Abstract
Annona muricata, commonly known as Graviola, soursop or guanabana is an evergreen tree native to the tropics. Its roots, bark, seed, leaves and pulp have been used in traditional African and South American pharmacopeias to treat a variety of diseases such as arthritis, parasitic infection, hypertension, fever or diabetes. Most notably extracts from several parts of the plant have been reported to be toxic to cell lines models for breast, colorectal, skin, head and neck, lung, liver, pancreatic and prostate cancer. Graviola extracts (GE) have been shown to affect several cancer-specific metabolic pathways which are key to cancer progression such as glucose metabolism, hypoxia response, oxidative stress, apoptosis and immune response. The present review attempts to summarize how Graviola alters those metabolic pathways. The possibility that GE modulate the expression of upstream epigenetic factors controlling the synthesis of cancer biomarkers will also be discussed.
Keywords
graviola, cancer cell metabolism, glycolysis, hypoxia, anti-cancer-drug
Abbreviations
GE: Graviola extract; GTE: Graviola tig extract; GRE: Graviola root extract; GLE: Graviola leaf extract; GFE: Graviola fruit extract; GSE: Graviola seed extract; GPE: Graviola pulp extract; GEE: Graviola exocarp extract; GFPE: Graviola fruit pericarp extract
Introduction
Annona Muricata, commonly known as Graviola, soursop, Brazilian pawpaw or guanabana is an evergreen tree native to the tropical regions of the Americas belonging to the custard apple/ Annonacea family. The tree height ranges from 5 to 8 m. Its heart-shaped fruit, green in color are appreciated for their acidulated taste and consumed in ice-cream or juice. In the traditional pharmacopeia of South America and Africa, the fruit is used as a natural remedy for a variety of ailments such as malaria, fever, diabetes, insomnia, rheumatism, hypertension, arthritis among others [1]. Studies conducted in vitro and in vivo have concluded that Graviola phytocomponents have anti-cancer and anti-tumor properties [2].
A clinical study conducted on a 66-year-old patient suffering from metastatic breast cancer (BC) has shown that the consumption of Graviola leaves boiled in water stopped the progression of chemotherapeutic-resistant metastatic tumors [3] and GE have ever since been prescribed in adjuvant therapy [1]. The cytotoxic properties of Graviola toward cancer cells is believed to be mediated by acetogenins, a class of long-chain fatty acids derivatives. Over 100 different types of acetogenins have been extracted from the leaves, bark, seeds, roots and fruits of Graviola. Aside from the acetogenins, Graviola phytocompounds also include flavonoïds (rutin, quercitin-3-glucoside), isoquinolines, alkaloīds and minerals (for a detailed description of Graviola phytocompounds, see [1]).
Used as the main therapeutic agents or as complements to classical treatments, natural products such as Graviola offer excellent alternative over synthetic because they tend to alter several signaling pathways which are key to cancer progression. This review aims at describing some of them (Figure1).
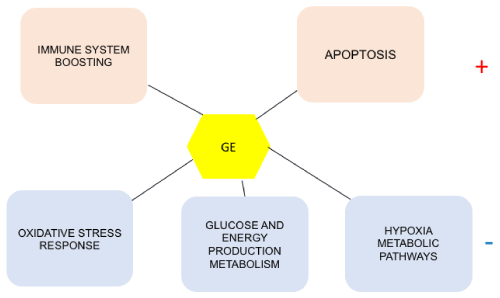
Figure 1. Some of the metabolic pathways affected by Graviola treatment in cancer cells
Graviola stimulates (+) apoptosis, boosts the cell immune system, inhibits (-) the oxidative
stress response, the glucose and energy production metabolism and the hypoxia pathways.
GE=Graviola Extract
Cytotoxicity of Graviola extracts (GE) toward cancer cells
The antiproliferative activity of Graviola extracts (GE) has been reported in a large variety of cancer cell lines including breast, colorectal, skin, head and neck, lung, liver, pancreatic and prostate cancer. The cytotoxic potency of GE varies depending on the part of the plant from which the extract was prepared. Pieme and al. have analyzed the cytotoxic effect of twig (GTE), root (GRE) and leaf extract (GLE) on HL-60 leukemia cells. In that study the cells were treated with GE for 72 h and their survival percentage was assessed by the resazurin reduction assay. The results suggest that GE from the different plant parts inhibit the proliferation of in a dose-dependent manner. The most potent extract is the GRE with an IC50= 9 ug/mL, followed by the GLE (IC50= 14 ug/mL), while the GTE to be much less cytotoxic (IC50= 49 ug/mL) [4]. In a study involving prostate cancer cell lines LNCaP and PC3 where the cytotoxicity of seed (GSE), pulp, (GPE), exocarp (GEE), and GLE was tested, all extracts induced a dose-dependent cell death but the GSE displayed the highest relative efficiency following a 48 h-treatment [5]. Methanol extracts from GSE, GLE and fruit pericarp (GFPE) have been shown to induce cytotoxicity in Multidrug resistant leukemia cell lines CCRF-CEM/ADR5000 with IC50 = 0.4 ug/mL for GSE, IC50 = 0.6 ug/mL for GLE and IC50 = 4.6 ug/mL for GFPE [6].
Cytotoxicity also seems to depend on the type of solvent used for the extraction. For example, Moghadamtousi, et al. studied the cell viability of several cancer cell lines following a 72h treatment with hexane, ethyl acetate or methanol leaf extracts GE by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay. While the methanol extracts proved to be poorly cytotoxic, low doses of the ethyl acetate showed a significant cytotoxicity activity towards lung cancer cell lines A549 (IC50 = 5.1 ug/mL), breast cancer cells MCF-7 (IC50 =6.4 ug/mL), hepatocarcinoma HepG2 (IC50 =9.3 ug/mL), and breast cancer cells MDA-MB-31 (IC50 =11.4 ug/mL). Interestingly, ethyl acetate extracts were much less cytotoxic to the non-cancer hepatic WRL-68 cell line (IC50 =47 ug/mL) than to cancer cell lines [7]. Similarly, Dai, et al. were able to show that \GFE) decreases the viability of EGFR-overexpressing BC MDA-MB-468 with an (IC50 =4.8 ug/ mL) while not altering the survival rate of non-tumorigenic BC MCF-10A epithelial cells [8] and a Graviola fruit peel extract proved to be toxic to HT-29 colorectal adenocarcinoma while not affecting non-malignant epithelial CCD-1074 and fibroblastic MRC-5 cell lines [9]. Such results suggest that low doses of GE could specifically target cancer cells lines while not affecting sane cells, a property that could qualify Graviola as an excellent anti-cancer drug candidate.
Graviola inhibits the production of energy in cancer cells
One of the key metabolic pathways promoting the growth of cancer is accelerated glycolysis. Glycolysis is the 10-step-process by which glucose is oxidized into two molecules of pyruvate. In the process 2 molecules of ATP are generated. The complete oxidation of pyruvate through the Krebs cycle and the mitochondrion respiratory chain, generates much more ATP than glycolysis and, in conditions where oxygen is present, respiration is prioritized over partial glycolytic oxidation. Glycolysis, however, is the major process used by the cell to generate energy in conditions of oxygen depletion (hypoxia). One of the metabolic differences between non-cancer and cancer cells is that in cancer cells, glycolysis is prioritized over respiration no matter the status of oxygen supplies: cancer cells have an hyperglycolytic metabolism which provides a constant and high supply of ATP and metabolic precursors, crucial to cancer tumor growth, proliferation and migration/metastasis. More generally, tumorigenesis results from a thorough reprogramming of energy metabolism through accelerated glucose uptake and intense glycolysis [10]. It should therefore be no surprise that one of the anti-cancer therapeutic strategies focuses on components with inhibitory effects on glucose metabolism. Importantly, cancer cells are often exposed to oxygen deprivation or hypoxia and hypoxic conditions have been shown to trigger the reprogramming of energy metabolism in the cancer cell [11]. Oxygen is supplied to the cells through blood by a system of intense vascularization. In rapidly growing tumors, vascularization becomes insufficient. Consequently, Hypoxia induces several complex intracellular signaling pathways by which the cancer cell turns to an hyperglycolytic/high-glucose-uptake metabolism to compensate for its impossibility to generate ATP through respiration. Hypoxia-inducible factor-1 (HIF-1) is a dimeric protein composed of subunits alpha and beta. HIF-1 activates the transcription of some hundred genes in response to hypoxic conditions. Among its target genes are glucose transporter- 1 (GLUT1), and of glycolytic enzymes hexokinase (HK), pyruvate kinase (PK) and lactate dehydrogenase (LDHA). Under hypoxia, glucose metabolic pathway is therefore reprogrammed toward intense glycolysis and glucose uptake, a condition favorable to cancer cell growth and migration [12]. A study conducted by Torres, et al. on pancreatic cancer cells lines (PC) FG/COLO357 and CD118/HPAF shows that GFE affected cell survival of both PC cell types with respective IC 50 values of 73μg/mL and 200μg/mL after a 48h treatment. The GFE-induced cytotoxicity is correlated with a decrease in glucose uptake and in ATP cell content, downregulation in the expression of protein subunit alpha of HIF-1 (HIF-1-alpha) and a decrease in the mRNA levels of GLUT 1 and GLUT 4, hexokinase II (HKII) and LDHA. Western blot analyses show that the expression of three proteins involved in hypoxia-associated pathways: Nuclear factor Kappa B (NF-Kb), ERK and PI3K/Akt. PC, are downregulated in PC treated with GFE compared to untreated cells. Additionally, the cells treated with GFE showed a decreased microtubule dynamics, reduced motility and migration potential, and decreased expression of proteins involved in cell invasion and metastasis such as: phosphorylated focal adhesion kinase (pFAK), matrix metalloproteinase 9 (MMP9) and mucin 4 (MUC4). GFE-mediated cytotoxicity is also correlated with and increased production of Reactive Oxygen Species (R.O.S), a decrease in the expression of Cyclin D1, cell cycle arrest in phase G1 and cell necrosis [13]. GFE therefore affects not only glycolysis but also multiple metabolic pathways involved in cancer cell progression.
Graviola inhibits the oxidative stress response
Hypoxia is one of the key conditions for the activation of the Nicotinamide adenine dinucleotide (NAPDH) oxidase complex (NOX) and cancer cell progression has been correlated with the aberrant activation of NOX. NOX is a membrane enzymatic complex of the mitochondrial electron transport chain which consists of catalytic (gp91phox, [NOX1-5], p22phox) and regulatory subunits (p47phox, p67phox, p40phox, Rac1/2). NOX generates superoxide and R.O.S. NOX-generated R.O.S are key elements in the maintenance of malignant cells: R.O.S act as secondary messengers and have been shown to be enhance the activity of oncogenes (Src and Ras) while inhibiting the activity of tumor suppressor genes (p53, PTEN, TSC2). NOX is also indirectly involved in the regulation of glucose metabolism since it activates and stabilizes subunit HIF1-alpha which in turn upregulates glycolytic enzymes and glucose transporters. NOX-related metabolism stands at the crossroads of the hypoxic and oxidative pathways and is therefore an important target for therapeutic drugs.
By using transgenic adenocarcinoma of the mouse prostate (TRAMP) as in vivo model for prostate cancer, Deep, et al. analyzed the expression of NOX 1 and p67phox in different stages of PCs and incomparison with normal prostate tissues. In sane tissues the expression of the two proteins were almost inexistent while their expression increased in high-grade tumors compared to low-grade ones, thus suggesting a correlation between the expression of NOX 1 and p67phox with the stage of the disease and with the aggressiveness of the tumor.
The authors also conducted in vitro experiments to test the inhibitory effect of Graviola pulp extract (GPE) on hypoxia-induced NOX activity. Three different prostate Cancer (PCa) cells lines (PCa22Rv1, LNCaP and PC3) were treated with doses of GPE between 1 to 5 ug/mL and the NOX-activity was assessed in each case by measuring the production of the superoxide anion. The results showed an inhibition of the hypoxia-induced NOX activity in the GPE-treated cells compared to untreated cells. PCa 22Rv1 cells treated with GPE showed a decreased expression of NOX subunits NOX 1, NOX2 and p47phox. As it was to expect, protein HIF-1 alpha was also downregulated. The inhibition of the NOX activity was correlated with GPE-treated PCa poor ability to form clones (clonogenicity) compared to untreated cells [5].
Graviola induces apoptosis
Apoptosis is responsible for homeostatic mechanism and maintenance of cell populations in tissues. Cancer cell progression is associated with uncontrolled cell proliferation and deregulation of apoptotic mechanisms and pro-apoptotic compounds have been considered as anti-cancer drugs. In vitro studies have shown that treatments with GE induces apoptosis in various cancer cell lines such as leukemia CCRF-CEM and HL-60, lung cancer A549 and hepatocarcinoma HepG2. GE-induced apoptosis has been correlated with a modification of the cell-cycle phases/ cell cycle arrest, disruption of mitochondrial membrane potential (MMP), accumulation of R.O.S, increased activities of pro-apoptotic proteins Casp3/7 and Caspase 9, upregulation of the transcription of pro-apoptotic gene Bax and downregulation of the anti-apoptotic Bcl2 [1,4,14-18]. A proteonomic study on HepG2 shows that a treatment with ethanol GE extract induces apoptosis through the Endoplasmic reticulum (ER) stress pathway [17]. Similarly, GLE has been shown to inhibit the process of metastasis and induces cell death through apoptosis in vitro and in vivo in a model of BC [19].
Graviola boosts the immune system
The immune system plays a central role in the control/elimination of diseased cells and there is often a correlation between cancer progression and the deficiency of a functional immune system. One anti-cancer strategy consists in using immune boosting compounds to target and eliminate the cancer cells. Najmuddin, et al. conducted an experiment in which mice with the 4T1 BC tumor were fed with GLE extracts. The authors were able to show that GLE-treatment resulted in a boosting of the cell immune system through an increase of white-blood cells, T-cells and natural killer cell population [19]. Likewise, active ingredients of GLE have been shown to enhance the immune activity of RAW 264.7 macrophage cells and treatment with 50% ethanol GSE induced the macrophages to differentiate. Both extracts activated the transcription of cytokines necrosis factor alpha (TNF-alpha) and interleukin-1(IL-1 beta) through a mitogen-activated protein (MAP) kinase pathways [1].
Table 1 summarizes the biomarkers altered by a Graviola treatment at the transcriptional level (T), protein level (P) or activity level (A).
Table 1. Biomarkers affected by GE.
Graviola extract (GE) treatment downregulates biomarkers involved in the pro-cancer metabolic pathways, while upregulating the markers involved in the destruction of cancer cells. GLE=Graviola Leaf Extract, GSE=Graviola Stem Extract, GFE=Graviola Fruit Extract, GPE= Graviola Pulp Extract, The biomarkers have been shown to be regulated at the transcriptional (T:), protein (P:) or Activity (A:) levels.
Metabolic pathways affected |
Biomarkers affected |
Type of GE |
Type of cancer cells |
Reference |
|
Downregulated: |
|
|
|
Glucose metabolism
Cell cycle
Tumor Metastasis |
T: GLUT 1,GLUT 4, HKII, PK, LDH-A, MUC 4.
P: HIF-1, ERK, ATk, pFAK, Cyclin D1, MUC 4. |
GLE GSE |
In vitro Pancreas
in vivo Pancreas |
[12,13] |
Apoptosis |
Upregulated: T: Bax
A: Caspase-3/7, Caspase-9. |
GFE |
In vitro Lungs |
[1,26] |
|
|
|
In vitro Prostate |
|
|
Downregulated: P, T: Bcl2 |
|
|
|
Oncogene activation |
Downregulated: T: EGFR, NOX 1
P: EGFR, p 47 phox, p67 phox, P: NOX2, H1F-1-alpha. |
GPE |
In vitro Breast, Pancreas carcinoma. |
[5,8] |
|
|
In vivo Mouse Breast Cancer xenografts |
|
Endoplasmic Reticulum stress pathway |
Upregulated:
P: GRP94, HSP70, PERK,CHOP,
DPI-related protein 5
Phosphorylation of PERK, eIF2- |
GLE |
In vitro Liver |
[18] |
Concerns over neurotoxicity
Some Graviola-extracted pharmaceutical compounds have shown powerful anti-proliferative and anti-tumor properties but one of the main concerns of using Graviola compounds or supplements as an anti-cancer drug is the reported neurotoxicity of Graviola acetogenins. An in vitro study by Hollerhage et al. on Lund human mesencephalic neurons showed that a dosis of 1 ug/mL of GPE induced death in 67% of the neurons [20]. Similarily, Lannuzel et al. have shown that annonacin, the major acetogenin of Graviola impairs the energy production in neurons by inhibiting the mitochondrial respiratory chain complex I. The study shows that annonacin is toxic to dopaminergic neurons at very low doses (IC50= 0.018 uM) and its consumption might be associated with forms of Parkinsonism [21]. Bustos et al. conducted an in vivo study to evaluate the effect of GE on the C. elegans NB327 mutant strain in which the of the dic-1 tumor suppressor gene is knocked down. Behavior analyses were conducted in animals which had been treated by the GE and compared to control animals. The results showed that when the mutant strain NB327 was exposed to GE (5 mg/ml) there was a significant decrease in average locomotion had a reduced number of progenies compared to the unexposed controls. The authors conclude of a possible neurotoxic effect in concentrations equal to or greater than 5 mg/mL [22]. Further studies should be conducted on the phytochemical composition of Graviola extracts to determine what cocktails of molecules could provide efficient therapeutic results without provoking side effects. According to Yang and al., flavonoïds present in the Graviola leaf could modulate the cytotoxicity of acetogenins and confers possible maximum therapeutic benefits [23]. Targeted-drug-delivery protocols where the compounds are specifically delivered to cancer cells while avoiding contact with some other cells such as neurons, might also be an option to consider.
Conclusions
Compounds extracted from different parts of the Graviola plant have been shown to inhibit key cancer metabolic pathways such as glucose intake, ATP synthesis, cancer cell survival, growth and metastasis which makes them a potentially excellent anti-cancer drug candidate. While it is known that Graviola affects the activity of key cancer protein complexes such as HIF-1 alpha or NOX, genetic biomarkers are not the only players controlling cell metabolism. Graviola alteration of multiple metabolic pathways at once could be due to its capacity to downregulate of one or several upstream epigenetic factors. Recent studies have shown that non-coding RNAs (microRNA and LncRNA), previously considered as “junk RNA” play a central role in the regulation gene expression at the transcriptional, post-transcriptional and epigenetic levels and might act as a proto-oncogene playing a critical role in the occurrence and development of human tumors [12,24]. LncRNAs correspond to an important population of the cell transcriptome which accumulation has been linked to cancer.
Li et al. Suggested the existence of a link between the upregulation of lncRNA urothelial cancer-associated 1 (UCA1) and the altered glucose metabolism in cancer bladder cells [25] and a study conducted on glioma cells suggests that UCA1 enhances the proliferation and cell cycle progression of cancer cells by upregulating Cyclin D1 transcription [24]. LncRNAs are considered as viable therapeutic targets to combat various aspects of cancer progression and there is currently a great interest in identifying molecules that act as transcriptional inhibitors of lncRNas [26]. Given the multiple impact of Graviola extracts on critical cancer metabolic pathways, there is a good probability that Graviola phytochemicals be such promising anti-cancer molecules.
Acknowledgements
The author would like to take the opportunity to acknowledge the contribution of UPRM undergraduate students Gabriela De Leon Albors, Adriana Diaz Bula and Bryan Vega Sanabria for their bibliographical help.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Competing interest
The authors declare that they have no competing interests.
References
- Moghadamtousi SZ, Fadaeinasab M, Nikzad S, Mohan G, Ali HM, et al. (2015) Annona muricata (Annonaceae): A review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci 16: 15625-15658. [Crossref]
- Qazi, AK, Siddiqui, J, Jahan, R, Chaudhary, S, Walker, LA, et al. (2018) Emerging Therapeutic Potential of Graviola and its Constituents in Cancers. Carcinogenesis 39: 522-533. [Crossref]
- Hansra DM, Silva O, Mehta A, Ahn E (2014) Patient with Metastatic Breast Cancer Achieves Stable Disease for 5 Years on Graviola and Xeloda after Progressing on Multiple Lines of Therapy. Adv breast cancer Res 3: 84-87.
- Pieme CA, Kumar SG, Dongmo MS (2014) Antiproliferative activity and induction of apoptosis by Annona muricata (Annonaceae) extract on human cancer cells. BMC Complement Altern Med 14: 516. [Crossref]
- Deep G, Kumar R, Jain AK, et al. (2016) Graviola inhibits hypoxia-induced NADPH oxidase activity in prostate cancer cells reducing their proliferation and clonogenicity. Sci Rep 6: 23135. [Crossref]
- Kuete V, Dzotam JK, Voukeng IK, Fankam AG, Efferth T (2016) Cytotoxicity of methanol extracts of Annona muricata, Passiflora edulis and nine other Cameroonian medicinal plants towards multi-factorial drug-resistant cancer cell lines. Springerplus 5: 1666. [Crossref]
- Moghadamtousi SZ, Kadir HA, Paydar M, Rouhollahi E, Karimian H (2014) Annona muricata leaves induced apoptosis in A549 cells through mitochondrial-mediated pathway and involvement of NF-? B. BMC Complement Altern Med 14: 795-801. [Crossref]
- Dai Y, Hogan S, Schmelz EM, Ju YH, Canning C, et al. (2011) Selective growth inhibition of human breast cancer cells by graviola fruit extract in vitro and in vivo involving downregulation of EGFR expression. Nutr Cancer 63: 795-801. [Crossref]
- Robles, J.F., Muñoz, K., Rivera N., Suarez, E (2017) Anti-proliferative Properties of Methanolic Extracts of Annona muricata in Colon, Lung and Skin Cancer Cell Lines. FEBS.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646-674. [Crossref]
- Muz B, Puente P de la, Azab F, Azab AK (2015) The role of hypoxia in cancer progression angiogenesis metastasis and resistane to therapy. Hypoxia 3: 83-9. [Crossref]
- Gao JL, Chen YG. Natural compounds regulate glycolysis in hypoxic tumor microenvironment. Biomed Res Int. 2015: 354143. [Crossref]
- Torres MP, Rachagani S, Purohit V, Pandey P, Joshi S et al. (2012) Graviola: A novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett 323: 29-40. [Crossref]
- Son YR1, Choi EH1, Kim GT2, Park TS2, Shim SM1 (2016) Bioefficacy of Graviola leaf extracts in scavenging free radicals and upregulating antioxidant genes. Food Funct 7: 861-871. [Crossref]
- Höllerhage M1, Rösler TW2, Berjas M3, Luo R4, Tran K5, et al. (2015) Neurotoxicity of Dietary Supplements from Annonaceae Species. Int J Toxicol 34: 543-550. [Crossref]
- Kim G-T, Tran NKS, Choi E-H, Song YJ, Song JH et al. (2016) Immunomodulatory Efficacy of Standardized Annona muricata (Graviola) Leaf Extract via Activation of Mitogen-Activated Protein Kinase Pathways in RAW 264.7 Macrophages. Evidence-Based Complement Altern Med. 2016: 2905127. [Crossref]
- Kuete V, Dzotam JK, Voukeng IK, Fankam AG, Efferth T (2016) Cytotoxicity of methanol extracts of Annona muricata, Passiflora edulis and nine other Cameroonian medicinal plants towards multi-factorial drug-resistant cancer cell lines. Springerplus 5: 1666. [Crossref]
- Liu N, Yang HL, Wang P, Lu YC, Yang YJ, et al. (2016) Functional proteomic analysis revels that the ethanol extract of Annona muricata L. induces liver cancer cell apoptosis through endoplasmic reticulum stress pathway. J Ethnopharmacol 189: 210-217. [Crossref]
- Syed Najmuddin SUF, Romli MF, Hamid M, Alitheen NB, Abd Rahman NMAN (2016) Anti-cancer effect of Annona Muricata Linn Leaves Crude Extract (AMCE) on breast cancer cell line. BMC Complement Altern Med 16: 311. [Crossref]
- Höllerhage M1, Rösler TW2, Berjas M3, Luo R4, Tran K5, et al. (2015) Neurotoxicity of Dietary Supplements from Annonaceae Species. Int J Toxicol 34: 543-550. [Crossref]
- Lannuzel A, Michel PP, Caparros-Lefebvre D, Abaul J, Hocquemiller R, et al. (2002) Toxicity of annonaceae for dopaminergic neurons: Potential role in atypical parkinsonism in Guadeloupe. Mov Disord 17: 84-90. [Crossref]
- Bustos AVG, Jiménez MG, Mora RMS (2017) The Kuete leaf ethanol extract affects mobility and reproduction in mutant strain NB327 Caenorhabditis elegans. Biochem Biophys Reports 10: 282-286. [Crossref]
- Yang C, Gundala SR, Mukkavilli R, Vangala S, Reid MD, Aneja R (2015) Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis 36: 656-665. [Crossref]
- Zhao W, Sun C2, Cui Z3 (2017) A long noncoding RNA UCA1 promotes proliferation and predicts poor prognosis in glioma. Clin Transl Oncol 19: 735-741. [Crossref]
- Li Z, Li X, Wu S, Xue M, Chen W (2014) Long non-coding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTOR-STAT3/microRNA143 pathway. Cancer Sci 105: 951-955. [Crossref]
- Xiao ZD, Zhuang L1, Gan B2 (2016) Long non-coding RNAs in cancer metabolism. Bioessays 38: 991-996. [Crossref]

