Abstract
Recent studies have clearly showed that rice can be another important pathway of MeHg exposure to inhabitants in higher Hg hotspots, such as Hg mining areas in Guizhou Province, China. However, the elevated Hg levels only account for about 1% of Chinese cultivation area and the majority of rice planting areas are ranked the low Hg areas. Therefore, it is important and necessary to verify MeHg accumulation in rice in low Hg areas because rice is the stable daily food in these extensive areas. Importantly, the human health-risk assessment of Hg exposure doesn’t consider the extreme values observed. Although these extreme values are fewer, it may result in critical Hg exposure for local inhabitants and may become the determinants or “drivers” of environmental decisions and policies, even though most individuals are exposed to far lower concentrations and risks. This study clearly confirmed rice has the highest ability to accumulate MeHg not only in Hg mining areas also in low Hg areas. The THg and MeHg concentrations in the rice samples collected from northeast plain rice advantage area were lower than that of Xinyang city, and significantly lower than that of reported from the rice from southern China. However, in extreme micro-environments, routine consumption of rice provides a THg and MeHg intake of 11.6 µg kg-1 and 1.8 µg kg-1 of body weight per day, respectively. The MeHg intake was 18 times of RfD of USEPA and 7.8 times of PTWI by JECFA. It clearly demonstrates that a few inhabitants, such as Gouxi and Dashui in Wanshan, are exposed to Hg and MeHg to a much serious health risk.
Key words
rice, total mercury, methylmercury, low mercury areas, mercury exposure, extreme micro-environments
Introduction
Mercury (Hg) bioaccumulation in aquatic food chains has been much studied and even found in Arctic biota [1] at concentrations so higher due to the long-range Hg transportation and deposition from anthropogenic sources. As a result of its neurotoxin that biomagnifies through aquatic food chains and can be toxic to wildlife and humans, methylmercury (MeHg) bioaccumulation and exposure attracts worldwide concern [2]. The net accumulation and transfer of MeHg in food chains is controlled by biotic and abiotic pathways that lead to its production and degradation [3]. It is well established that consumption of fish, fish products and marine mammal tissues are the major MeHg exposure pathway to most human populations [2,4,5]. Of MeHg consumed from fish, 95%, on average, is absorbed by humans ingested [6]. Based primarily on the relationship between the intake of mercury from fish and mercury levels in blood and hair associated with the onset of clinical disease, a provisional tolerable weekly intake (PTWI) for MeHg is 1.6 μg. kg−1.week−1 issued by the Joint Expert Committee on Food Additives (JECFA) of the Food and Agriculture Organization and the World Health Organization [7]. In 1997, the United States Environmental Protection Agency (USEPA) set the critical limit of 0.1 μg. kg−1.d−1 as reference dose (RfD) for MeHg [8].
Recently, many studies have clearly showed that rice can be another important pathway of MeHg exposure to inhabitants in higher Hg hotspots, such as Hg mining areas in Guizhou province [9,10]. Actually, the elevated Hg levels only occur in Hg mining areas, urban soil and surrounding region of coal combustion in China, which only covers approximate 800 km2 and account for about 1% of Chinese cultivation area. However, China is the second-largest area of rice cultivation with rice paddies of approximately 32,000 km2, accounting for about 20% of the world’s rice-producing area and 23% of all cultivated land in terms of the total area [11]. THg and MeHg concentrations in soils from those majority of the China are lower ranging from 20-200 µg kg-1 and 0.09-0.23 µg kg-1 [12], which are comparable to or slightly higher than that in background soils worldwide which varied between 0.01-0.5 mg kg-1 [12]. So, the majority of rice planting areas are ranked the low Hg areas. Therefore, it is important and necessary to verify MeHg accumulation in rice in these extensive low Hg areas because rice is the stable daily food in these areas.
Furthermore, the human health-risk assessment of Hg exposure in high Hg areas has been conducted by several studies [13]. It demonstrates that the general inhabitants in Hg mining areas are exposed to Hg and MeHg to a certain level and they are not under a serious health risk [9,14]. However, it respects the general population health - risks of Hg exposure because all of the studies used parameter of the average Hg and MeHg concentrations in rice and the average daily rice intakes [14] and doesn’t consider the extreme conditions(e.g. highest concentrations of MeHg in rice; highest routine rice intakes in poor rural areas) has been observed. Although these extreme conditions are fewer, it may result in critical Hg exposure for local inhabitants because rice is the stable routine food. Therefore, the health-risk assessment of Hg exposure should pay more attention to extreme conditions of Hg and MeHg exposure from rice.
To address the issues, the primary objectives of this study are (1) to confirm MeHg accumulation in rice through a small synthetical field(SF) and (2) to verify the variation and consistency of MeHg accumulation in more rice samples from Chinese low Hg areas and (3) to access the potential rice intake health-risk of Hg exposure in the extreme conditions.
Materials and methods
Sample collection and pretreatment
Xinyang city (31°23°40´~32´ N, 113°42°55~115´ E) with an area of about 18 800 km2, lies in the south bank of Huai River, is the only one southern city of Henan Province. There are big differences in climate (cold and dry in northern China; warm and rainy in southern China), geography and stable daily foods between southern China and northern China. As one part of the three Chinese rice advantage areas (Figure 1a), rice is widespread cultivated and stable daily food in the two districts and eight counties (Figure 1b), accounting for more than the city’s total grain output of 70%. THg concentrations in soil of Henan province are reported to be in the range of 19.2-449.4 µg kg-1 [15], which are comparable to that in background soils worldwide and it belongs to the low Hg areas.
Three sampling campaigns were conducted in our studies. The first sampling campaign was conducted in a small SF investigation, which consists of three separate rice paddy field, one corn upland, one sweet potato upland and a pond (Figure 1c). The SF is located in Changling village of Xixian county of xinyang city with an area of 0.6 km2 (length: 200 m, width: 300 m). It possesses similar Hg sources, such as similar atmosphere Hg deposition, similar soil and similar irrigation water, which is an ideal field to compare the Hg and MeHg accumulation in rice paddy with the upland plants (Figure 1c). Therefore, rice tissues, edible corms, sweet potatoes and the corresponding soil from the root zones were sampled. The second sampling campaign was conducted during rice harvest season to investigate the Hg and MeHg accumulation in the whole Xinyang city in 2016. 2-5 control sites, which are selected randomly to sample rice plants and the corresponding soil for each county of Xinyang city (Figure 1b). 2-6 rice plants, as well as the corresponding soil from the root zone (10-20 cm depth), were collected during each sampling campaign randomly for each county or district. The third sampling campaign was collecting the edible rice samples from markets or by posting from other province to verify the variation and consistency of THg and MeHg accumulation in low Hg areas.
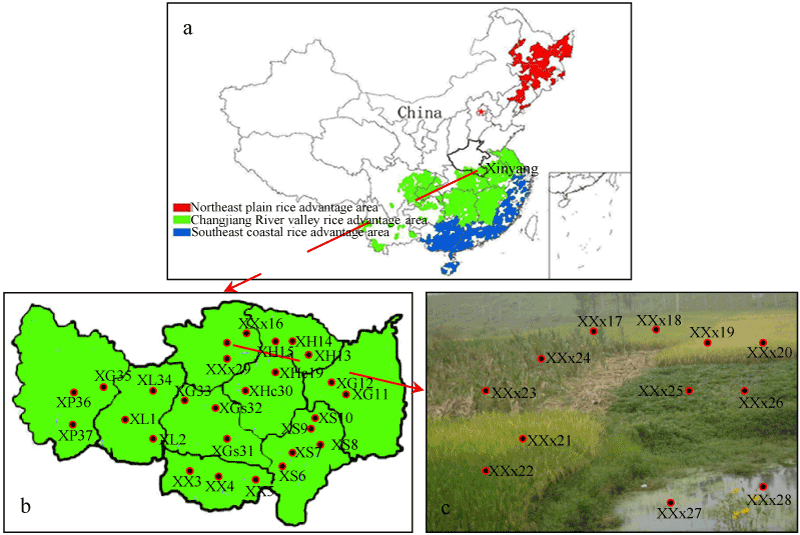
Figure 1. Map of study regions and sampling sites. ( ) “XXx17” indicates a sampling site and the sample number. The first “X” represents Xinyang city. The second “X” is the administrative county of xinyang city, such as Xinxian county, Xixian county. If the second letter is same as the front county according to the sampling sequence number, we use the third letter “x” to distinguish each other. The “17” is the sampling sequence number.
Rice plants were cleaned using drinking water, followed by ultrasonically assisted rinses with de-ionized water and then divided into four parts using a pair of stainless steel scissors in situ: root, stem, leaf and tassel. After being brought back to the laboratory, the rice tissue samples were finally air-dried and stored in polyethylene bags to avoid cross contamination. The tassels were separated to stalks (small pieces of stem and connect the stem and the seed) and seeds by hand with disposable polyethylene glove, and then the seeds were separated to hull and rice (edible part) from their hulls using a surgical knife in the laboratory one by one. Soil samples were collected by hand with the disposable polyethylene glove at the same site as the rice plant sample, and then were sealed, double-bagged, stored in an ice-cooled container before being shipped to the laboratory within 24 hours, and then stored in a refrigerator at -17 ºC prior to being freeze-dried.
Rice plant tissues were weighted and then ground to 200 mesh. Similarly, freeze-dried soil samples were homogenized to 200 mesh with a mortar before chemical analysis. Precautions were taken in order to avoid any cross contamination during sample preparation. The grinder was thoroughly cleaned after processing each sample. Powdered samples were transferred into an open plastic dish and separately enclosed in polyethylene bags, then placed in a desiccator kept at +4 ºC in the dark.
THg and MeHg analysis
The THg and MeHg analysis are similar to our previous studies [16,17]. Briefly, for THg analysis a rice plant sample was digested in a freshly prepared mixture of HNO3/H2SO4 (4:1, v/v) at 95°C. Similarly, soil was digested using a fresh mixture of HCl and HNO3 (1:3, v/v). THg was determined by cold vapor atomic fluorescence spectrometry (Brooks, Model III, Brooks Rand Labs) preceded by BrCl oxidation, SnCl2 reduction, pre-concentration, and finally thermo-reduction to Hg0. THg in water samples was quantified within 28 days of sampling following the approved methodology [18].
For MeHg analysis, rice plant samples were treated using a KOH-methanol/solvent extraction technique [19]. MeHg was determined using aqueous ethylation, purge, trap, and GC-CVAFS detection (Brooks Rand Model III, Brooks Rand Labs) following the U.S. Environmental Protection Agency method 1630 [18,19].
Quality control
Quantification for THg and MeHg in rice tissues sample was conducted using daily calibration curves with the coefficient of variation (r2) ≥ 0.99. Quality control measures consisted of method blanks, field blanks, triplicates, matrix spikes, and several certified reference materials. Field blanks of water samples were 0.13 ng L-1 for THg. The method detection limits (3´s) were 0.01 μg kg-1 for THg and 0.002 μg kg-1 for MeHg in rice tissues. The precision and bias for triplicate samples were less than 7.2% for THg and MeHg analysis. The recoveries for matrix spikes ranged from 96 to 110% for THg analysis, and from 86 to 105% for MeHg. The following certified reference materials(CRMs) were employed: soil (GB W07405, National Research Centre for Certified Reference Materials), poplar leaf (GBW07604, National Research Centre for Certified Reference Materials), Estuarine sediment (ERM-CC580, Institute for Reference Materials and Measurements) and Fish protein (Dorm-3, National Research Council of Canada).
Statistical analysis was performed using SPSS 13.0 software (SPSS). The measurements in samples are generally described by giving the mean ± standard deviation (SD). Relationships between covariant sets of data were subjected to regression analysis. Correlation coefficients (r) and significance probabilities (p) were computed for the linear regression fits. Differences are declared as significant in case that p < 0.05. Moreover, the data was analyzed by principal component analysis.
Results and discussion
MeHg accumulation in plant tissues in low Hg area
Mercury in soil depends mainly on local geochemical background [20]. The background Hg value of soil in China is reported to be in the range of 20 to 200 µg kg-1 by State Environmental Protection Administration of China [21]. Results showed that total Hg concentrations are the highest in the topsoil [8]. Mercury in topsoil usually comes from the deposition of atmospheric Hg and reflects more recent Hg deposition, which causes an accumulation of Hg in the soil [22]. Studies have found that Hg levels in root were directly associated with Hg levels in soil [23] and Hg in rice paddy soil was the main source of inorganic Hg in root [24]. So, we sampled the corresponding soil of the plants and as a section of plant tissues following the first and second sampling campaign. Concentrations of THg in rice tissues (Oryza sativa L.), corm (Zea mays L.), sweet potato (Ipomoea batatas) from the synthetical field, and from the 30 control sampling sites in Xinyang city were showed in Figure 2.
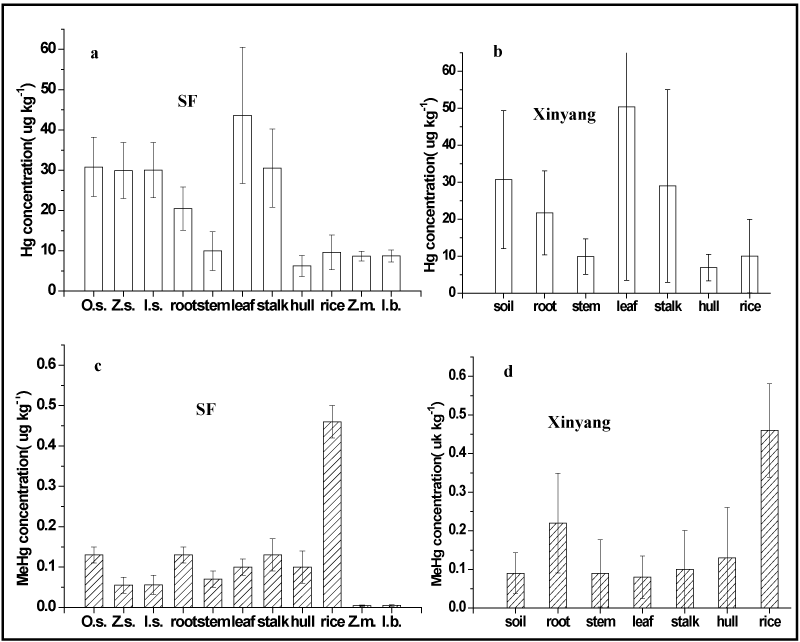
Figure 2. Concentrations THg and MeHg in rice tissues (Oryza sativa L.), corm (Zea mays L.), sweet potato (Ipomoea batatas) and the corresponding soil from synthetical field(SF), and the 30 control sampling sites in Xinyang city.
The THg in rice corresponding soil (30.82 ± 7.35 µg kg-1), corn corresponding soil (29.94 ± 6.94 µg kg-1), sweet potato corresponding soil (30.02 ± 29.94 µg kg-1) from SF (Figure 2a) and the rice corresponding soil from the 30 control sampling sites(30.75 ± 18.67 µg kg-1) in Xinyang city (Figure 2b) are no obvious variation and lower than the national Hg concentration in class I soils (150 µg kg-1) for the natural background value, which are consistent with THg in background soils worldwide. The lower THg in topsoil suggested that the topsoil was not been impacted with rapidly growing industries in recent decades as China has undergone massive industrialization. Similarly, the average THg concentration in rice from SF (9.63 ± 0.48 µg kg-1), edible corn (8.66 ± 1.2 µg kg-1) and sweet potato (8.73 ± 1.49 µg kg-1), and rice from the 30 control sampling sites in Xinyang (10.04 ± 9.91 µg kg-1) were no significant variation and lower than the maximum permissible limit of 20 µg kg-1 issued by Chinese National Standard Agency (22) and significantly lower the rice from Wanshan Hg mine areas(10.3-1120 µg kg-1). THg distribution in rice tissues exhibited the following distribution patterns: leaf > stalk > soil> root >stem>rice>hull at the CPF in Xinyang city. The results were consistent with the THg distribution at the control site, but varied from THg distribution at the artisanal Hg mining site and the abandoned Hg mining sites [25].
The highest average THg concentration was observed in leaf (50.38 ± 46.89 µg kg-1) which was up to 199.58 µg kg-1. Except for THg in leaves, we found that THg in stalk (29.01 ± 36.03 µg kg-1) was also higher than THg in root (20.49 ± 5.34 µg kg-1) and other rice tissues (Figure 2b). The results implied that there are at least two pathways to accumulate Hg in leaf and stalk. The elevated Hg in leaf and stalk mainly accumulated from the atmosphere other than the corresponding soil or root tissue. Findings were consistent with previous researches that demonstrated leaf THg mainly originate from air [24]. The lowest one was found in hull (6.22 ± 2.9 µg kg-1), with the range 3.63-8.81 µg kg-1. Meng et al. [25] regarded that the THg in hull might also originate from air.
Similarly, MeHg concentrations of rice corresponding soil (0.13 ± 0.02 µg kg-1), corm corresponding soil (0.055 ± 0.02 µg kg-1), sweet potato corresponding soil (0.056 ± 0.02 µg kg-1) from the SF, and from the 30 control sampling sites (0.09 ± 18.67 µg kg-1) in Xinyang city was also no obvious variation and much lower than that of Hg mining areas.
However, the highest average MeHg concentration was founded in rice (0.46 ± 0.04 µg kg-1) with the maximum of 0.51 µg kg-1, which was 8.2 times in upland crops and 3.5 times in the corresponding soil. Rice paddy field is a typical manmade seasonal wetland. During rice growing period, the anaerobic conditions created by the seasonal flooding may contribute to the high methylation ability in a high level in paddy soils. Furthermore, the irrigation water, precipitation and fertilizing activities provided more Hg to the rice paddy field. Therefore, rice paddy fields were favorable for the methylation of Hg as a result of the gradients in the seasonal conversion of oxidation-reduction conditions [9,13]. Our results clearly demonstrated that only rice could assimilate and accumulate MeHg in its edible portion [26]. The elevated levels of MeHg clearly confirmed that rice has the highest ability to accumulate MeHg not only in Hg minging areas also in low Hg areas.
For MeHg in rice tissues, MeHg in root (0.13 ± 0.02 µg kg-1) was commensurate with the MeHg in rice corresponding soil (0.13 ± 0.02 µg kg-1). The lowest one was found in stem (0.07 ± 0.02 µg kg-1). MeHg distribution in rice tissues exhibited the following trend: rice > stalk > root > soil >hull&leaf>stem at the SF, which is different from the distribution pattern of THg. The general tendency of MeHg concentration in rice tissues may reflect the MeHg uptakes into the rice tissues. The difference between THg and MeHg distribution may imply that the mechanisms of inorganic Hg and MeHg uptakes into the tissues of rice plant are completely different. Meng et al. [25] regards that MeHg in soil was first absorbed by roots, then translocated to the above-ground parts (leaf and stalk), and finally most of the MeHg was transferred to rice during the ripening period. This was quite similar to the transport of nutrients to seed in plants, since seed is the final destination of nutrients, especially the endosperm.
General Hg exposure from rice in low Hg areas
There are three rice advantage areas in China (Figure 1a), such as northeast plain rice advantage area, Changjiang River valley rice advantage area and southeast coastal rice advantage area, accounting for the whole rice planting area of 97% in china. The northeast plain rice advantage area includes Liaoning, Jilin and Heilongjiang Province. The Changjiang River rice advantage area includes Jiangsu, Anhui, Hubei, Hunan, Jiangxi, Chongqing, Sichuan, Guizhou, Yunnan and Henan Province and so on. The southeast coastal rice advantage area includes Shanghai, Zhejiang, Fujian, Guangxi, Guangdong and Hainan Province.
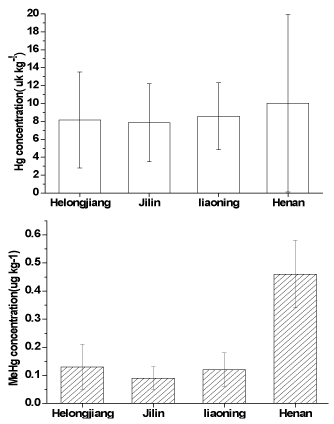
Figure 3. Comparison between THg and MeHg concentrations in the rice samples collected from northeast plain rice advantage area and Henan provinces.
Mercury exposure from rice in the Changjiang River rice advantage area and southeast coastal rice advantage area, especially in Hg mining areas in Guizhou province, has been well reported [13,14]. As studied above, rice has the highest ability to accumulate MeHg not only in Hg mining areas also in low Hg areas. Therefore, Hg exposure from rice in northeast plain rice advantage area needs to study. In this work, we collected and analyzed the rice samples from northeast plain rice advantage area following the third sampling campaign. The results were showed in Figure 3.
THg concentrations in the rice samples (Mean: 8.22 ± 2.68 µg kg-1; range from 3.76 to 14.23 µg kg-1, n=29) collected from northeast plain rice advantage area were lower than that from Xinyang city and the national limits, and significantly lower than that from the Hg mining areas in Guizhou Province [9,13,24,27,28]. The THg concentrations were comparable with that from 20 provinces in China (with a mean of 5.8 µg kg-1) [29] and that reported in the third national Total Diet Study conducted in China in 2000 (with a mean of 9.0 µg kg-1) [30]. The lower Hg levels are related to the geochemical background in north China because of the Global Circum-Pacific Hg Belt crossing southern China [14,26].
The MeHg concentrations in the rice samples collected from northeast plain rice advantage area (mean:0.12 ± 0.06 µg kg-1; range of 0.04-0.26 µg kg-1) were lower that of Xinyang city, and significantly lower than that of reported from the rice from Changjiang Rive valley rice advantage area and southeast coastal rice advantage area, such as Guangdong (1.86 µg kg-1), shanghai (2.11 µg kg-1) , Jiangsu (1.78 µgkg-1), Guangxi (3.14 µg kg-1), Hunan (3.33 µg kg-1), Jiangxi (2.04 µg kg-1), Guizhou (1.76 µg kg-1) and Henan (0.46 µg kg-1) province [14].
Zhang et al. [13] have reported that rice is by far the most important source of MeHg in the southern China (94–96%). So, Probable Daily Intakes (PDIs) of MeHg from rice was calculated for northeast plain rice advantage area and Henan province. This calculation is based on the absorption of MeHg in bodies was 95%, routine consumption of 0.187 kg per day of rice [31] and each adult’s body weight was 60 kg for the northern population. For an adult, routine consumption of rice provide a MeHg intake of 0.014 µg kg-1 of body weight per day for inhabitants of Xinyang city and 0.0004 µg kg-1 of body weight per day for inhabitants of body weight per day for northeast plain rice advantage area, which was much lower than the RfD of 0.1 µg kg-1 of body weight per day for the MeHg exposure reference dose (RfD) recommended by the USEPA [8]. It implied that the general inhabitants in northeast plain rice advantage area are to be in low risk of MeHg exposure.
MeHg exposure in extreme conditions
The human health-risk assessment of Hg exposure in Hg mining areas was conducted by several studies. Li et al. [14] reported that rice consumption contributes to low level MeHg exposure in southern China based on average MeHg and daily intake rates to access the health- risk, which represent the general population in southern China in low risk of MeHg exposure. Although fewer similar to the events of “Minamata Disease” hasn't been found in these higher Hg hotspots also demonstrate the inhabitants in Hg mining area are exposed to Hg and MeHg to a certain level and they are not under a serious health risk, the maximal values, such as the maximum of Hg and MeHg concentration observed in extreme rice conditions and the maximum of rice intake in poor rural areas, should be considered to access the health-risk of Hg exposure.
Due to date, the Highest elevated THg concentrations in rice was up to 1120 µg kg-1, and More importantly, the highly toxic MeHg concentrations can reach as high as 174 µg kg-1 [28], which was reported at abandoned Hg mining areas in Gouxi of Wanshan, Guizhou Province, southern China. Horvat et al. [27] also reported THg and MeHg value as high as 569 and 144 µg kg-1 in the rice harvested from the Dashuixi in Wanshan, respectively. Qiu et al. [28] also reported that the daily routine consumption of rice in Dashui, for instance, was up to 0.62 kg of dry weight per day for adults. Actually, the daily routine consumption of rice may be at least up to 1.0 kg in the poorest district because rice is the only caloric source (fewer vegetable, fewer meat and so on). These highest values may become the determinants or “drivers” of environmental decisions and policies, even though most individuals are exposed to far lower concentrations and risks.
According to the above extreme conditions observed, routine consumption of rice provide a THg and MeHg intake of 11.6 µg kg-1 and 1.8 µg kg-1 of body weight per day, respectively. The MeHg intake was 18 times of RfD of USEPA and 7.8 times of PTWI by JECFA. It clearly demonstrates that a few inhabitants, such as Gouxi and Dashui in Wanshan, are exposed to Hg and MeHg to a much serious health risk. Zhang et al. [13] estimated that approximately 22,400 residents in Guizhou (0.06% of the total population) are exposed to Hg concentrations of ≥ 0.23 µg kg-1.day-1.bw, and approximately 107,200 residents (0.28% of the total population) are exposed to ≥ 0.1 µg kg-1.day-1. Future research should take much attention of those inhabitants living in the extreme regions where rice is the staple food.
Implications for health-risk assessment
Owing to the bioaccumulation, biomagnifications, severe toxicity and longtime exposure of MeHg from rice, however, the present low MeHg levels in rice in low Hg areas also should be taken more attentions since rice is the stable food for the inhabitants in the rice advantage areas.
MeHg is bonded to the thiol group of the cysteine residues in fish protein and not be removed and destroyed by cooking or cleaning processes [32]. Of MeHg consumed, 95%, on average, is absorbed [6]. However, rice is a new important pathway of MeHg exposure to inhabitants and poor micronutrients associated with fish (e.g., n−3 long-chain polyunsaturated fatty acid, selenium, the essential amino acids), many knowledge about rice intakes need to understand, such as the Hg absorption, distribution, metabolism,excretion by bodies and longtime Hg exposure from rice and so on. The mercury isotopic analysis may provide essential information about the mechanism of mercury intakes and toxicity in the gastrointestinal tract and in mammalian tissues.
Since the PTWI for MeHg to 1.6 μg·kg−1·week−1 is based primarily on the fish intake exposure issued by the JECFA, it should be given more emphasis on assessing the health effects of MeHg exposure in the rice-consumption population and limits of MeHg for rice in the future. In addition, a detail investigation, including different ages, gender of Hg exposure from rice and related diseases, should be carried out in those extreme micro-environments, such as Gouxi, Dashuixi of Wanshan. It may become the determinants or “drivers” of environmental decisions and policies of Hg and MeHg limits in rice, even though most individuals are exposed to far lower concentrations and risks.
Conclusions
We clearly verified that rice has the highest ability to accumulate MeHg not only in Hg minging areas also in low Hg areas. It should be given more emphasis on accessing the health- risk of low level MeHg exposure in the rice-consumption population. The THg and MeHg concentrations in the rice samples collected from northeast plain rice advantage area were lower than that of Xinyang city, and significantly lower than that of reported from the rice from Changjiang Rive valley rice advantage area and southeast coastal rice advantage area. Similarly, the general inhabitants in northeast plain rice advantage area are to be in low risk of MeHg exposure. However, in extreme micro-environments, routine consumption of rice provide a THg and MeHg intake of 11.6 µg kg-1 and 1.8 µg kg-1 of body weight per day, respectively. The MeHg intake was 18 times of RfD of USEPA and 7.8 times of PTWI by JECFA. It clearly demonstrates that a few inhabitants, such as Gouxi and Dashui in Wanshan, are exposed to Hg and MeHg to a much serious health risk.
Acknowledgments
The work described here was supported by the National Natural Science Foundation of China (41372360, 41573006) and the Scientific and Technical Project of the Education Department of Henan Province (12A610006).
References
- Riget F, Asmund G, Aastrup P (2000) Mercury in Arctic char (Salvelinus alpinus) populations from Greenland. Sci Total Environ 245: 161-172. [Crossref]
- Mergler S, Pleyer U (2007) The human corneal endothelium: new insights into electrophysiology and ion channels. Prog Retin Eye Res 26: 359-378. [Crossref]
- Fitzgerald W, Lamborg C (2007) Geochemistry of Mercury in the Environment. Treatise on Geochemistry 9: 1-47.
- Bloom N, Lasorsa B (1999) Changes in mercury speciation and the release of methyl mercury as a result of marine sediment dredging activities. Science of the Total Environment 237/238: 379-385.
- Lockhart WL, Stern GA, Low G, Hendzel M, Boila G, et al. (2005) A history of total mercury in edible muscle of fish from lakes in northern Canada. Sci Total Environ 351-352: 427-63. [Crossref]
- WHO (1991) International programme on chemical safety: Environmental health criteria 118-Inorganic Mercury. Geneva.
- JECFA (2003) Summary and conclusions of the sixty-first meeting of the joint FAO/WHO expert committee on food additives. Rome, Italy.
- USEPA (United States Environmental Protection Agency) (1997) Mercury study report to the congress, volume v: health effects of mercury and mercury compounds. Washington, DC, USA.
- Feng XB, Li P, Qiu GL, Wang SF, Li GH, et al. (2008) Human exposure to methylmercury through rice intake in mercury mining areas, Guizhou province, China. Environ Sci Technol 42: 326-332. [Crossref]
- Windham-Myers L, Fleck JA, Ackerman JT, Marvin-DiPasquale M, Stricker CA, et al. (2014) Mercury cycling in agricultural and managed wetlands: a synthesis of methylmercury production, hydrologic export, and bioaccumulation from an integrated field study. Sci Total Environ 484: 221-231. [Crossref]
- Zhang W, Yu Y, Huang Y, Li T, Wang P (2011) Modeling methane emissions from irrigated rice cultivation in China from 1960 to 2050. Global Change Biology 17: 3511-3523.
- Lin Y, Vogt R, Larssen T (2012) Environmental mercury in China: a review. Environ Toxicol Chem 31: 2431-2444. [Crossref]
- Zhang H, Feng X, Larssen T, Qiu G, Vogt R (2010) In Inland China, Rice, Rather than Fish, Is the Major Pathway for Methylmercury Exposure. Environ Health Perspect 118: 1183-1118.
- Li P, Feng X, Yuan X, Chan H, Qiu G, et al. (2012) Rice consumption contributes to low level methylmercury exposure in southern China. Environ Int 49: 18-23. [Crossref]
- Zhang M, Yao S, Zhang J, Sun X (2009) Investigation of mercury content in plough layers of main soil types in Henan province. Shandong Agriculture Sciences 3: 93-96.
- Tang S, Feng X, Qiu J, Yin G, Yang Z (2007) Mercury speciation and emissions from coal combustion in Guiyang, southwest China Original Research Article. Environ Res 105: 175-182.
- Tang S, Huang Z, Liu J, Yang Z, Lin Q (2012) Atmospheric mercury deposition recorded in an ombrotrophic peat core from Xiaoxing'an Mountain, Northeast China. Environ Res 118: 145-148.
- USEPA (2001) Method 1630: Methyl Mercury in Water by Distillation, Aqueous Ethylation, Purge and Trap, and CVAFS. Draft January 2001. U.S. Environmental Protection Agency, Office of Water, Office of Science and Technology Engineering and Analysis Division (4303), 1200 Pennsylvania Avenue N.W., Washington, DC 20460, pp: 1-41.
- Liang L, Horvat M, Cernichiari E, Gelein B, Balogh S (1996) Simple solvent extraction technique for elimination of matrix interferences in the determination of methylmercury in environmental and biological samples by ethylation gas chromatography cold vapor atomic fluorescence spectrometry. Talanta 43: 1883-1888. [Crossref]
- Burak D, Fontes M, Santos N, Monteiro L, Martins E, et al. (2010) Geochemistry and spatial distribution of heavy metals in Oxisols in a mineralized region of the Brazilian Central Plateau. Geoderma 160: 131-142.
- CSEPA(State Environmental Protection Administration of China) (1994) The Atlas of Soil Environmental Background Value in the People’s Republic of China. China Environmental Science Press, Beijing, China.
- Lin X, Brooks J, Bronson M, Ngu-Schwemlein M (2012) Evaluation of the association of mercury(II) with some dicysteinyl tripeptides. Bioorg Chem 44: 8-18. [Crossref]
- Fay L, Gustin M (2007) Assessing the influence of different atmospheric and soil mercury concentrations on foliar mercury concentrations in a controlled environment. Water, Air, Soil Pollut 181: 373-384
- Meng B, Feng XB, Qiu GL, Cai Y, Wang DY, et al. (2010) Distribution patterns of inorganic mercury and methylmercury in tissues of rice (Oryza Sativa L.) plants and possible bioaccumulation pathways. J Agric Food Chem 58: 4951-4958. [Crossref]
- Meng B, Feng XB, Qiu GL, Liang P, Li P, et al. (2011) The process of methylmercury accumulation in rice (Oryza sativa L.). Environ Sci Technol 45: 2711-2717.
- Feng X, Li G, Qiu G (2004) A preliminary study on mercury contamination to the environment from artisanal zinc smelting using indigenous methods in Hezhang county, Guizhou, China. Part 1. Mercury emission from zinc smelting and its influences on the surface waters. Atmos Environ 38: 6223-6230.
- Horvat M, Nolde N, Fajon V, Jereb V, Logar M, et al. (2003) Total mercury, methylmercury and selenium in mercury polluted areas in the province Guizhou, China. Sci Total Environ 304: 231-256. [Crossref]
- Qiu G, Feng X, Li P, Wang S, Li G, et al. (2008) Methylmercury accumulation in rice (Oryza sativa L) grown at abandoned mercury mines in Guizhou, China. J Agric Food Chem 56: 2465-2468. [Crossref]
- Qian Y, Chen C, Zhang Q, Li Y, Chen Z, Li M (2010) Concentrations of cadmium, lead, mercury and arsenic in Chinese market milled rice and associated population health risk. Food Control 21: 1757-1763.
- Li XW, Gao JQ, Chen JS (2006) Chinese total diet study in 2000--the dietary mercuric intakes. Wei Sheng Yan Jiu 35: 323-325. [Crossref]
- CNBS (National Statistics Bureau of China) (2013) China statistical yellowbook. Chinese Statistics Press, Beijing, China.
- Harris HH, Pickering IJ, George GN (2003) The chemical form of mercury in fish. Science 301: 1203. [Crossref]



