Background: Melanotic neuroectodermal tumor of infancy (MNTI) is a rare, pigmented tumor, usually located in premaxilla during the first year of life. The cranial bones are less frequently involved. They usually have aggressive growth with bone destruction and expansion to neighboring tissues. They may have relapses, but most are benign. The goal of this publication is to provide immunohistochemical findings in two cases.
Subjects and methods: The biopsies from A 16-month girl with a pigmented maxillary tumor and a 4 month baby with a tumor in the right temporal bone were analyzed in the bone pathology section of the pathologic anatomy Institute in the Central University of Venezuela. The tissues were processed and by the histopathologic analysis and the immunohistochemical study, the diagnosis of MNTI was done in both cases.
Results: The morphology of both tumors showed small round cells and large, pigmented epithelial cells. The immunohistochemical study showed positive expression for NSE in the round small cells component of both tumors. HMB45 and CKAE1-AE3 were positive in the epithelial component.
Conclusion: Regardless of whether most MNTI cases can show a benign course, its clinical, radiological, and histopathological characteristics mimic a malignant tumor. Given the rarity of these lesions, a deep understanding of the tumor by pediatricians, dentists, radiologists, and pathologists is needed to avoid unnecessary therapies. Immunohistochemical study is a valuable tool for diagnosing this tumor.
melanotic neuroectodermal tumor, inmunohistochemistry, pediatric cases
The melanotic neuroectodermal tumor of the infancy (MNTI) is an uncommon tumor observed in newborns, formed by a biphasic cell population of neuroblastic and pigmented epithelial cells [1]. MNTI was first described by Krompecher in 1918 as a congenital melanocarcinoma of the maxilla in a 2-month-old boy and was later designated as Melanotic neuroectodermal tumor of infancy by Borello and Gorlin in 1963. Other terms are melanotic adamantinoma, progonoma, retinal anlage tumor and pigmented epulis of infancy, which are no longer in use [1-5].
The most common anatomic locations are the craniofacial bones, represented by the maxilla (70%) and the mandible (10%) with an aggressive local behavior. Other locations include cranium 10%, brain 1% and involves less frequently extracranial locations such as the skin, uterus, epididymis, and mediastinum, representing 1 to 4% of the cases. More than 90% of the cases appear during the first year of life, with slightly predominance in males. However, few cases have been reported in adults [6,7].
MNTI clinically presents as a pigmented solitary rapidly growing mass in the maxillary anterior alveolar ridge with a smooth surface or intact mucosa. The symptoms may appear between the second week and the fifth month. Some cases could show-elevated urinary vanillylmandelic acid levels that eventually may decrease after treatment is started. The imaging studies show a poorly defined radiolucent area with bone destruction [8].
Histopathologic sections show a non-encapsulated lesion, with a biphasic pattern composed of neuroblastic and melanotic cells. Necrotic areas as well as mitosis are rare or absent. Immunohistochemical analysis shows multiphenotypic expression of tumor cells, being represented by neural, melanocytic and epithelial markers, but without photoreceptor differentiation. The large epithelial cell component is immunoreactive for cytokeratins, HMB45, and vimentin, with infrequent expression of epithelial membrane antigen (EMA). The small neuroblastic cell component shows positivity for neuron specific enolase (NSE) and CD57 (HNK1). The small cells may express, glial fibrillary acidic protein (GFAP) and desmin in focal areas [9-11].
Even though not currently relevant for diagnostic purposes, the electron microscopic study shows epithelial cells with ribosomes, Golgi apparatus, prominent rough endoplasmic reticulum immersed in a moderate cytoplasm with electron dense granules and variable amounts of prominent melanosomes and premelanosomes. The neuroectodermal cells exhibited round nuclei with dense chromatin, scarce cytoplasm and less developed organelles, as well as free ribosomes and glycogen granules. A basal lamina or desmosomes are not observed. A mature cell population presents large nuclei with abundant euchromatin and nucleoli. In addition, the cytoplasm shows dendritic-like processes and a moderate quantity of organelles, microfilaments, 20 nm (diameter) microtubules and dense secretory granules attached to the membrane [12,13]. The purpose of the present study was to perform an histological and immunohistochemical study on the neoplastic cell population in two cases of MNTI.
Two cases diagnosed at the Bone pathology section and approved by the Technic Council of the Pathological Anatomy Institute, Faculty of Medicine, Central University of Venezuela were included for diagnosis and immunohistochemical analysis. The material was fixed (10% buffer formalin), paraffin embedded, sectioned and routinely stained for histopathologic examination. In addition, special stains (PAS and trichromic) were also performed.
For the immunohistochemical technique, 3 µm sections were done of each selected paraffin block, using the Envision System (Dako, USA), following standardized protocol with adequate controls and using the following antibodies: S100 protein, HMB45, Neuron specific enolase (NSE), Cytokeratin AE1-AE3 (CKAE1-AE3), (GFAP), chromogranin, synaptophysin and p53.
Case 1
A 16-month-old female patient with a reddish- and slow-growing pigmented tumor mass located in the maxilla (Figure 1) was examined. Acomputed tomography(CT) scanshowed a destructive lytic and hyperdense bone lesion that compromised predominantly the left side of the maxillary (Figure 2). An excisional biopsy was performed, and a lesion of solid consistency is found that encompasses the superior alveolar border and extends to the posterior third of the hard palate. The surgical sample was represented by an ovoid fragment of 3.8 x 2.7 x 1.5 cm, with a smooth outer surface (Figure 3). When sectioned, the cut surface was solid, homogeneous, and grayish, with radiant black stretch marks (Figure 4). In a separate container, referred to as "palatal mucosa", multiple irregular fragments were obtained, measuring the largest 1.5 x 0.7 x 0.3 cm. In addition, alveolar ridge and two teeth were received, the largest measured 0.5 x 0.3 x 0.3 cm and the smallest 0.4 x 0.3 x 0.3 cm.
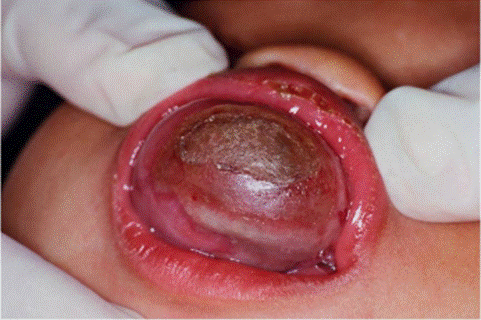
Figure 1. Case 1. A maxillary reddish/pigmented tumor mass.
Figure 2. Case 1. Image of a CT scan showing a heterogeneous destructive, lytic and hiperdense lesion of the maxillary bone.
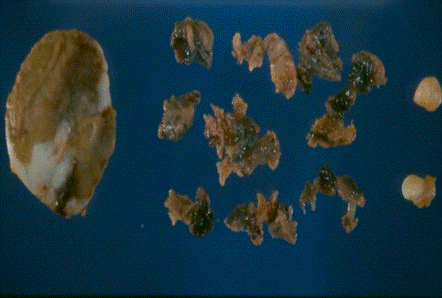
Figure 3. Gross image of surgical resection sample. At left the larger fragment is the tumor and at right side multiple fragments of soft tissue, bone and teeth from maxillary region.
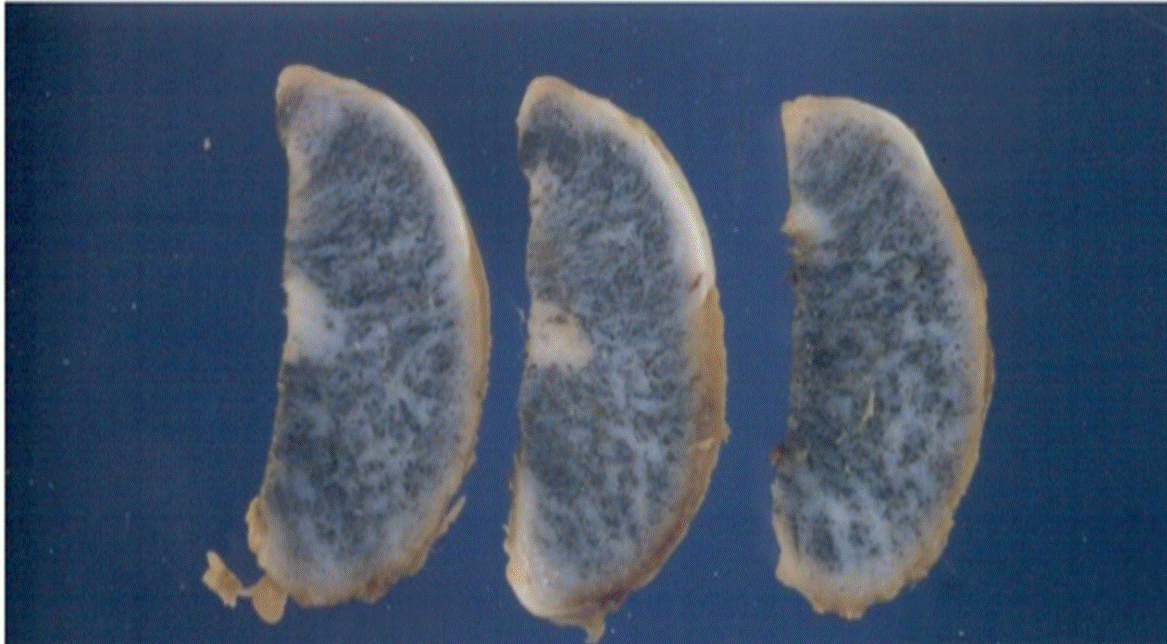
Figure 4. Case 1. Three sections of the tumor. The surface shows pigmented stretch marks.
Histological sections showed two cellular components, the first consisting of epithelial cells with abundant pigmented cytoplasm and the other component were immature, small, bluish, round cells with hyperchromatic nuclei and sparse cytoplasm. The cells were distributed in nests, surrounded by dense fibrous stroma (Figure 5). In the immunohistochemical study positive expression of NSE was observed in small cells (Figure 6) and the expression of protein S100 and synaptophysin was weak to moderate in isolated cells. The epithelial component was strongly positive for HMB45 (Figure 7) and moderately positive for CK AE1-AE3 (Figure 8). The expression for p53 was negative.
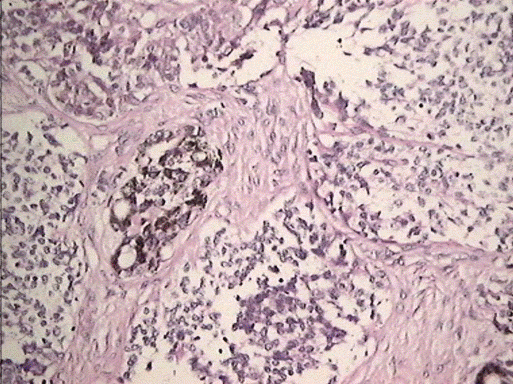
Figure 5. MNTI. Case 1. Epithelial cells alternating with small blue cells. H-E. 200X.
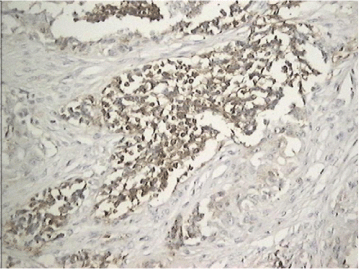
Figure 6. MNTI. Case 1. Immunohistochemistry. Small cells with positive expression for NSE.
Figure 7. MNTI. Case 1. Immunohistochemistry. Epithelial pigmented cells with strong expression for HMB45.
Figure 8. MNTI. Case 1. Immunohistochemistry. Positive expression of AE1-AE3 cytokeratin in epithelial cells.
The child was surgically re-intervened three months later to expand the resection margins. The patient was then treated with a soft prosthetic appliance to facilitate feeding while planning definitive surgical treatment. She remains disease-free after six years of follow up.
Case 2
A four-month-old baby was evaluated for a slow-growing lesion in the left temporal region. Clinical examination revealed a bulging lesion 3 centimeters in diameter. Plain X-ray of the skull showed a delimited radiodense lesion in the temporal bone (Figure 9). The lesion was resected, and a provisional diagnosis of Langerhans cell disease was made. A paraffin block was sent in consultation to the bone pathology section of the Pathological Anatomy Institute at Central University of Venezuela. Routine H-E stains were analyzed, and microscopic study revealed a tumor consisting of small, round tumor cells and pigmented epithelial cells surrounded for a dense fibrous stroma (Figure 10). An immunohistochemical study was performed with the following markers: CK AE1-AE3, HMB45, S100, NSE, CD68, Myogenin and CD1a. The epithelial cells were positive for CKAE1-AE3 and HMB45 (Figure 11). Small tumor cells were positive for S100 and NSE. CD1a and myogenin were negative. After a year of follow-up, the patient reveals no recurrence after surgical removal. No radiotherapy or chemotherapy was given.
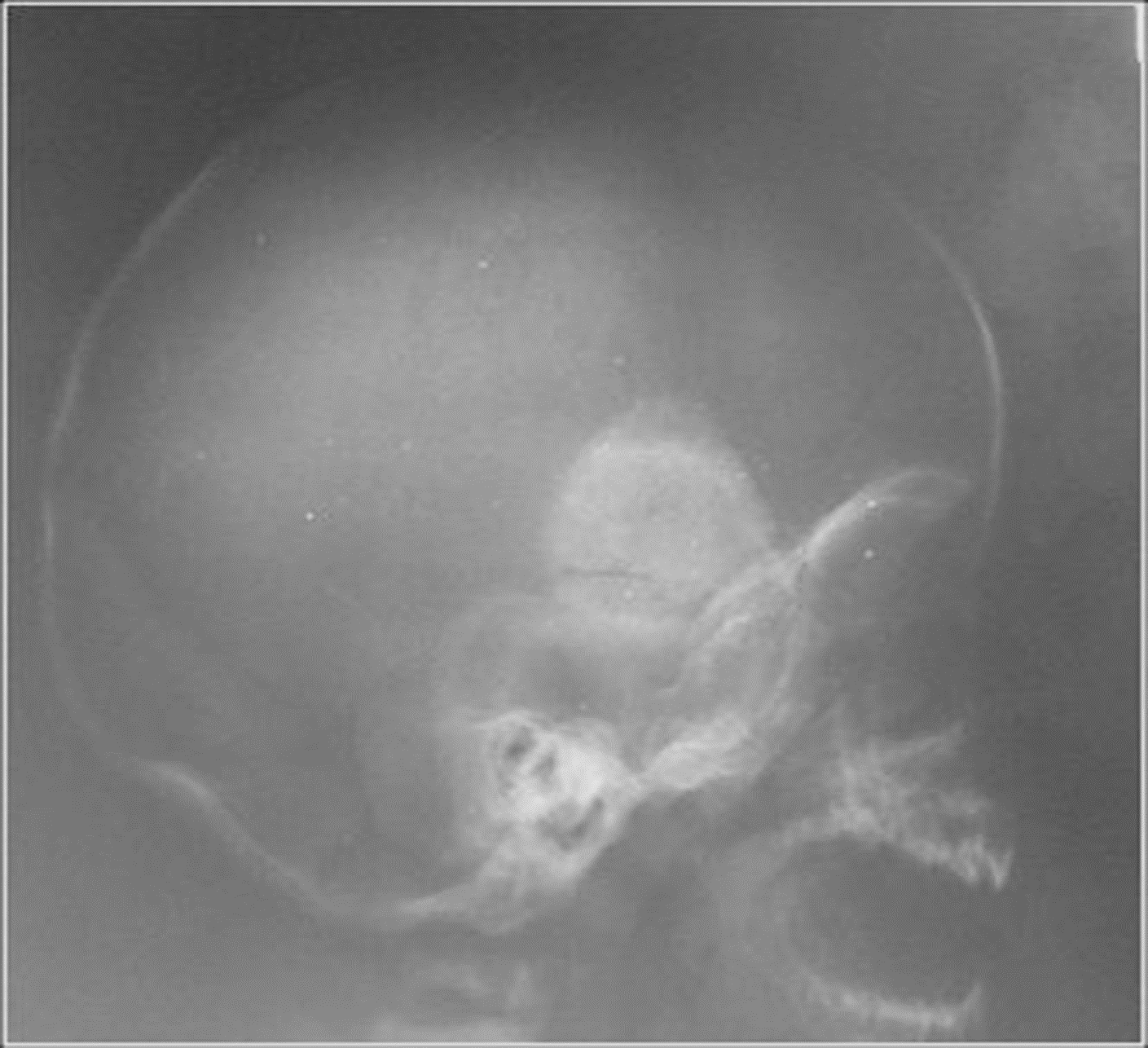
Figure 9. MNTI. Case 2. Lateral, RX of cranium showing radiodense, delimited, tumoral lesion in temporal bone.

Figure 10. MNTI. Case 2. Round small cells and isolated epithelial cells surrounded by fibrous dense stroma. H-E 100X.

Figure 11. MNTI. Case 2. Immunohistochemistry. Strong positive expression for HMB45 in epithelial cells.
The MNTI is an uncommon neoplasm with an aggressive behavior, high recurrence rate [12-16], and rarely metastasize, usually estimated between 3.7 and 6.6% of the cases [17-19]. The histologic spectrum is characterized by an epithelial component, with dense cytoplasmic melanin granules; the so called epithelial melanocytic cells, which show immunoreactive expression of HMB45 and CKAE1/AE3, with a weaker staining pattern for NSE and Vimentin. The neuroblastic cells, the second component of the tumor, are smaller in size, and express NSE and other neural markers. Pettinato et al. [13] analyzed 10 cases of MNTI, reporting that both cell components were positive for HMB45, NSE and synaptophysin. Agarwal et al. [14] reported one case showing HMB45 positivity in the melanin-containing peripheral polygonal cells and for synaptophysin in the small neuroblast-like cells. In the present case, we found HMB45 and CKAE1-AE3 in the large cells and S100 protein, synaptophysin and NSE in the small cell component. Kapadia et al. [9] reported 20 cases of MNTI. Of these 12/12 cases were positive for cytokeratin, HMB45 and Vimentin in 7/8 cases, mainly in the large cell component; NSE in 7/12 cases, S100 was focally positive in 12/12 cases. All the studied cases were children. These findings are in agreement with the present case.
Pettinato et al. [13] performed an IHC, flow cytometric and EM study in 10 cases of MNTI and described three cell types in all studied cases: undifferentiated cells, small cells displaying neuroblastic differentiation and enlarged melanin producing cells. The undifferentiated cells showed poorly developed organelles, free ribosomes and glycogen granules. A subpopulation of more mature cells displayed dendritic like cells processes, primitive intercellular junctions and dense secretory granules. The larger melanin producing cells were polygonal, cuboidal or dendritic in shape containing numerous premelanosomes and prominent endoplasmic reticulum. A DNA study was conducted on 8 cases, two of which had DNA aneuploidy. These 2 patients showed recurrence. The treatment of choice has been surgery, assessing free margins. Radiotherapy has been used in the past, however, is no longer used
Recently, Strieder et al. [15] reported two cases of MNTI, using a different approach. They evaluated the immunohistochemical characteristics of the stroma and immune cells, with the purpose of achieving a better understanding of the role that the microenviroment plays in these lesions. Their study showed CD138 expression in stromal fibroblasts and HLA-DR, XIIIa, CD68 and CD163 expression in cells exhibiting a dendritic morphology. The former finding may be associated with proliferation of neoplastic cells through activation of the signaling pathway via fibroblast growth factor 2 (FGF2) and stromal cell-derived growth factor-1 (SDF1). The second finding suggests the presence of M2-polarized macrophages-like cells, a protumor phenotype that contributes to the survival of tumor cells and the progression of MNTI. The presence of neural markers in neuroblastic cells and vanillylmandelic acid (VMA) in urine supports a neuroectodermal origin [1,8,12-15,18-21]. Currently no specific genetic alteration can help to diagnosis MNTI however recently was referred a case with dominant neuroblastic component which showed loss of heterozygosity with deletion of chromosome 1p and gain of chromosome 7q [22]. A BRAFV600E mutation in MNTI has recently been demonstrated. This molecular finding can contribute to personalized treatment [23]. So far, a chemotherapeutic treatment protocol has not been developed aimed especially at MNTI. Metformin has recently been used successfully in two cases of MNTI [24].
Differential diagnosis includes Ewing sarcoma and neuroblastoma (NB). Both tumors are rare as primary in the maxillary bone. Ewing sarcoma appears as a solid tumor of small, round cells without epithelial pigmented cells, is CD99 and FLI1 positive and HMB45 and Cytokeratin negative and has translocation t(11,22)(q24,q12). MNTI expresses HMB45 and Cytokeratin in epithelial cells and lacks a specific genetic translocation. Metastatic NB shows neuronal arrangement with Homer-Wright rosettes, necrosis, and central calcifications. Neuroblastic cells express synaptophysine, NSE and other neural markers, but this neoplasm lacks melanocytic cells. The rhabdomyosarcoma frequently develops in the infancy; the alveolar pattern may be confusing with the MNTI, however the rhabdomyosarcoma does not show positivity for HMB45. The latter may show positivity for desmin, myogenin, MyoD1, although some cases of MNTI may have some myogenic features. Another tumor to consider in differential diagnosis is the desmoplastic round cell tumor (DRCT), which is rare as a primary maxillary tumor, but could occasionally be metastasis of an intra-abdominal primary tumor. DRCT is a polyphenotypic tumor in which distinctive dot-like desmine staining as well as other intermediate filaments can be demonstrated. The tumor lacks melanin pigment and has genetic translocation t(11,22)(p13,q12). The malignant ectomesenchymoma is an infancy tumor composed of a mixture of neuroectodermal and malignant mesenchymal cells. A rhabdoid component is present in this tumor, while is absent in MNTI.
Differential diagnosis between MNTI and melanoma is raised in such cases by the presence of pigmented tumor cells, but melanoma is extremely rare in childhood and even more on oral mucosa. When melanoma occurs in childhood it is usually congenital and most often associated with large congenital melanocytic nevus. Melanoma lack neuroblastic cells as in TNMI.
In small biopsies where round cells are the only tumoral component, the diagnosis of lymphoma must be considered. The maxillary lymphomas represent 7% of all malignant bone tumors and 5% of the extranodal forms. The expression of lymphoid markers contributes to the diagnosis.
Current research of these lesions is limited due to the scarce number of cases reported in the literature, which is no surprise given the rarity of these tumors. This lack of knowledge and familiarity augments the possibility of potential diagnostic pitfalls that may delay treatment or provoke unnecessary therapies. When the pathologist analyzes these types of tumors it must recognize the histologic components, immunohistochemical expression and ultrastructural characteristics of MNTI and correlate with clinical data to obtain a correct diagnosis. A multidisciplinary approach, accompanied by a profound understanding of the disease by clinicians, radiologists and pathologists is necessary, using all possible tools to reach an accurate diagnosis and providing a better care for our patients.
MNTI is a rare tumor that must be recognized for histopathological characteristics. It is of remarkable importance to pathologists to recognize the cellular biphasic components by checking for the presence of small neuroblastic cells and large melanotic epithelial cells. Immunohistochemistry is a useful tool for an accurate diagnosis that allows the medical team to choose the best treatment.
We would like to thank Dr. Ghislaine Cespedes and the staff of the Dr Jose Antonio O' Daly Institute of Pathological Anatomy, especially technicians working in immunohistochemistry for their invaluable help.
- Borello ED, Gorlin RJ (1966) Melanotic neuroectodermal tumor of infancy. A neoplasm of neural crest origin. Cancer 19: 196-205. [Crossref]
- Krompecher E (1918) Zur Histogenese und morphologie der adimantinome und sonstiger kiefergeschwülste. Beitr Path Anat Allg Pathol 64: 165-197.
- Babu B, Avinash M (2012) Melanotic neuroectodermal tumor of infancy: A rare case report with differential diagnosis and review of the literature. Contemp Clin Dent 3: 108-112. [Crossref]
- Lurie HI (1961) Congenital melanocarcinoma, melanotic adamantinoma, retinal anlage tumor, progonoma, and pigmented epulis of infancy. Cancer 1961; 14: 1090-1108. [Crossref]
- Stowens D (1957) A pigmented tumor of infancy. The melanotic progonoma. J Pathol Bact 73: 43.
- Antunes AC, Freitas RM, Oliveira PP, Reboucas RG (2005) Melanotic neuroectodermal tumor of infancy. Arq Neuropsiquiatr 63: 670-672.
- Kumari RN, Sreedharan S, Balachandran D (1993) Melanotic neuroectodermal tumor of infancy: a case report. Am J Surg Pathol 17: 566-573. [Crossref]
- Madrid C, Aziza J, Hlali A, Bouferrache K, Abarca M (2010) Melanotic neuroectodermal tumour of infancy: A case report and review of the aetiopathogenic hypotheses. Med Oral Patol Oral Cir Bucal 15: e739-e742. [Crossref]
- Kapadia SB, Frisman DM, Hitchcock CL, Ellis GL, Popek EJ (1993) Melanotic neuroectodermal tumor of infancy. Clinicopathological, immunohistochemical, and flow cytometric study. Am J Surg Pathol 17: 566-573. [Crossref]
- Suarez MA, Restrepo L, Penagos P, Rubio A (2002) Tumor neuroectodermico primitivo melanótico de la infancia. Reporte de un caso. Rev Col Cancerol 6: 41-44.
- Kantar M, Sezak M, Turhan T, Kitis O, Mutluer S, et al. (2008) Melanotic progonoma of the skull in infancy. Childs Nerv Syst 11: 1371-1375. [Crossref]
- Nozicka Z, Spacek J (1978) Melanotic neuroectodermal tumor of the infancy with highly differentiated neural component. Light and electron microscopy study. Acta Neuropathol 44: 229-233. [Crossref]
- Pettinato G, Manivel JC, d'Amore ES, Jaszcz W, Gorlin RJ (1991) Melanotic neuroectodermal tumor of infancy. A reexamination of a histogenetic problem based on immunohistochemical, flow cytometric, and ultrastructural study of 10 cases. Am J Surg Pathol 15: 233-245. [Crossref]
- Agarwal P, Saxena S, Kumar S, Gupta R (2010) Melanotic neuroectodermal tumor of infancy: Presentation of a case affecting the maxilla. J Oral Maxillofac Pathol 14: 29-32. [Crossref]
- Strieder L, Carlos R, León JE, Ribeiro-Silva A, Costa V, et al. (2016) Protumorigenic M2-like phenotype cell infiltration in the melanotic neuroectodermal tumor of infancy. Oral Surg Oral Med Oral Pathol Oral Radiol 121: 173-179. [Crossref]
- Hamilton S, MacRae D, Agrawal S, Matic D (2008) Melanotic neuroectodermal tumour of infancy. Can J Plast Surg 16: 41-44.
- Nicosia G, Spennato P, Aliberti F, Cascone D, Quaglieta L, et al. (2017) Giant Melanotic neuroectodermal tumor of infancy (melanotic progonoma) of the head and neck: report of a malignant case. J Neurosurg Pediatr 19: 538-545. [Crossref]
- Cutler LS, Chaudry AP, Topazian R (1981) MNTI an Ultrastructural study, literature review, a reevaluation. Cancer 48: 257-270.
- Dehner LP, Sibley RK, Sauk JJ, Vicker RA, Nesbit ME, et al. (1979) Malignant MNTI: a clinical, pathological, ultrastructural and tissue culture study. Cancer 43: 1389-1410. [Crossref]
- Soles BS, Wilson A, Lucas DR, Heider A (2018) Melanotic Neuroectodermal Tumor of infancy. Arch Pathol Lab Med 142: 1358-1363.
- Chaudhary A, Wakhlu A, Mittal N, Misra S, Mehrotra D, et al. (2009) Melanotic neuroectodermal tumor of infancy: 2 decades of clinical experience with 18 patients. J Oral Maxillofac Surg 67: 47-51. [Crossref]
- Neven J, Hulsbergen-van der Kaa C, Groot-Loonen J, de Wilde PC, Merkx MA (2008) Recurrent melanotic neuroectodermal tumor of infancy: a proposal for treatment protocol with surgery and adjuvant chemotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106: 493-496. [Crossref]
- Gomes CC, Diniz M, Ferreira de Menezes GH, Castro WH, Gomes RS (2015) BRAFV600E Mutation in Melanotic Neuroectodermal Tumor of infancy: Toward Personalized Medicine? Pediatrics 136: e267-e269. [Crossref]
- Liang Y, Tian R, Wang J, Shan Y, Gao H, et al. (2020) Melanotic neuroectodermal tumor of infancy successfully treated with metformin. Medicine 99: e22303. [Crossref]











