Beta-blockers are drugs indicated in the treatment of multiple cardiovascular pathologies. The review highlights the mechanisms of action of Nebivolol and its particular potential effect on β3 receptors that increases nitric oxide that may mark very significant differences, which are traduced to benefits in clinical results.
Nebivolol has an special place in the treatment of adrenergic hypertension associated with tachycardia and emotional stress, more frequently in young individuals, and may be considered adequate even in patients with glucose and lipid metabolism disorders due to its pleiotropic effect.
In patients with heart failure, Nebivolol showed effectiveness and safety in patients over 75 years old.
Nebivolol showed a reduction of cardiomyocyte apoptosis and improvement of contractile function through a mechanism related to β3 receptor agonism after an acute coronary syndrome.
Because of all these actions Nebivolol should be considered not only a third-generation Beta-blocker.
Beta-blockers are drugs indicated in the treatment of multiple cardiovascular pathologies, with different level of evidence. Despite the fact that they are divided into first-generation to third-generation drugs and according to their selectivity by beta and alpha receptors, they are often mentioned as belonging to the same group [1].
The purpose of this review is to highlight that the mechanisms of action of the beta-blockers may mark very significant differences, which are seen in clinical results [2,3]. In this sense, Nebivolol seems to be a drug that due to its pharmacodynamics and its particular potential effect on β3 receptors and the increase of nitric oxide (NO) [4], may be considered in a different place in comparison to a conventional beta-blocker.
Propranolol is the oldest beta-blocker, dating back to the 1960s. It has an equal affinity for ß1 and ß2 receptors and is a non-selective beta-adrenergic antagonist. It is highly lipophilic and has a half-life of 3 to 5 hours, even though the duration of action is higher. It is indicated in cases of high blood pressure [5], angina [6] and some arrhythmias [7,8], frequently with two or more daily doses taken orally. It was one of the first beta-blockers that showed benefits.
Atenolol is a selective β1 receptor inhibitor, hydrophilic, highly used in spite of not having shown decrease of arrhythmias or mortality after myocardial infarction [9]. Compared with angiotensin-converting-enzyme inhibitors (ACE inhibitors), atenolol does not improve the arteriolar resistance in hypertensive patients [10] and has a short duration of action that does not allow for a homogeneous antihypertensive effect during 24 hours [11]. What is more, it was associated with an increase in diabetes and stroke risk and total mortality when compared with other agents [12], especially in elderly patients [13].
Bisoprolol, like atenolol, is also a second-generation beta-blocker, with greater selectivity for β1 receptors, is lipophilic and has a half-life of 11 to 17 hours. There are randomized clinical trials that have proven the benefits of bisoprolol in cases of heart failure [14]. However, despite the fact that the studies conducted on high blood pressure do not have the same methodological rigor, it is also approved for its use in cases of hypertension [15].
Carvedilol is a non-selective beta-blocker with additional blockades of ἀ receptors, which gives it a vasodilatory effect. At high doses, it blocks the entry of calcium. Milligram per milligram, it is two to four times more potent than propranolol as ß antagonist [16,17]. In the U.S. Carvedilol Heart Failure Program [18], it is used in the treatment of heart failure, and it is also indicated for high blood pressure and myocardial ischemia.
Even though beta-blockers have been used for almost half a century for the treatment of HBP, they are not drugs considered as first-line treatment by national [19] and international [20,21] treatment guidelines.
The antihypertensive effect of second-generation beta-blockers is achieved through the reduction of the cardiac minute volume, the heart rate and the contractility, with no effect on peripheral vascular resistance [22]. Third-generation beta-blockers have vasodilatory properties through blockades of ἀ receptors (carvedilol) or through the increase of nitric oxide (nebivolol), which lowers the peripheral resistance maintaining the minute volume [23].
The low effectiveness for the prevention of strokes with beta-blockers has been attributed to a low ability to decrease the central systolic pressure and the pulse pressure. However, they have proved effective in the prevention of cardiovascular events in patients with a recent myocardial infarction or with heart failure [24].
The national and international treatment guidelines [16,20] clearly state that beta-blockers with a vasodilatory effect have advantages over other beta-blockers since they reduce the central pressure of the pulse and the aortic rigidity [25-27]. There is evidence that the central aortic pressure is an independent predictor of cardiovascular structural damage and clinical events [28-31].
Nebivolol has a greater selectivity of β1 receptor blockages than other beta-blockers [32] (Figure 1).
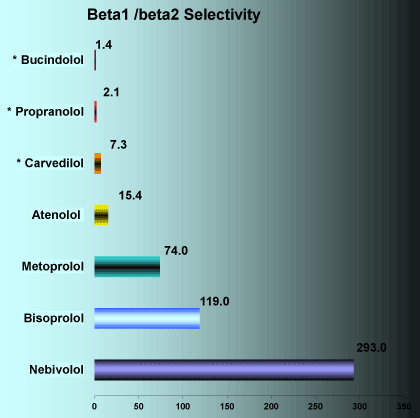
Adapted from data of the British Journal of Pharmacology (2001) 133, 1330 ± 1338
Figure 1. Comparison of Beta-blockers blockade selectivity of β1 receptors.
Unlike carvedilol, Nebivolol exercises its vasodilatory effect through the production of nitric oxide derived from the endothelium by stimulating the nitric oxide synthase (NOS) mediated by β3 receptor agonism [33,34] (Figure 2).
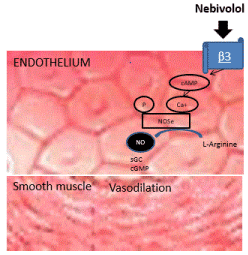
Figure 2. Vasodilation mediated by β3 receptors and NO of Nebivolol.
Endothelial dysfunction caused by oxidative stress is an essential mechanism involved in high blood pressure [35] and is associated with the prognosis in cardiovascular disease [36]. Nebivolol showed reduction of oxidative stress, which may also be an explanation for the better metabolic profile of Nebivolol in connection with glucose and lipids [37-39].
The studies that assessed Nebivolol against placebo in patients with high blood pressure showed a significant reduction of systolic (SBP) and diastolic blood pressure (DBP) [40], with a broad safety margin, and few adverse effects (headache 7.1% versus 5.9 with placebo, fatigue 3.6% vs. 1.5% with placebo and dizziness 2.9 vs. 2.0% with placebo), without differences in the treatment discontinuation rate (2.6% with Nebivolol versus 2.0% with placebo). These results were concordant in the subgroup analysis [41] and especially beneficial in the group of young patients [42], a special target for the incidence of adrenergic hypertension.
Compared with angiotensin-converting-enzyme inhibitors (ACE inhibitors), Nebivolol showed a greater percentage of patients that reached the target values and was comparable to the angiotensin 2 receptor antagonists (AT2) and calcium channel blockers [43]. Maximum antihypertensive action is observed between week 2 and 8 of treatment, which is intermediate between the ACE inhibitors (slower) and amlodipine (faster) [44].
The action on nitric oxide may be of great benefit, preventing erectile dysfunction [45] reported with other beta-blockers [46,47], which is one of the main adverse effects feared by young patients.
These additional benefits reinforce the potential role of Nebivolol in the treatment of adrenergic hypertension associated with tachycardia and emotional stress, more frequently in young individuals, and may be considered adequate even in patients with glucose and lipid metabolism disorders [48] due to its pleiotropic effect [49]. In patients with hypertension and type 2 diabetes, Nebivolol has demonstrated reductions in mean glucose levels and HbA1c across several age groups [50] and increases in HDL cholesterol (5 mg/dl) were observed [51].
There are no doubts about the benefit of beta-blockers in the treatment of heart failure [52]. They reduce mortality and morbidity by approximately 30% over 5 years [53]. They have a grade I recommendation, with a level of evidence A in all international and national treatment guidelines (20) together with the ACE inhibitors. The beneficial effect in this pathology would be associated with a reduction of adrenergic stimulation modulating the sympathetic-vagal balance and the variability of the heart rate, in addition to improving heart performance [44]. The deleterious effects of beta-blockers are related to the reduction of inotropism and chronotropism, which is why they must be administered in patients that are hemodynamically stable.
The distinctive pharmacologic profile of Nebivolol is explained by some relevant hemodynamic effects: 1- The highly selective ß1 blockade reduces heart rate at rest and on exertion as well as SBP and DBP, without causing adverse effects related to the β2 receptor blockade [54] and maintaining the balance with the alpha receptors at vascular level. 2- Vasodilation mediated by NO results in the reduction of peripheral vascular resistance, the improvement of remodeling and of arterial stiffness [55,56] and in the increase of systolic volume and ejection fraction maintaining the minute volume [44,57].
When compared to other beta-blockers, Nebivolol did not cause negative hemodynamic effects such as increase of pulmonary artery pressure and pulmonary capillary pressure (PCP) and decrease of cardiac minute volume [58], unlike Metoprolol tartrate. The hemodynamic profile and tolerance to exertion was also better in comparison to Atenolol [59], and when compared to Carvedilol (CARNEBI (Multiparametric comparison of CARvedilol, vs. NEbivolol, vs. BIsoprolol in moderate heart failure cardiopulmonary trial), patients with moderate HF had better physical capacity [60].
Nebivolol is a 1:1 racemic combination of a D- and an L-isomer. The D-isomer grants it a blocking effect for ß1 receptors and the L-isomer is mainly responsible for the stimulating action of NOSe [56] (Figure 3).
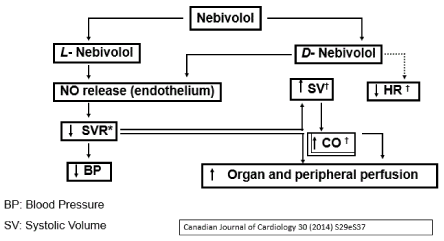
Figure 3. Synergy of combined action of beta-blockade and increase of NO with Nebivolol.
When studying the randomized clinical trials (RCT) conducted on patients with heart failure, it is observed that they included younger populations than the average age of patients that are hospitalized in real life due to this pathology. The average age of RCT patients is around 60 years old and only 25% are > 75 years old [44].
In our country, according to the last census [61], there are more than 4 million people older than 70, and in accordance with the prevalence of the disease, it is estimated that there are more than 300,000 people with heart failure in that age group [62].
Although some of the studies that have assessed the treatment with beta-blockers in HF have analyzed the results in elderly patients, these studies were not designed to reach statistically significant conclusions [63,64].
The SENIORS study (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors with Heart Failure) [65] enrolled patients with heart failure, and established as inclusion criteria that the patients were 70 years old or older and incorporated a high percentage of women and patients with preserved systolic function. It showed a reduction of 14% in the mortality from all causes, and there were no more adverse effects than with placebo. This last datum is also relevant considering that, in the treatment with other beta-blockers, advanced age was a determining factor of intolerance risk [66].
The SENIORS study reported an improvement in cardiac function and diameter, but the hospitalization rate had not decreased. In patients under 75 years old (median age of the population) and with ejection fraction <35%, a decrease of the primary final event of 38% (hazard ratio of 0.62) was observed, in concordance with other beta-blockers in previous studies [44].
Treatment with beta-blockers is indicated in patients with acute and chronic coronary artery disease [67-69]. Although in some countries Nebivolol is not indicated in this pathology, there is evidence that, in comparison to Atenolol, it improves exercise tolerance and the time until the onset of angina in the exercise test in patients with stable coronary artery disease [70] and also improves coronary flow reserve [71].
Even in patients with cardiac syndrome X and endothelial dysfunction, Nebivolol was associated with an improvement of the exercise time, less exercise-induced ischemia and less angina attacks than Metoprolol [72].
In acute coronary syndromes, a small-scale study showed that patients that had an infarction with ventricular dysfunction had fewer events (infarction/death/hospitalization due to coronary syndrome/stroke or need of revascularization) over 12 months than those patients treated with Metoprolol and similar to those treated with Carvedilol [73].
The additional benefit may be related to an antiplatelet effect showed with Nebivolol [74,75].
However, there is a new paradigm related to the agonist effect of β3 receptor.
For many years we understood the hemodynamic and myocardial functioning based on two beta receptors at myocardial level: β1 and β2 and the ἀ receptors at a vascular level, together with the neurohumoral behavior of the renin-angiotensin-aldosterone system (RAAS).
The endogenous catecholamines act at a myocardial level through the union to these β myocardial receptors regulating heart rate and contractility. Special situations may cause an increase of the catecholamine levels and an increase of the cardiac activity, as occurs, for instance, in adrenergic hypertension or in heart failure. In this last case, a mechanism that is initially compensatory becomes harmful due to cellular toxicity and apoptosis, consequence of the activation and “overstimulation” of β1 receptors at myocardial level.
The discovery of β3 receptors in atria and in ventricles [76] forces us to redefine the model. These receptors, encoded in chromosome 8 [77], were known in the adipose tissue, where they regulate thermogenesis, and were also described at the muscular level of bladder and gallbladder.
In the myocardium, it was observed that in situations where there are high levels of catecholamines, the upregulation of β3 receptors occurs [78,79]. The union to these receptors causes attenuation of inotropism, favoring a counterbalance of β1 receptors against "deleterious overexposure" of catecholamines that may derive in hypertrophy, fibrosis and apoptosis. Thus, the β3 receptor agonism may exercise a protective effect at myocardial level [80,81]. The activation of one or another β receptor depends on the clinical situation and the circulating catecholamine levels.
The β3 effect is connected with the activation through NO. There are 3 isoforms of NO synthase (NOS). In patients with heart failure, the NOSe (endothelial) and ONSn (neural) are the ones responsible for the protective effect through the increase of NO at myocardial level [82,83].
Several studies have shown that Nebivolol causes its effect through the combined action of β1 receptors blockade and β3 receptors agonism. At a vascular level, the increase of NO causes vasodilation, and at a myocardial level, it favors the necessary balance to achieve a balanced inotropic effect, preventing the increase of catecholamines from becoming deleterious (Figure 4).
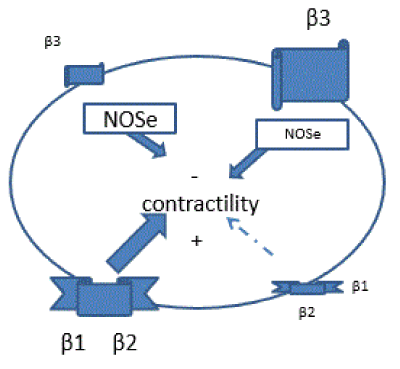
Figure 4. Modifications of β receptors in the cardiomyopathy.
β3 receptor agonism at a coronary vascular level causes vasorelaxation mediated by nitric oxide (NO), especially in microvasculature. Considering that an important component of cardiac remodeling is the adaptation of the capillary density under hemodynamic stress and neoangiogenesis [84], the action at the vascular level of β3 agonists may be part of the improvement of post-infarction remodeling [85] observed with Nebivolol, jointly with a paracrine effect at fibroblast level [86] that improves the formation of scar tissue and peripheral vasodilation contributing to the improvement of ventricular relaxation.
In experimental studies in animals with recent myocardial infarction, treatment with Nebivolol showed a reduction of cardiomyocyte apoptosis and improvement of contractile function through a mechanism related to β3 receptor agonism [87].
These same mechanisms could explain part of the benefit observed in the SENIOR study in patients with chronic heart failure [65].
Beta-blockers proved beneficial in different clinical scenarios. A big part of the benefit was explained by the antiarrhythmic and antihypertensive effect of these drugs, added to a decrease of the deleterious action of catecholamines against excessively high levels.
The initial approach was to differentiate beta-blockers according to their affinity to β1 and β2 receptors. Then, focusing on the neurohumoral hypothesis and reassessing the hemodynamic control of ventricular dysfunction, an action on peripheral resistance was considered essential, and carvedilol differed due to its vasodilatory effect through blockade of ἀ receptors at vascular level. However, NO is a more potent vasodilator and is physiologically present in situations of ischemia. The discovery of the β3 agonist action with the consequent action of NO at myocardial and vascular level could change the paradigm. Nebivolol acting as agonist would have additional beneficial effects, at the level of ventricular remodeling, in patients with different degrees of ventricular dysfunction and in subpopulations specifically assessed as elderly patients.
Authors would like to thank Teva laboratory.
- Goodman, Gilman (1993) Las bases farmacológicas de la terapéutica. 8va edición.chapter 10. Hoffman BB, Lefkowit RL. Ed. Panamericama.
- Larochelle P, Tobe SW, Lacourcière Y (2014) I-Blockers in hypertension: studies and meta-analyses over the years. Can J Cardiol 30: S16-22. [Crossref]
- Poirier L, Tobe SW (2014) Contemporary use of I-blockers: clinical relevance of subclassification. Can J Cardiol 30: S9-9S15. [Crossref]
- Fongemie J, Felix-Getzik E (2015) A review of nebivolol pharmacology and clinical evidence. Drugs 75: 1349-1371. [Crossref]
- Holland OB, Kaplan NM (1976) Propranolol in the treatment of hypertension. N Engl J Med 294: 930-936. [Crossref]
- Parker JO (1987) Nitrate therapy in stable angina pectoris. N Engl J Med 316: 1635-1642. [Crossref]
- Delacrétaz E1 (2006) Clinical practice. Supraventricular tachycardia. N Engl J Med 354: 1039-1051. [Crossref]
- Page RL (2004) Clinical practice. Newly diagnosed atrial fibrillation. N Engl J Med 351: 2408-2416. [Crossref]
2021 Copyright OAT. All rights reserv
- Biccard BM, Sear JW, Foex P (2006) Are lipophilic beta-blockers preferable for peri-operative cardioprotection? S Afr J Anaesth Analg 12: 141-146. [Crossref]
- Schiffrin EL, Deng LY, Larochelle P (1994) Effects of a beta-blocker or a converting enzyme inhibitor on resistance arteries in essential hypertension. Hypertension 23: 83-91.
- Neutel JM, Schnaper H, Cheung DG, Graettinger WF, Weber MA (1990) Antihypertensive effects of beta-blockers administered once daily: 24-hour measurements. Am Heart J 120: 166-171. [Crossref]
- Bangalore S, Parkar S, Grossman E, Messerli FH (2007) A meta-analysis of 94, 492 patients with hypertension treated with beta blockers to determine the risk of new-onset diabetes mellitus. Am J Cardiol 100: 1254-1262. [Crossref]
- Kuyper LM, Khan NA (2014) Atenolol vs nonatenolol β-blockers for the treatment of hypertension: a meta-analysis. Can J Cardiol 30: S47-53. [Crossref]
- CIBIS-II Investigators and Committees (1999) The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomised trial. Lancet 353: 9-13. [Crossref]
- 2013 ESH/ESC Guidelines for the management of arterial hypertension. European Heart J 34: 2159-2219.
- Nichols AJ, Gellai M, Ruffolo RR Jr (1991) Studies on the mechanism of arterial vasodilation produced by the novel antihypertensive agent, carvedilol. Fundam Clin Pharmacol 5: 25-38. [Crossref]
- Frishman WH (1998) Carvedilol. N Engl J Med 339: 1759-1765. [Crossref]
- Bristow MR, Gilbert EM, Abraham WT, Adams KF, Fowler MB, et al. (1996) Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 94: 2807-2816. [Crossref]
- REVISTA ARGENTINA DE CARDIOLOGÍA / VOL 81 SUPLEMENTO 2 [VOL 81 SUPPLEMENT 2] / AUGUST 2013.
- Marcelo Mallagray, Daniel Piskorz, Carlos Secotaro, Pablo Clementi. Consejos para el manejo, tratamiento de la Hipertensión Arterial y Prevención de Enfermedades Cardiovasculares. Revista FAC 2007.
- 2013 ESH/ESC Guidelines for the management of arterial hypertension. European Heart J 34: 2159-2219.
- De Caterina AR, Leone AM (2011) The role of Beta-blockers as first-line therapy in hypertension. Curr Atheroscler Rep 13: 147-153. [Crossref]
- Vanhoutte PM, Gao Y (2013) Beta blockers, nitric oxide, and cardiovascular disease. Curr Opin Pharmacol 13: 265-273. [Crossref]
- Law MR, Morris JK, Wald NJ (2009) Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 338: b1665. [Crossref]
- Boutouyrie P, Bussy C, Hayoz D, Hengstler J, Dartois N, et al. (2000) Local pulse pressure and regression of arterial wall hypertrophy during long-term antihypertensive treatment. Circulation 101: 2601-2606.
- Dhakam Z, Yasmin, McEniery CM, Burton T, Brown MJ, et al. (2008) A comparison of atenolol and nebivolol in isolated systolic hypertension. J Hypertens 26: 351-356. [Crossref]
- Kampus P, Serg M, Kals J, Zagura M, Muda P, et al. (2011) Differential effects of nebivolol and metoprolol on central aortic pressure and left ventricular wall thickness. Hypertension 57: 1122-1128. [Crossref]
- London G, Blacher J, Pannier B, Guerin AP, Marchais SJ, Safar ME (2001) Arterial wave reflections and survival in end-stage renal failure. Hypertension 38: 434-438. [Crossref]
- Kingwell BA, Waddell TK, Medley TL, Cameron JD, Dart AM (2002) Large artery stiffness predicts ischemic threshold in patients with coronary artery disease. J Am Coll Cardiol 40: 773-779. [Crossref]
- Jankowski P, Kawecka-Jaszcz K, Bryniarski L, Czarnecka D, Brzozowska-Kiszka M, et al. (2004) Fractional diastolic and systolic pressure in the ascending aorta are related to the extent of coronary artery disease. Am J Hypertens 17: 641-646. [Crossref]
- The CAFE investigators, for the anglo-scandinavian cardiac outcomes trial (ASCOT) investigators (2006) Differential impact of blood pressure lowering drugs on central aortic pressure and clinical outcomes. Circulation 113: 1213-1225.
- Brixius K, Bundkirchen A, BoÈ lck B, Mehlhorn U, Schwinger RHG (2001) Nebivolol, bucindolol, metoprolol and carvedilol are devoid of intrinsic sympathomimetic activity in human myocardium. Br J Pharmacol 133: 1330-1338. [Crossref]
- Bowman AJ, Chen CP, Ford GA (1994) Nitric oxide mediated venodilator effects of nebivolol. Br J Clin Pharmacol 38: 199-204. [Crossref]
- Cockcroft JR, Chowienczyk PJ, Brett SE, Chen CP, Dupont AG, et al. (1995) Nebivolol vasodilates human forearm vasculature: evidence for an L-arginine/NO-dependent mechanism. J Pharmacol Exp Ther 274: 1067-1071. [Crossref]
- Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, Touyz RM (2015) Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol 31: 631-641. [Crossref]
- Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T (2001) Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104: 2673-2678. [Crossref]
- Serg M, Kampus P, Kals J, Zagura M, Zilmer M, et al. (2012) Nebivolol and metoprolol: long-term effects on inflammation and oxidative stress in essential hypertension. Scand J Clin Lab Invest 72: 427-432. [Crossref]
- Stears AJ, Woods SH, Watts MM, Burton TJ, Graggaber J, et al. (2012) A double-blind, placebo-controlled, crossover trial comparing the effects of amiloride and hydrochlorothiazide on glucose tolerance in patients with essential hypertension. Hypertension 59: 934-942. [Crossref]
- Fonseca VA (2010) Effects of beta-blockers on glucose and lipid metabolism. Curr Med Res Opin 26: 615-629. [Crossref]
- Weiss RJ, Saunders E, Greathouse M (2011) Efficacy and tolerability of nebivolol in stage I-II hypertension: a pooled analysis of data from three randomized, placebo-controlled monotherapy trials. Clin Ther 33: 1150-1161. [Crossref]
- Germino FW, Lin Y, Pejovic V, Bowen L (2012) Efficacy and tolerability of nebivolol: does age matter? A retrospective analysis of three randomized, placebo-controlled trials in stage I–II hypertension. Ther Adv Cardiovasc Dis 6: 185-199. [Crossref]
- Giles TD, Khan BV, Lato J, Brener L, Ma Y, Lukic T (2013) Nebivolol monotherapy in younger adults (younger than 55 years) with hypertension: a randomized, placebo-controlled trial. J Clin Hypertens (Greenwich) 15: 687-693. [Crossref]
- Ambrosioni E, Bacchelli S, Esposti DD, Borghi C (2001) Beta-blockade in hypertension and congestive heart failure. J Cardiovasc Pharmacol 3: S25-31. [Crossref]
- Münzel T, Gori T (2009) Nebivolol: the somewhat-different beta-adrenergic receptor blocker. J Am Coll Cardiol 54: 1491-1499. [Crossref]
- Sharp RP, Gales BJ (2017) Nebivolol versus other beta blockers in patients with hypertension and erectile dysfunction. Thera Adv Urol 9: 59-63. [Crossref]
- Boydak B, Nalbantgil S, Fici F, Nalbantgil I, Zoghi M, et al. (2005) A randomised comparison of the effects of nebivolol and atenolol with and without chlorthalidone on the sexual function of hypertensive men. Clin Drug Investig 25: 409-416. [Crossref]
- Brixius K, Middeke M, Lichtenthal A, Jahn E, Schwinger RH (2007) Nitric oxide, erectile dysfunction and beta-blocker treatment (MR NOED study): benefit of nebivolol versus metoprolol in hypertensive men. Clin Exp Pharmacol Physiol 34: 327-331. [Crossref]
- Schmidt A, Graf C, Brixius K, Scholze J (2007) Blood pressure-lowering effect of nebivolol in hypertensive patients with type 2 diabetes mellitus: The YESTONO Study. Clin Drug Invest 27: 841-849. [Crossref]
- Celik T, Iyisoy A, Kardesoglu E, Fici F (2009) The anti-inflammatory effects of Nebivolol in human coronary smooth muscle cells: clinical implications. Int J Cardiol 133: 415-416. [Crossref]
- Ladage D, Reidenbach C, Rieckeheer E, Graf C, Schwinger RH, et al. (2010) Nebivolol lowers blood pressure and increases weight loss in patients with hypertension and diabetes in regard to age. J Cardiovasc Pharmacol 56: 275-281. [Crossref]
- Peter P, Martin U, Sharma A, Dunne F (2006) Effect of treatment with nebivolol on parameters of oxidative stress in type 2 diabetics with mild to moderate hypertension. J Clin Pharm Ther 31: 153-159. [Crossref]
- Chatterjee S, Biondi-Zoccai G, Abbate A, D'Ascenzo F, Castagno D, et al. (2013) Benefits of β blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ 346: f55. [Crossref]
- Shibata MC, Flather MD, Wang D (2001) Systematic review of the impact of beta blockers on mortality and hospital admissions in heart failure. Eur J Heart Fail 3: 351-357. [Crossref]
- Zuber MEP (2004) Changes in peak respiratory flow and quality of life during nebivolol therapy. Heart Drug 4: 103-108.
- Münzel T, Gori T (2009) Nebivolol: the somewhat-different beta-adrenergic receptor blocker. J Am Coll Cardiol 54: 1491-1499. [Crossref]
- Howlett JG (2014) Nebivolol: vasodilator properties and evidence for relevance in treatment of cardiovascular disease. Can J Cardiol 30: S29-37. [Crossref]
- Brune S, Schmidt T, Tebbe U, Kreuzer H (1990) Hemodynamic effects of nebivolol at rest and on exertion in patients with heart failure. Angiology 41: 696-701. [Crossref]
- Triposkiadis F, Giamouzis G, Kelepeshis G, Sitafidis G, Skoularigis J, et al. (2007) Acute hemodynamic effects of moderate doses of nebivolol versus metoprolol in patients with systolic heart failure. Int J Clin Pharmacol Ther 45: 71-77. [Crossref]
- Nodari S, Metra M, Dei Cas L (2003) b-Blocker treatment of patients with diastolic heart failure and arterial hypertension. A prospective, randomized, comparison of the long-term effects of atenolol vs. nebivolol. Eur J Heart Fail 5: 621-627. [Crossref]
- Contini M, Apostolo A, Cattadori G, Paolillo S, Iorio A, et al. (2013) Multiparametric comparison of CARvedilol, vs. NEbivolol, vs. BIsoprolol in moderate heart failure: the CARNEBI trial. Int J Cardiol 168: 2134-2140. [Crossref]
- CENSO 2012 ARGENTINA [2012 ARGENTINE CENSUS]. http://www.indec.gov.ar
- Insuficiencia Cardiaca Crónica. Dr. Fernando de la Serna. Cap. 1: Epidemiología de la IC. Editorial Federación Argentina de Cardiología. 3ra. Edición 2010
- Hjalmarson A, Goldstein S, Fagerberg B, et al. (2000) Effects of controlled release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: The Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA 283: 1295-1302.
- Packer M, Fowler MB, Roecker EB, Coats AJ, Katus HA, et al. (2002) Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 106: 2194-2199. [Crossref]
- Ghio S, Magrini G, Serio A, Klersy C, Fucili A, et al. (2006) Effects of nebivolol in elderly heart failure patients with or without systolic left ventricular dysfunction: results of the SENIORS echocardiographic substudy. Eur Heart J 27: 562-568. [Crossref]
- Krum H, Hill J, Fruhwald F, Sharpe C, Abraham G, et al. (2006) Tolerability of beta-blockers in elderly patients with chronic heart failure: the COLA II study. Eur J Heart Fail 8: 302-307. [Crossref]
- Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, et al. (2011) ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 32: 2999-3054. [Crossref]
- ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 33: 2569-2619.
- Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, et al. (2013) 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 34: 2949-3003. [Crossref]
- Van Bortel LM, van Baak MA (1992) Exercise tolerance with nebivolol and atenolol. Cardiovasc Drugs Ther 6: 239-247. [Crossref]
- Galderisi M, D'Errico A (2008) Beta-blockers and coronary flow reserve: the importance of a vasodilatory action. Drugs 68: 579-590. [Crossref]
- Sen N, Tavil Y, Erdamar H, Yazici HU, Cakir E, et al. (2009) Nebivolol therapy improves endothelial function and increases exercise tolerance in patients with cardiac syndrome X. Anadolu Kardiyol Derg 9: 371-379. [Crossref]
- Ozaydin M, Yucel H, Kocyigit S, Adali MK, Aksoy F, et al. (2016) Nebivolol versus carvedilol or metoprolol in patients presenting with acute myocardial infarction complicated by left ventricular dysfunction. Med Princ Pract 25: 316-322. [Crossref]
- Ignjatovic V, Pavlovic S, Miloradovic V, Andjelkovic N, Davidovic G, et al. (2015) Influence of different β-blockers on platelet aggregation in patients with coronary artery disease on dual antiplatelet therapy. J Cardiovasc Pharmacol Ther 21: 44-52. [Crossref]
- Karabacak M, Dogan A, Aksoy F, Ozaydin M, Erdogan D, Karabacak P (2014) Both carvedilol and nebivolol may improve platelet function and prothrombotic state in patients with nonischemic heart failure. Angiology 65: 533-537. [Crossref]
- Gauthier C, Tavernier G, Charpentier F, Langin D, Le Marec H (1996) Functional beta3-adrenoceptor in the human heart. J Clin Invest 98: 556-562. [Crossref]
- Chantal Dessy, Jean-Luc Balligand (2010) Beta3-adrenergic receptors in cardiac and vascular tissues: Emerging concepts and therapeutic perspectives. Adv Pharmacol 59: 135-163. [Crossref]
- Strosberg AD (1997) Structure and function of the beta 3-adrenergic receptor. Annu Rev Pharmacol Toxicol 37: 421-450. [Crossref]
- Stéphane Moniotte, Lester Kobzik, Olivier Feron, Jean-Noël Trochu, et al. (2001) Upregulation of b3-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation 103: 1649-1655.
- Gauthier C, Tavernier G, Charpentier F, Langin D, Le Marec H (1996) Functional beta3-adrenoceptor in the human heart. J Clin Invest 98: 556-562. [Crossref]
- Gauthier C, Leblais V, Kobzik L, Trochu JN, Khandoudi N, et al. (1998) The negative inotropic effect of beta3-adrenoceptor stimulation is mediated by activation of a nitric oxide synthase pathway in human ventricle. J Clin Invest 102: 1377-1384. [Crossref]
- Niu X, Watts VL, Cingolani OH, Sivakumaran V, Leyton-Mange JS, et al. (2012) Cardioprotective effect of beta-3 adrenergic receptor agonism: role of neuronal nitric oxide synthase. J Am Coll Cardiol 59: 1979-1987. [Crossref]
- Zhang Z, Ding L, Jin Z, Gao G, Li H, et al. (2015) Nebivolol protects against myocardial infarction injury via stimulation of Beta 3-adrenergic receptors and nitric oxide signalling. PLOS ONE 9: e98179.
- Sorrentino SA, Doerries C, Manes C, Speer T, Dessy C, et al. (2011) Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional beta1- blockade. J Am Coll Cardiol 57: 601-611. [Crossref]
- Balligand JL (2013) Beta3-adrenoreceptors in cardiovasular diseases: new roles for an "old" receptor. Curr Drug Deliv 10: 64-66. [Crossref]
- Jean-Luc Balligand (2016) Cardiac salvage by tweaking with beta-3-adrenergic receptors. Cardiovascular Research 111: 128-133. [Crossref]




