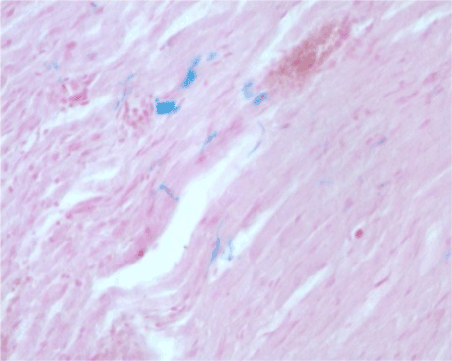
Figure 2b. MNPs found inside nervous fibre
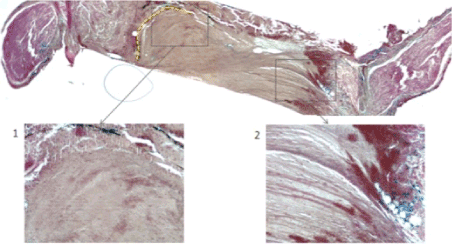
Figure 2c. The MNPs that were not engulfed, had been moved away from the migration front
Unfortunately, in this case, the stain was not specific enough for nervous fiber; in fact, nervous fibers were stained in blue, but also non-nervous cells inside the conduit appeared blue, even if slightly lighter (Figure 2d).
Figure 2d. nervous fibers were stained in blue, but also non-nervous cells inside the conduit appeared blue, even if slightly lighter
To mark more specifically nervous fibers and to define more accurately the state of nerve regeneration, the next sample will be prepared with immunochemistry using anti-neurofilament antibodies.
Use of a silicone tube for MNPs implant
No studies have described a strategy of MNPs application in vivo on a nervous lesion yet, so it was necessary to conceive a new system that keeps the transected axons in contact with MNPs and protects nervous regeneration. For this purpose, it was adopted a silicone tube sutured ti the nervous stumps, bridging the lesion. As previously demonstrated, nervous tubulization has numerous advantages for regeneration: the creation of a closed space that physically supports and guides nervous fibers across the nervous gap, limitation of axonal dispersion, storage of neurotrophic and neurotrophic factors and prevention of fibroblastic invasion. Moreover, silicone is elastic, relatively inert, cheap, easily available and easily to suture. Thanks to the presence of a closed space inside the tube, MNPs do not disperse and after a week they result significantly engulfed by nervous and Schwann cells.
The most important disadvantage of silicone, like all synthetic non-biodegradable materials, is the possible induction of foreign body reaction, formation of a large amount of scarring tissue and consequent chronic compression of the nerve [18].
In this study, a week after tubulization, it was observed the formation of fibrotic material around the tube, but it was not enough to compress neural stumps. In the case of future studies on the use of MNPs to enhance nerve regeneration in vivo, it would be useful to choose a tube of different materials like natural or synthetic biodegradable materials.
MNPs internalization
The preliminary phase of this study aims to evaluate the behavior of MNPs inside the nervous gap. The histological studies have evidenced that a week after application MNPs have been engulfed mainly by non-neuronal round cells, probably Schwann cells. Anyway, MNPs have been found also inside nervous fibers, even if in smaller quantities.
These results differ from previous in vitro studies where MNPs used to enhance nerve regeneration were quickly engulfed by neuronal cells PC12 by endocytosis, so MNPs were found also in the neuronal growth cone15. In consideration of the key role of Schwann cells in the regeneration process, this element could result positive. The magnetic force applied to MNPs engulfed by Schwann cells could be advantageous for nerve regeneration, stimulating the migration of these cells inside the nervous gap and favoring their alignment. Furthermore, thanks to modern techniques of MNPs functionalization, nanoparticles can be covered with functional groups specifically recognized by nervous cells, to enhance MNPs internalization.
Future
The next step of this study will be the application of an external magnetic force that will attract MNPs internalized in neuronal and Schwann cells in the proximal nerves stump, directing the growth of the nerve fibers along the longitudinal axis of the nerve towards the distal stump, guiding and accelerating the nerve regeneration process. With this aim, it is planned to create a magnetic bracelet for the foreleg of the rat. Anyway, to prevent eventual auto-mutilations of the rat, an alternative system could be the application of the magnetic field directly on the silicone tube using a magnetic tape around the tube.
The results of the in vitro study conducted by Riggio et al. [15] demonstrated that MNPs could be engulfed by nervous cells PC12 and that, under the influence of an external magnetic field, these particles created a tensive force able to enhance and direct axonal growth. In this way, it was confirmed that tension could be a powerful factor of stimulation for axonal elongation, just like in 1979 Bray had already demonstrated [14]. The force created with the dual use of MNPs and the magnetic field could be a new therapeutical instrument against peripheral nerve lesions. This study represents the first example of animal experimentation of this therapeutical strategy, using an experimental model the lesion of the median nerve of the rat. This article reports the results of the preliminary phase of the study. In this phase, MNPs were applied in the nervous gap inside a silicone tube sutured to the nervous stumps. The results of the histological studies of the tube and the nervous stumps harvested a week after implantation enables us to draw some important conclusions.
Firstly, MNPs were proved to not interfere with the normal regeneration process, as after a week the observed regeneration is compatible with the results of previous studies in the literature. Schwann cells migrate from the proximal stump following the disposition of collagen fibers.
Secondly, it was demonstrated that MNPs were engulfed mostly by Schwann cells around the nervous fiber, and in a smaller amount, they were internalized by nervous fibers.
To confirm these data, further investigation will be necessary: in particular immunochemistry with anti-neurofilament antibodies and examination under an electron microscope.
In the next phase of the experimental study, after the application of MNPs, nerve lesion will be exposed to an external magnetic field, to create a tensile force on the axons that have engulfed MNPs, favoring its growth. This strategy of stimulation of axonal regeneration could contribute to improving the treatment of peripheral and central nervous lesions.
The use of rats as an experimental model has also some limits. These animals are commonly adopted for experimental purposes, but their neuro-regenerative capacity is much more important than in the human being.
Finally, it is important to highlight that nanotechnology is taking its first steps, so many aspects, in particular, nanotoxicology should be further investigated to use MNPs safely.
- Isaacs J (2010) Treatment of acute peripheral nerve injuries: current concepts. J Hand Surg Am 35: 491-492.
- Sinis N, Schaller H, Schulte-Eversum C (2007) Comparative neuro tissue engineering using different nerve guide implants. Acta Neurochir Suppl 100: 61-64.
- Merolli A, Masciangelo M, Morini A (2011) La rigenerazione dei nervi periferici mediante guide neurali artificiali. l’applicazione clinica e la ricerca sperimentale GIOT 37: 198-205.
- Marcoccio I, Vigasio A (2010) Muscle-in-vein nerve guide for secondary reconstruction in digital nerve lesions. J Hand Surg Am 35: 1418-1426.
- Dahlin LB (2008) Nerve injuries. Curr Orthop 22: 9-16.
- Brunelli G, Battiston B, Vigasio A, Brunelli G, Marocolo D (1993) Bridging nerve defects with combined skeletal muscle and vein conduits. Microsurgery 14: 247-251.
- Battiston B, Geuna S, Ferrero M, Tos P (2005) Nerve repair by means of tubulization: literature review and personal clinical experience comparing biological and synthetic conduits for sensory nerve repair. Microsurgery 25: 258-267.
- Neubauer D, Graham JB, Muir D (2007) Chondroitinase treatment increases the effective length of acellular nerve grafts. Exp Neurol 207: 163-170.
- Dahlin L, Johansson F, Lindwall C, Kanje M (2009) Future Perspective in Peripheral Nerve Reconstruction. Int Rev Neurobiol 87: 507-530.
- Lundborg G, Dahlin LB, Danielsen N (1991) Ulnar nerve repair by the silicone chamber technique. Case report. Scand J Plast Reconstr Surg Hand Surg 25: 79-82. [Crossref]
- Daly W, Yao L, Zeugolis D, Windebank A, Pandit A (2012) A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface 7: 202-221.
- Suter DM, Miller KE (2011) The emerging role of forces in axonal elongation. Prog Neurobiol 94: 91-101. [Crossref]
- Bray D (1979) Mechanical tension produced by nerve cells in tissue culture. J Cell Sci 37: 391-410.
- Bray D (1984) Axonal growth in response to experimentally applied mechanical tension. Dev Biol 102: 379-389.
- Riggio C, Calatayud MP, Giannaccini M (2013) The orientation of the neuronal growth process can be directed via magnetic nanoparticles under an applied magnetic field. Nanomedicine 2014: 1-10.
- Sugimoto T, Matijevic E (1980) Formation of Uniform Spherical Magnetite Particles by Crystallization from Ferrous Hydroxide Gels. J Colloid Interface Sci 74.
- Calatayud MP, Riggio C, Raffa V (2013) Neuronal cells loaded with PEI-coated Fe3O4 nanoparticles for magnetically guided nerve regeneration. J Mater Chem 1: 3607-3616.
- Merle M, Dellon A, Campbell J, Chang P (1989) Complications from silicon-polymer intubulation of nerves. Microsurgery 10: 130-133.





