Introduction
Vesicovaginal fistula (VVF) is the most common subtype of female urogenital fistula. It consists in an atypical connection (fistulous tract) between the bladder and the vagina that allows continuous leakage of urine into the vaginal vault. VVF has been known since ancient times. In 1663, Hendrik von Roonhuyse first described surgical repair. First reports of successful repairs emerged during the 19th century James Marion Sims published his now famous surgical series describing his method of surgical treatment of VVF using silver wire in a transvaginal approach. But it was not until his 30th attempt at closure of VVF that he achieved success.
We present a case of VVF and a review of literature with focus on epidemiology, etiology, classification, medical presentation, diagnosis and management.
Case report
The patient, 38-year-old womanhad undergone laparoscopic total hysterectomyin June 2017, due to myoma and metrorrhagia. She was referred to our hospital in September 2017by the Gynecology group because of continuous and abundant urinary loss through the vagina in the past 75 days.On physical exam, she had a Foley catheter for the past month, profuse amount of liquid in the posterior fornix. There was increase of leakage after valsalva maneuvers. It was possible to identify the fistulous orifice at the anterior vaginal wall, close to the anterior fornix.
At this point, the Foley catheter was replaced and urethrocystography (UCG) was performed. The patient return after 3 weeks without any urinary loss, and the UCG couldn’t show the fistulous path.With the Foley catheter withdraw, the patient remitted symptoms.
A corrective procedure was proposed, using Martius flap. We initiated with an urethrocystoscopy. The fistulous orifice, 5mm wide, was found above the vesical trigone (Figure 1). A hydrophilic guide wire was used to catheterize the orifice (Figure 2). The guide wire went through the vaginal orifice (Figure 3). It was followed by a 10 Fr Foley silicone catheterization (Figure 4). After inflating the Foley balloon, it was used for traction in order to expose and facilitate the dissection process. A second Foley catheter was placed, for vesical drainage, as usual. Inverted “U” incision at the anterior vaginal wall was performed(Figure 5), followed by fistulous path dissection until bladder wall and excision. Bladder wall was closed in two layers, Martius flap (Figure 6 and 7) was interposed between the bladder and vagina (Figure 8). Vaginal wall was closed in two layers [1-5].
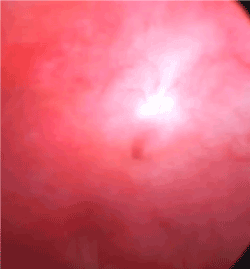
Figure 1: Cystoscopy shows fistulous orifice.
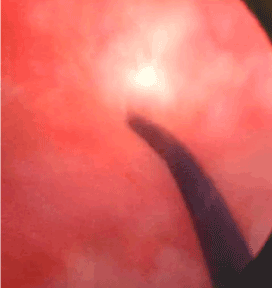
Figure 2: Fistulous path being catheterized by hydrophilic guide wire.
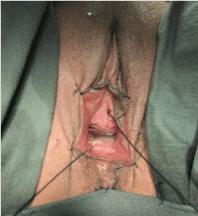
Figure 3: Guide wire at the fistulous path.
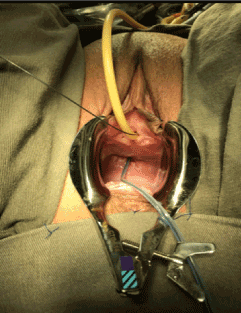
Figure 4: Silicone foley catheterization of the orifice.
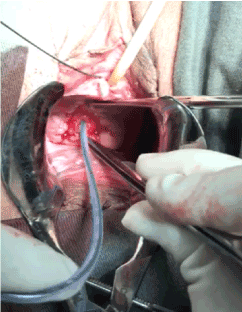
Figure 5: Inverted “U” incision at anterior vaginal wall.
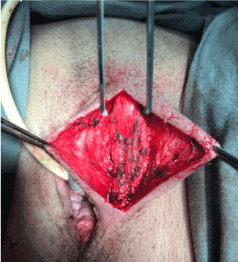
Figure 6: Labia majora incision and Bulbocarvenosus muscle dissection.
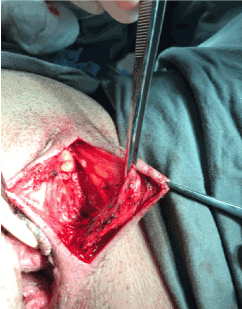
Figure 7: Flap mobilization.
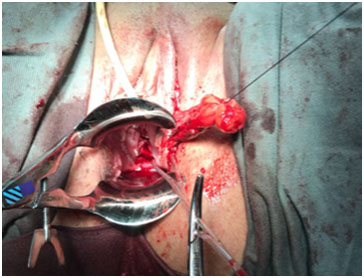
Figure 8: Tunnel creation below labia minora, followed by flap positioning and fixation.
The patient received hospital discharge on the second day, without complaints. She returns one week later, to check the operative wound. The Foley catheter was removed after two weeks.
A month after procedure, there were no symptoms relapse and the patient is satisfied with the results.
Background and epidemiology
VVF is an abnormal fistulous tract extending between bladder and vagina that enables continuous involuntary discharge of urine into the vagina. The incidence of VVF is decreasing all over the world in the past decades. Although, statistics suggest that there are over three million women with non-treated fistula and up to 130 thousand new cases every year on Africa. This data represents a major public health problem, as it impacts on quality of life.
The causes of VVF usually vary between developed and developing countries, as iatrogenic operatory vesical lesion respond for 75% of VVF in developed countries, a VVF case series in Nigeria suggest that 96% were related to labor issues [6-9].
Etiology
Developing countries: usually associated with labor trauma. It’s well known that cephalopelvic disproportion, especially if full pelvic growth isn’t achieved, can lead to genital tissue lesion. Prolonged labor can also determine genital lesion, usually related to larger time of tissue hypoxia, edema and necrosis.
Developed countries: the major cause of VVF is inadvertent bladder injury during pelvic surgery (90%). Commonly bladder wall laceration or suture placement can provoke a hematoma or necrosis, subsequently evolving with site infection. Gynecologic procedures are the most common iatrogenic factor (75%), followed by radiotherapy in 10% of cases. Other types of procedures (urologic, gastrointestinal) also contribute to the incidence of VVF[10-12].
There are some risk factors related to VVF such as prior pelvic or vaginal surgery, diabetes, arteriosclerosis, carcinoma, endometriosis, anatomic distortion, infection and cuff abscess.
Classification
VVF may be classified using Goh’s or Waaldijk’s system.
Goh’s system divides fistulas by anatomic localization, size and particular findings.
Type 1: Distal edge of fistula >3.5cm from external urinary meatus
Type 2: Distal edge of fistula 2.5–3.5cm from external urinary meatus
Type 3: Distal edge of fistula 1.5−<2.5cm from external urinary meatus
Type 4: Distal edge of fistula < 1.5 cm from external urinary meatus
(a) Size <1.5cm, in the largest diameter
(b) Size 1.5–3cm, in the largest diameter
(c) Size >3cm, in the largest diameter
i. None or only mild fibrosis (around fistula and/or vagina) and/or vaginal length > 6 cm, normal capacity.
ii. Moderate or severe fibrosis (around fistula and/or vagina) and/or reduced vaginal length and/or capacity.
iii. Special consideration e.g. postradiation, ureteric involvement, circumferential fistula, previous repair.
Waaldijk’s system is simpler, describing location and size.
Location | Type |
Not involving the urethral sphincter | I |
Involving the urethral sphincter | II |
• with partial involvement of the urethra | IIA |
• with (sub)total involvement of the urethra | IIB |
Fistula involving a ureter or other remote site | III |
Subtypes for IIA and IIB | Finding |
a | without a circumferential defect |
b | with a circumferential defect |
Size classification:
(1) Small
(2) Medium
(3) Large
(4) Extensive
Medical presentation
The most frequent clinical finding is uncontrolled leakage of urine through the vagina. It can be generally proceeded by pelvic procedure, radiotherapy or complicated labor. Recurrent cystitis, pyelonephritis and hematuria can also be present [12-17].
The time to present symptoms may vary according to the etiology. VVF caused by direct trauma (laceration of the bladder, point of suture) will develop symptoms in a short period of time (7-30 days). A anterior vaginal wall laceration associated with obstetric fistulas presents within 24h. In the other hand, fistulas due to radiation may present 30 days to 30 years later.
Workup
All investigation should initiate with a complete physical exam. The presence of fluid collection in the exam of the vaginal vault, along with the history can indicate further investigation. The fluid can be tested for urea, creatinine or potassium concentration to determine the probability of VVF. Also, urine should be collected for culture, and if positive, should be treated before surgical procedure.
Indigo carmine or Methylene blue dye can be used to confirm the presence of fistula.
After confirmation, differential diagnosis should be performed, in order to clarify the fistula origin (vesicovaginal, urethrovaginal, or ureterovaginal fistulas and fistula formation between the urinary tract and the cervix, uterus, vagina, vaginal cuff, or ureteral fistula to a fallopian tube). Therefore, urethrocystoscopy must be considered. The fistula orifice can be determined by catheterization with hydrophilic guide wire.
Patients with malignancy risk should be biopsied.
Radiologic studies should be performed previous to repair. Urethrocystography filling and voiding phases)will demonstrate the fistula path, while intravenous urogram (IVU) or abdominal computerized tomography could help to investigate ureteral fistulas. If ureteral fistula is highly suspected, retrograde ureteropyelography can be performed to complement investigation.
Management
The first and most important statement is that there is no preferable surgical approach. It may be defined including many variables, such as surgeon expertise, patient condition, type of fistula e duration.
In 10% of cases, fistula will close spontaneously after 2 weeks to 2 months of indwelling catheterization and anticholinergic medication. The conservative treatment is mostly indicated if fistula orifice is small (less than 2-3mm), prompt diagnosed and without epithelization. With the delayed diagnosis, it becomes necessary to fulgurate the fistula mucosa, followed by catheterization during 4 weeks.
As a complementary conservative treatment, fibrin sealant can be used as adjuvant therapy for larger fistula or if there is inflammation around the orifice.
If the VFF maintains patency after 3 weeks, it seems that conservative management will fail and surgical repair becomes necessary.
In cases of non-complicated fistula, early repair is advised, once there is no evidence that the time of repair modifies the outcome in long time studies and improves patient quality of life. On the contrary, in the management of obstructed labor-induced fistula, is accepted that surgical procedure must be postponed for 3-6 months if cystitis, isquemic tissue, edema or inflammation. Longer periods, 6-12 months, are recommended to treat radiation-induced fistula, which are frequently related to poor vascularization, endarteritis and prolonged inflammatory response.
Vaginal versus Abdominal approach: non-complicated fistula is well managed with vaginal repair and complex fistula is treated with abdominal approach or vaginal approach using flaps.
The vaginal approach has many advantages such as reduced morbidity, short operative time, minimal blood loss, reduced pain, possibility of posterior abdominal approach is not compromised, and concomitant vaginal procedures are possible. It shouldn’t be the first choice of repair if low bladder capacity and/or compliance, need of ureteral reimplantation, pelvic structures impairment or vaginal stenosis.
The abdominal approach is indicated for narrow vaginas, VVF close to the ureter meatus, large orifices (greater than 5cm), multiple orifices fistula, and need of other abdominal procedure, refractory and recidivist fistula. O’Connor technique is well accepted for the treatment of supra-trigonal VVF and is performed through suprapubic incision. The bladder is dissected extraperitonealy, followed by bipartition. The fistula’s orifice is indentified and dissected. After excision, a double layer suture with absorbable wire and tissue interposition is done.
Some modifications were added during time in order to improve successof the procedure, such as the tissue interposition. HenrichMartius described in 1928 a labial flap using the bulbocarvernosus muscle. This procedure increased cure rate up to 97% of distal VVF. Peritoneal flap, omental flap, Gracilis muscle flap, Rectus abdominis flap are other possible tissue for interposition.
The technological advance during the last decades created the opportunity of different strategies, such as laparoscopic and robotic approaches. They both are transperitoneal abdominal approaches. Many specialists advocate the benefits of minimally invasive techniques, showing reduced pos operatory pain, brief inpatient stay, lower blood loss and faster recover with same success rate.
Conclusion
VVF still is a public health problem, especially in developing countries. It brings major impact on quality of life of millions of patients.In some cases, if early diagnosed, can be treated with conservative measures. In the order hand, the delayed diagnostic along with complications may represent a clinical and surgical challenge.
References
- Wall LL (2006) Obstetric vesicovaginal fistula as an international public-health problem. Lancet368:1201-1209. [Crossref]
- Lee WJ, Smith AD, Cubelli V, Badlani GH, Lewin B, et al. (1987) Complications of percutaneous nephrolithotomy. AJR Am J Roentgenol 148: 177-180.[Crossref]
- Gerber GS, Schoenberg HW (1993) Female urinary tract fistulas. J Urol 149: 229-236.[Crossref]
- WC Keettel, FG Sehring, CA deProsse, Scott JR(1978)Surgical management of urethrovaginal and vesicovaginal fistulae. Am J ObstetGynecol 131:425-431.[Crossref]
- WJ Harris (1995)Early complications of abdominal and vaginal hysterectomy. ObstetGynecol Survey 50:795-805.[Crossref]
- HP Drutz, Mainprize TC (1988) Unrecognized small vesicovaginal fistula as a cause of persistent urinary incontinence. Am J ObstetGynecol 158:237-2401988.[Crossref]
- GoodwinWE, ScardinoPT (1980)Vesicovaginal and ureterovaginal fistulae: a summary of 25 years of experience. J Urol 123:370-374 1980.[Crossref]
- Davits RJ, MirandaSI (1991) Conservative treatment of vesicovaginal fistulae by bladder drainage alone. Br J Urol 68:155-156.[Crossref]
- BlaivasJG, HeritzDM, RomanziLJ (1995)Early versus late repair of vesicovaginal fistulae: vaginal abdominal approaches. J Urol 153:1110-1112.[Crossref]
- KristensenJK, LoseG (1994)Vesicovaginal fistulae: the transperitoneal repair revisited. Scand J UrolNephrolSuppl157:101-105 1994.[Crossref]
- Waaldijk K (1994) The immediate surgical management of fresh obstetric fistulas with catheter and/or early closure. Int J GynaecolObstet 45: 11-16.[Crossref]
- Waaldijk K (1995) Surgical classification of obstetric fistulas. Int J GynaecolObstet 49: 161-163.[Crossref]
- Waaldijk K (2004)The immediate management of fresh obstetric fistulas. Am J ObstetGynecolObstet191: 795-799.[Crossref]
- Nezhat CH, Nezhat F, Nezhat C, Rottenberg H (1994) Laparoscopic repair of a vesicovaginal fistula: a case report. ObstetGynecol 83: 899-901.[Crossref]
- Nabi G, Hemal AK (2001) Laparoscopic repair of vesicovaginal fistula and right nephrectomy for nonfunctioning kidney in a single session. J Endourol 15: 801-803.[Crossref]
- Sundaram BM1, Kalidasan G, Hemal AK (2006) Robotic repair of vesicovaginal fistula: case series of five patients. Urology 67: 970-973.[Crossref]
- Gupta NP, Mishra S, Hemal AK, Mishra A, Seth A, et al. (2010) Comparative analysis of outcome between open and robotic surgical repair of recurrent supra-trigonal vesico-vaginal fistula. J Endourol 24: 1779-1182.[Crossref]








