Abstract
Dypsnea is a common and distressing symptom in patients receiving palliative care, with significant impact on patients and their carers. A variety of causes may be implicated in the development of dyspnea. Multiple inter-related factors contribute to the experience of breathlessness, of which, the presence of a substantial component of anxiety can make dyspnea more distressing, leading to the development of total dyspnea. It is therefore imperative that a palliative care physician be competent in the assessment and management of patients with dyspnea. In particular, the concept of total dyspnea and its application in management of dyspnea will be reviewed through this case study.
Keywords
Anxiety, Breathlessness, Dyspnea, Palliative care, Psychological distress, Total dyspnea
Introduction
Dyspnea, is an unpleasant sensation defined as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity” [1]. It is of considerable burden - with high prevalence in advanced cancer, heart failure and chronic lung disease that increase towards the end of lif [2,3], lead to significant healthcare utilization [4], and adversely impacts on patients’ quality of life [2].
Multiple physiological, psychological, social and spiritual factors interact to give rise to the experience of dyspnea. In this case study, in which significant psychosocial distress contributed to the experience of dyspnea, the management of breathlessness will be reviewed. In particular, the utility of the concept of total dyspnea, a useful framework that helps assess the impact of dyspnea and guide management strategies, will be explored [5].
Case Study
Mr C was a 55-year-old Chinese male with locally invasive esophageal cancer with metastasis to lung, left adrenal and lymph nodes. At diagnosis in April 2017, he had multiple complications including hypercalcemia and dysphagia on nasogastric tube feeding. There was malignant infiltration of the trachea on bronchoscopic evaluation, but no intervention was performed initially as patient was asymptomatic at that point and planned for palliative chemotherapy.
In May 2017, he was readmitted for right upper limb venous thrombosis. During the same admission he developed hemoptysis and stridor, requiring an emergent tracheostomy. Following which, he received 10 fractions of palliative radiotherapy in May 2017, followed by second line chemotherapy in July 2017. He was then referred to home hospice team for symptom management and psychosocial support at home.
Psycho-Social History
Married with a daughter in her twenties, Mr C worked in a food & beverage company. He shared a close relationship with his wife and daughter. They also received good support from his wife’s siblings who would help to care for patient during the period that his wife was away at work. There was no financial concern as he had adequate insurance cover.
Disease and Symptom Progression
Mr C made repeated visits to emergency department for breathlessness from mucous plugging due to copious tracheostomy secretions. His disease continued to progress, with development of tracheo-oesophageal fistula (TOF), right upper lobe invasion and left brachiocephalic venous thrombosis. Oesophageal stenting to occlude the TOF was considered but deemed unsuitable due to markedly narrowed oesophageal lumen. He was seen by the inpatient palliative medicine team for breathlessness. His wife and daughter were morphine-phobic as they had a relative with cancer who was perceived to have deteriorated after starting morphine. Hence, he was started on fentanyl infusion instead and eventually converted to a patch of 12 mcg/hour with use of oxycodone syrup as breakthrough, on discharge.
In view of rapid disease progression and multiple complications, option of best supportive care was offered. However, patient and family chose to pursue a trial of immunotherapy (nivolumab) in August 2017. He had to be readmitted after developing breathlessness during the first infusion of nivolumab. This was eventually attributed to superior vena cava obstruction (SVCO) and concomitant pneumonia in view of clinical findings of neck/bilateral upper limb swelling, as well as radiological evidence of infection.
He was started on a fentanyl infusion for his breathlessness while the pneumonia was treated with a course of intravenous antibiotics. Family declined the use of steroids for SVCO as they were concerned about the possibility of reducing the efficacy of immunotherapy. Primary oncologist felt that stenting might not be useful due to presence of brachiocephalic thrombosis. However, despite suboptimal symptom control as reported by patient and frequent breakthrough doses taken, patient’s daughter was averse to an increase of fentanyl and had stopped the administration of fentanyl purges on a several occasions when patient requested for it. She recounted multiple times when patient’s symptoms improved immediately after the doses were administered, and described patient as having an anxious pre-morbid personality. These led to a strong perception that his need for breakthroughs were predominantly psychological. As such, she felt that there was no need to further increase his fentanyl. Eventually, he was converted to a fentanyl patch of 37mcg/hr and discharged home with home hospice follow up.
The home hospice team continued to support the family at home. Further exploration with Mr C revealed that he was conflicted about his goals of care as he found it difficult to achieve both his desire for comfort and life prolongation. He saw the repeated admissions/interventions as burdensome and uncomfortable, and wished to consider best supportive care, yet because of his daughter (who still hoped that he would fight on), he felt he had to persevere with treatment and even downplay his symptoms. This gave rise to significant emotional distress. When this was shared with his daughter, she began to see how her father was suffering and recognized that relieving his symptoms was an important goal as well. She agreed to for a trial of steroids at home for the SVCO, which helped to improve patient’s dyspnea.
Patient eventually chose to receive a 2nd cycle of nivolumab but was admitted prior to that for seizures and hemoptysis. On that same night of hospitalization, he had an episode of massive hemoptysis and passed away.
Discussion
Impact of Dyspnea
Dyspnea affects a large population of patients, across a range of diagnoses from cancer to cardiorespiratory causes [2]. Among patients receiving hospice care, almost 50% was affected by breathlessness and a quarter of them was reported to be severe [6]. The prevalence and severity of breathlessness also increased significantly between 3 months and 1 month before death, particularly so in cancer patients [3]. It adversely impacts on the quality-of-life on individual patients and is associated with increased mortality and risk of in-hospital death [2]. Additionally, it poses significant burden on healthcare systems, with dyspnea being the 4th most common reason for palliative care patient to visit the emergency department in the last 6 months of life [7].
These were certainly evident in Mr C who experienced significant distress from breathlessness, made multiple emergency department visits in the last months of life and eventually passed away in hospital.
Pathophysiology and Perception of Dyspnea
3 main physiological abnormalities lead to the development of dyspnea: increased load requiring greater respiratory effort, an increase in proportion of respiratory muscle required to maintain a normal workload and an increase in ventilatory requirements. Of note, a patient may experience dyspnea even in the absence of known cardiopulmonary process, when dyspnea may arise from systemic effects of illness e.g. cachexia and asthenia [2].
Furthermore, the central nervous system (CNS) plays an important role in the perception of dyspnea as outlined in the neurophysiological model below. Firstly, the activation of areas of the brain such as the cortex and limbic system produces the sensation of dyspnea perceived by an individual. As the limbic system is involved in emotional processing, similar physiologic disturbances in respiration may be experienced differently. Correspondingly, the experience of dyspnea may be modulated by emotions and vice-versa [4,8]. In particular, anxiety play an important role in the development of dyspnea and in turn, can be exacerbated by its presence [2]. As such, it is important to assess for and address any element of anxiety as part of the holistic management of dyspnea. Secondly, the evidence demonstrating the role of endogenous opioids in ameliorating dyspnea and presence of opioid receptors in CNS and peripheries including the lung, form the basis for the use of opioids in dyspnea management [2,8].
Total Dyspnea
The concept of total dyspnea, first proposed by Abernethy and Wheeler, takes its origins from the total pain model. By identifying the patient’s experiences in the same four domains – physical, psychological, social and spiritual – the physician is able to formulate a global management strategy with appropriate interventions selected to address the issues in each realm [5].
Applying A Multidimensional Approach To Assessing Dyspnea
The following diagram (Figure 1) highlights the multidimensional experience of dyspnea for Mr C.
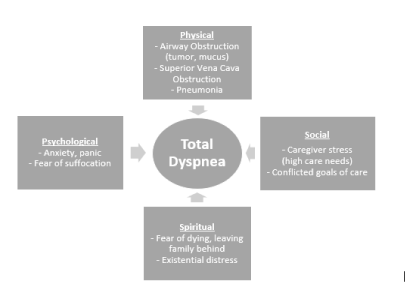
Figure 1. Multidimensional experience of dyspnea for Mr C, highlighting the various contributory factors in the physical, psychological, social and spiritual domains. (based on concept of Total Dyspnea by Abernethy et al [5])
In this case, Mr C had a malignant airway obstruction causing increased work load. Although it was bypassed by a tracheostomy, the presence of respiratory secretions with episodic mucous plugging contributed to his dyspnea. The subsequent development of SVCO and pneumonia further exacerbated his symptoms, through a combination of increased work of breathing and ventilator requirements.
His psycho-emotional distress, deepened the impact of dyspnea on him and his family. The acuity and intensity of his dyspnea, which he sometimes described as suffocating, heightened his anxiety. For example, he frequently worried about the not being able to clear his tracheostomy secretions in time, or that his oxygen concentrator was malfunctioning. As his daughter perceived the psychological distress to be the major source of his dyspnea, she saw the palliation of his symptom using pharmacological means as inappropriate which further made him worry that he would not be able to get the symptomatic treatment when he needed it.
While he received good support and shared close relationships with his family, his symptoms and increasing care needs (tube feeding, tracheostomy management, subcutaneous injections etc) placed tremendous caregiving strain on his family. Also, he had differing goals of care from his family and not being to reconcile the goals intensified his suffering. During periods of severe breathlessness, Mr C would often experience a fear of dying and leaving his family behind. This existential distress exacerbated the accompanying anxiety and may have worsened his sense of dyspnea.
Brief Review of Dyspnea Management
Whenever possible and appropriate, symptoms should be alleviated through disease-modifying therapies that target the underlying cause of dyspnea. In parallel, measures aimed at symptomatic relief should be implemented and these can broadly classified into pharmacological versus non-pharmacological measures [9].
Pharmacological Measures
Opioids
Opioids are the mainstay for pharmacological relief of dyspnea. There are several postulated mechanisms – affecting the responses of brainstem respiratory centres to different ventilatory stimuli, blunting of perceptual sensitivity to sensations of breathlessness via modulation of central processing as well as acting on peripheral opioid receptors in the lung, vasodilatory effect on pulmonary vasculature and an anxiolytic effect [8-10]. While there is evidence for a small clinically significant effect for oral and parenteral opioids compared to placebo as reported by a 2016 Cochrane review, the strength of evidence is limited by small sample sizes and relatively short duration of studies. Despite the presence of peripheral opioid receptors in the lung, there is insufficient evidence for the efficacy of nebulised opioids over placebo [10]. The evidence pool for fentanyl is even more limited, mainly from observational studies of small sample sizes [11].
Some clinicians may have concerns about significant respiratory depression, but a systematic review found no serious adverse effects in patients with advanced COPD given opioids for breathlessness [12]. Additionally, another study found that low dose opioids (MEDD <30 mg morphine) were not associated with increased admissions or deaths and would be safe for symptom reduction in severe respiratory disease [13].
Benzodiazepines
Because of the frequent co-existence of anxiety in dyspneic patients, benzodiazepines may have a role in its management. A Cochrane review did not find a beneficial effect of benzodiazepines for relief of breathlessness but this was based on small number of studies with limited participants [14]. However, a 2006 trial by Navigante showed a modest benefit in reducing dyspnea intensity and breakthrough dyspnea when midazolam was used with morphine, in a terminally ill population with less than a week’s prognosis [15]. Overall, benzodiazepines should not be used as first line therapy for dyspnea but can be considered as an adjunct to opioids or if used mainly for anxiety.
Oxygen Therapy
The benefit of oxygen supplementation in presence of significant hypoxemia has been demonstrated, especially in patients with COPD. The landmark trial by Abernethy in 2010, however, found no additional symptomatic relief of oxygen over room air for relieving refractory dyspnea in patients with life-limiting illnesses and less severe hypoxemia (PaO2 >55 mmHg). Both dyspnea and quality of life improved over study period for both oxygen and room air, suggesting that benefit may be derived from sensation of moving air alone. Of note, those with higher baseline dyspnea benefited most, and most improvements occurred within the first 72 hours [16]. As such, use of palliative O2 in the absence of hypoxemia should not be routine; if used, potential benefit versus burden and costs should be considered carefully and to be discontinued if no benefit is derived after a trial [9].
Non-Pharmacological Measures
Hand-held Fan
The use of a hand-held fan to direct airflow across the nose and face for 5 minutes (compared to blowing towards their leg) was shown in a randomized cross-over trial to reduce sensation of breathlessness. It is a simple, cheap and generally acceptable measure to relieve dyspnea, that may also help patient maintain a sense of control. Providing a brief explanation explaining the possible mechanisms for its effectiveness, proper instruction and demonstration are thought to be important as well [4,17].
Breathing Techniques, Positioning and Energy Conservation
Various breathing techniques such as pursed lip breathing, breathing control and paced breathing etc are recommended in guidelines and can be taught to patients and caregivers [4]. While the effects on breathlessness and quality-of-life were inconsistent, functional exercise capacity (measured by 6 minute walk test) improved compared to no intervention in COPD patients, as reported in a Cochrane review [18]. Similarly, specific positions such as forward lean sitting can be useful in relieving in relieving breathlessness and are recommended in guidelines as well. Energy conservation techniques help patients pace and adapt their activities in an efficient way, and allows effective self-management of breathlessness [4].
Psychotherapeutic Interventions
Cognitive-behavioural and self-management techniques may empower patients to develop mastery over their symptom. In particular, it has been shown to improve breathlessness, anxiety and depression and reduce health-care utilization in COPD patients [4].
Others
There are many other non-pharmacological measures used to manage dyspnea including neuromuscular electrical stimulation, acupuncture, and music therapy etc. to more complex multicomponent pulmonary rehabilitation (in COPD patients), which are outside the scope of this discussion [19].
Multidisciplinary Interventions
Breathlessness support services have been increasingly advocated as multifaceted interventions to manage the multidimensional nature of dyspnea. Underpinned by a palliative care approach, it uses evidence-based non-pharmacological and pharmacological interventions to support patients with advanced disease in an integrated fashion. It has been shown in randomized controlled trials to improve quality of life, reduce symptom impact and support carers [4,20].
Managing Mr C’s Multidimensional Dyspnea
Having holistically assessed the different factors contributing to Mr C’s experience of dyspnea, it is imperative to address each of them. Addressing only the physical aspects alone was unlikely to significantly alleviate the suffering he was experiencing.
Physical factors were addressed with secretions management (suctioning, reducing feeds and treating infections etc.), steroids for SVCO, supplemental oxygen in view of hypoxia, and use of opioids. Patient and family were also taught positioning and energy conservation measures by the physiotherapist. The use of adjunctive anxiolytics such as on-demand lorazepam was considered, given the significant degree of anxiety patient experienced. This was discussed with his daughter who declined as she felt that psychological distress should not be managed with medications.
Mr C’s psycho-emotional distress was further explored by the team, including a senior counsellor. He was found to be conflicted about his goals of care, which made him distressed. He found it difficult to discuss his wishes with his family who he could tell was very fixated on “fighting on” even if it came at the price of poor symptom control. In fact, when asked how she felt about patient’s breathlessness, his daughter verbalized that the “focus should be on targeting the cancer, and not the symptoms”. As such, the team helped to facilitate discussions between patient and his family. In particular, patient was allowed to articulate his emotions and wishes including a desire for better symptom control. It gave him a sense of assurance that his family would not compromise on his symptom control, having heard him voice his suffering. This helped to attenuate the sense anxiety that usually accompanied his bout of breathlessness.
Mr C had progressive cancer with multiple medical complications. There were many important physical factors leading to dyspnea such as airway obstruction from tumor and respiratory secretions, intervening pneumonia and SVCO (from both malignant compression and intravascular thrombosis) which mandated prompt medical interventions on many fronts. The medical complexity might potentially leave the medical team fixated on palliating the physical aspects and miss the opportunity of addressing problems in other dimensions given the rapid progression. Eventually, it became apparent that Mr C’s breathlessness was multidimensional in nature, with significant pyscho-social-spiritual components that needed to be addressed. Indeed, when some of these issues, in particular the conflicted goals of care, were addressed, patient’s breathlessness improved. By applying the framework of total dyspnea, suffering in all domains can be holistically assessed and an encompassing management strategy can be formulated.
By the same token, managing the complexities of dyspnea require a multi-pronged approach. Given the growing evidence for breathlessness support services, a service that delivers the multiple evidence-based measures in an integrated fashion may be of relevance locally. These services are based in the ambulatory setting and designed to be administered over 2-12 weeks; while it may not be suitable for patients near end-of-life, it could potentially attenuate the suffering experienced by those who continue to live with the debilitating effects of breathlessness, for example in patients with advanced COPD who suffer from frequent bouts of exacerbations and hospitalisations.
Conclusion
Dyspnea is complex, multidimensional symptom that warrants a holistic assessment to search for contributory factors beyond just the physical component. Frequently, the presence of related psychological, social and spiritual issues exacerbates the suffering associated with dyspnea and heightens its debilitating effects on patients and their families/caregivers. Without addressing the inter-related issues, the patient’s suffering may not be adequately alleviated. The concept of total dyspnea provides a useful framework for palliative care providers when caring for patients experiencing dyspnea and prompts a thorough assessment. It is only when a patient’s dyspnea can be managed in totality that we can hope to provide some relief for their multifaceted distress.
Declarations
Competing Interest: The author declare that he has no competing interests.
Ethical Approval: Not applicable
Funding Information: This study received no funding / grants.
References
- https://www.atsjournals.org/doi/abs/10.1164/rccm.201111-2042ST#readcube-epdf
- Kamal AH, Maguire JM, Wheeler JL, Currow DC, Abernethy AP (2011) Dyspnea review for the palliative care professional: assessment, burdens, and etiologies. J Palliat Med 14: 1167-1172. [Crossref]
- Currow DC, Smith J, Davidson PM, Newton PJ, Agar MR, et al (2010) Do the Trajectories of Dyspnea Differ in Prevalence and Intensity By Diagnosis at the End of Life? A Consecutive Cohort Study. J Pain Symptom Manage 39:680-690.
- Chin C, Booth S (2016) Managing breathlessness: a palliative care approach. Postgrad Med J 92: 393-400. [Crossref]
- Abernethy AP, Wheeler JL (2008) Total dyspnoea. Curr Opin Support Palliat Care 2: 110-113. [Crossref]
- Kutner JS, Kassner CT, Nowels DE (2001) Symptom burden at the end of life: hospice providers’ perceptions. J Pain Symptom Manage 21: 473-480. [Crossref]
- Barbera L, Taylor C, Dudgeon D (2010) Why do patients with cancer visit the emergency department near the end of life? CMAJ 182: 563-568. [Crossref]
- Elia G, Thomas J (2008) The symptomatic relief of dyspnea. Curr Oncol Rep 10: 319-325. [Crossref]
- Kamal AH, Maguire JM, Wheeler JL, Currow DC, Abernethy AP (2012) Dyspnea review for the palliative care professional: treatment goals and therapeutic options. J Palliat Med 15: 106-114. [Crossref]
- http://doi.wiley.com/10.1002/14651858.CD011008.pub2
- Simon ST, Köskeroglu P, Gaertner J, Voltz R (2013) Fentanyl for the relief of refractory breathlessness: a systematic review. J Pain Symptom Manage 46: 874-886. [Crossref]
- Ekström M, Nilsson F, Abernethy AA, Currow DC (2015) Effects of Opioids on Breathlessness and Exercise Capacity in Chronic Obstructive Pulmonary Disease. A Systematic Review. Annals Am Thorac Soc 12: 1079-1092. [Crossref]
- Ekström MP, Bornefalk-Hermansson A, Abernethy AP, Currow DC (2014) Safety of benzodiazepines and opioids in very severe respiratory disease: national prospective study. BMJ 348: g445.
- http://doi.wiley.com/10.1002/14651858.CD007354.pub3
- Navigante AH, Cerchietti LCA, Castro MA, Lutteral MA, Cabalar ME (2006) Midazolam as adjunct therapy to morphine in the alleviation of severe dyspnea perception in patients with advanced cancer. J Pain Symptom Manage 31: 38-47. [Crossref]
- Abernethy AP, McDonald CF, Frith PA, Clark K, Herndon JE, et al (2010) Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. The Lancet 376: 784-793. [Crossref]
- Galbraith S, Fagan P, Perkins P, Lynch A, Booth S (2010) Does the use of a handheld fan improve chronic dyspnea? A randomized, controlled, crossover trial. J Pain Symptom Manage 39: 831-838. [Crossref]
- Holland AE, Hill CJ, Jones AY, McDonald CF (2012) Breathing exercises for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 10: CD008250. [Crossref]
- Bausewein C, Booth S, Gysels M, Higginson I (2008) Non-pharmacological interventions for breathlessness in advanced stages of malignant and non-malignant diseases. Cochrane Database Syst Rev 16: CD005623. [Crossref]
- Bausewein C, Schunk M, Schumacher P, Dittmer J, Bolzani A, et al (2018) Breathlessness services as a new model of support for patients with respiratory disease. Chron Respir Dis 15: 48-59. [Crossref]

