Background: Neuroendocrine carcinoma (NEC) of the oropharynx is extremely rare, with only 42 cases reported since first identified in 1972. There is no consensus on optimal management.
Methods: Two cases of oropharyngeal NEC were identified at our institution. We reviewed the presenting features, radiological findings, histopathology and management, before conducting a literature review to evaluate treatment and survival for such patients.
Results: Final diagnoses were confirmed to be T2 N1 M0 poorly differentiated NEC of the left palatine tonsil and T2 N2c M0 large cell NEC of the left soft palate. Following discussion in our multidisciplinary meeting, chemotherapy combined with radiotherapy was deemed the most appropriate treatment.
Conclusions: NEC of the oropharynx is a rare and aggressive condition with a poor prognosis. Standardised treatment guidelines have not yet been established but the overriding opinion favours chemotherapy combined with radiotherapy. For locally advanced disease, surgery appears to have little impact on overall survival. HPV status should be checked in confirmed cases to help better understand the association between HPV and oropharyngeal NEC with regard to prognosis.
neuroendocrine, small cell, oropharynx, carcinoma, malignancy
Neuroendocrine carcinoma (NEC) is a malignant epithelial neoplasm with neuroendocrine morphology. Small cell NEC most commonly originates in the pulmonary tract but can also arise from gastrointestinal, genitourinary, breast, head and neck sites [1].
The incidence of NEC in the head and neck is low and mainly arises in the larynx followed by the salivary glands, nose and paranasal sinuses [2].
NEC affecting the oropharynx is extremely rare. Only 42 cases have been reported since it was first identified by Koss et al. [3]. Misawa et al. [4] have summarised the first 40 cases and reported two further cases in 2016; giving a total of 42 cases. Due to its rarity, standardised treatment guidelines have not yet been established. Here we report 2 further cases to add to the current body of literature with an emphasis on diagnosis and management.
Case 1
A 53-year-old man presented to our maxillofacial surgery department with a sensation of a lump in his throat, odynophagia and a right neck lump. He was otherwise fit and well with a past medical history of hypertension only. Clinical examination revealed an ulcerating mass in his left soft palate and right level II cervical lymphadenopathy.
Following discussion in our head and neck multidisciplinary team (MDT) meeting, a PET CT scan was organised which confirmed intense metabolic activity in the left soft palate, right level II neck region and in the left parapharyngeal lymph nodes. There were no liver, lung or bone lesions.
A biopsy of the left soft palate lesion was performed, and the final histology was confirmed to be T2 N2c M0 large cell neuroendocrine carcinoma of the left soft palate with high proliferation index (Figures 1 and 2).
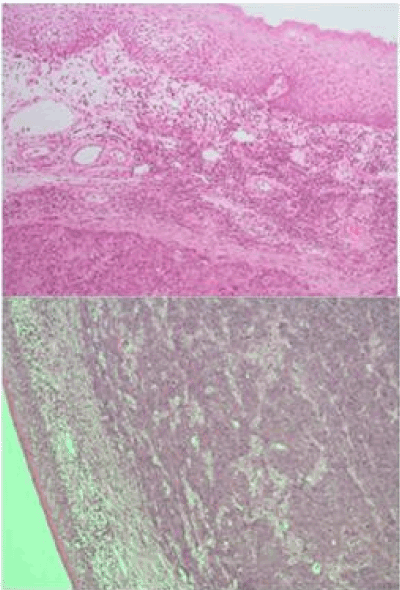
Figure 1. Poorly differentiated carcinoma undermining surface non-dysplastic stratified squamous epithelium (H&E X50). Case 1 above, Case 2 below.
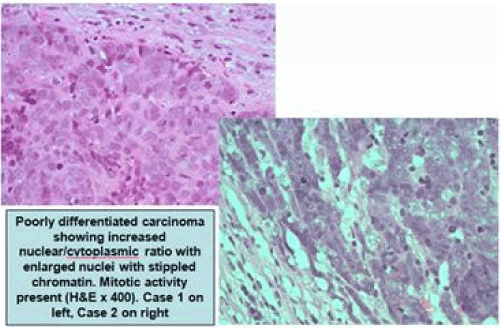
Figure 2. Poorly differentiated carcinoma showing increased nuclear/cytoplasmic ratio with enlarged nuclei with stippled chromatin. Mitotic activity present (H&E X400). Case 1 on left, Case 2 on right
Characteristic immunohistochemical features were also seen including positive staining for broad-spectrum cytokeratins (confirming poorly differentiated carcinoma), specific neuroendocrine markers, such as synaptophysin and the majority of the involved nuclei stained positively for the proliferation marker Ki67 (Figures 3-5).
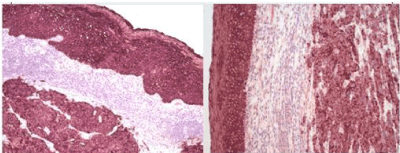
Figure 3. Positive immunohistochemical staining for broad-spectrum cytokeratins confirms poorly differentiated carcinoma (surface non-dysplastic epithelium also stains positively/internal positive control) (MCK X100). Case 1 on left, Case 2 on right.
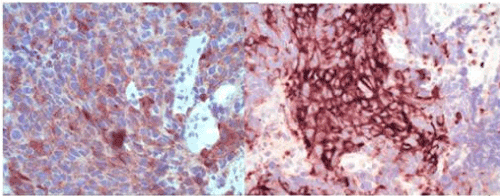
Figure 4. Positive immunohistochemical staining for specific neuroendocrine marker synaptophysin (Synaptophysin X400). Case 1 on left, Case 2 on right.
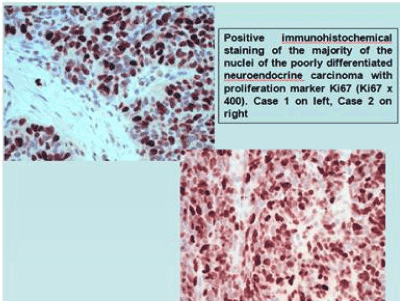
Figure 5. Positive immunohistochemical staining of the majority of the nuclei of the poorly differentiated neuroendocrine carcinoma with proliferation marker Ki67 (Ki67 X400). Case 1 on left, Case 2 on right.
After seeking advice from our neuroendocrine MDT Lead, the head and neck MDT recommendation was for radical non-surgical treatment in the form of chemoradiation. The patient received induction chemotherapy with cisplatin and etoposide for three cycles followed by radical chemo-radiotherapy with 60 grays (Gy) in 30 fractions with concurrent cisplatin and etoposide at week one and four.
The induction chemotherapy was well tolerated with very rapid clinical resolution of tumour- related pain and odynophagia. The patient was able to eat a normal diet after the first cycle with a significant reduction in tumour bulk.
A 3-month post-treatment PET CT scan showed an excellent anatomical and metabolic response to the administered therapy at the site of the primary tumour as well as previously involved lymphadenopathy; with significant reduction of tracer uptake in the palate, right level II neck and left parapharyngeal lymph nodes.
To date, the patient has achieved a disease-free survival of 18 months after completion of treatment. Despite mild xerostomia, he can eat and drink as normal. His level of function has also returned to his premorbid state and he has been able to return to employment.
Case 2
A 62-year-old man presented to our ENT department with a two-month history of odynophagia, left-sided sore throat and left otalgia. He was an ex-smoker, having stopped 12 years ago and consumed 6-8 units of alcohol per week. He had a past medical history of well controlled asthma and tuberculosis in childhood at the age of 10.
Full ENT clinical examination revealed asymmetry of the palatine tonsils with the left larger than the right, but the tonsils appeared smooth with no evidence of ulceration or exophytic mass. There were no other abnormal findings and no cervical lymphadenopathy was detected clinically.
Baseline MR and PET CT imaging were organised prior to bilateral tonsillectomy for histology. The initial MRI scan showed a sizable left tonsil mass measuring 2.3 centimetres in maximum craniocaudal diameter with some central ulceration. A prominent left level II lymph node was also noted. The PET CT scan confirmed intense metabolic activity at the site of the left tonsil mass with cervical left level II nodal involvement. There was no evidence of more distant disease.
The final histology was confirmed to be T2 N1 poorly differentiated NEC of the left palatine tonsil (Figures 1 and 2). The right tonsil showed reactive lymphoid hyperplasia only. As in case 1, characteristic immunohistochemical features were also seen (Figures 3-5).
As in case 1, our head and neck MDT recommendation were for radical non-surgical treatment in the form of chemoradiation. Similarly, the patient received induction chemotherapy with cisplatin and etoposide for two cycles followed by radical chemo-radiotherapy with 70 grays (Gy) in 35 fractions with concurrent cisplatin at week one and five.
Unfortunately, there were some delays in treatment after the second cycle of induction chemotherapy as the patient experienced diplopia with cerebellar signs. Following prompt review by our neurology colleagues, this was thought secondary to a paraneoplastic syndrome and was successfully treated with plasma exchange.
A 6-month post-treatment PET CT scan confirmed an excellent response at the site of the primary tumour and at the site of the regional left level II nodal involvement. This was seen on both anatomical and metabolic imaging.
The patient is managing a satisfactory diet but sometimes struggles with bulky and tough solids, such as meats. His xerostomia is well managed with artificial saliva substitutes and good oral hydration. To date, a disease-free survival of 10 months has been achieved and the patient is under regular follow up.
NEC of the oropharynx is rare. Typical histopathological appearances can be seen in figures 1 and 2. Immunohistochemical analysis usually demonstrates positive staining for general neuroendocrine markers including synaptophysin, CD56 and chromogranin [1].
Current evidence suggests that oropharyngeal NEC is more aggressive than the more commonly encountered SCC (squamous cell carcinoma) with a poorer prognosis. It has a propensity for development of early regional lymphatic and systemic metastases; and therefore, should be considered a ‘systemic’ disease from the outset [2]. One review suggested a median survival of 18 months for small cell NEC of the tonsil [5]. In our report, cases 1 and 2 have achieved a disease-free survival of 18 and 10 months respectively after completion of treatment to date.
In view of its aggressive nature, the common treatment modalities of surgery or radiotherapy for head and neck SCC targeting local disease may be suboptimal for oropharyngeal NEC. Thus, it has been proposed that systemic chemotherapy should be part of the treatment protocol for all cases of NEC, especially of the small cell variety [5]. This certainly guided our choice of treatment.
Moreover, our choice of induction chemotherapy agents was guided by data from the more extensively studied small cell lung cancer; which, in general, is very sensitive to platinum- based chemotherapy agents coupled with etoposide. In concordance with this, our neuroendocrine MDT lead agreed that induction chemotherapy cycles with cisplatin and etoposide followed by chemoradiation with further cycles of cisplatin and etoposide should be adequate radical treatment for the neuroendocrine tumours described in our reported cases. Notably, a recent study which analysed the largest known series of small cell carcinoma of the head and neck, showed that in patients with locally advanced disease, there was no survival benefit with the addition of surgery to chemoradiation [6].
There is emerging evidence in the literature of an association between oropharyngeal NEC and high-risk human papillomavirus (HPV) [7,8]. Due to the rarity of this tumour and therefore low numbers of reported cases, it is difficult to draw any meaningful conclusions regarding whether high-risk HPV association confers an even more aggressive disease course and therefore poorer prognosis. Kraft et al. [8] reported a series of 8 cases of HPV-associated oropharyngeal NEC. Of the 3 cases who later developed distant metastases, all were found to be HPV-associated. Of course, reliable prognostication is not possible due to low case numbers. However, it is clear from the literature that while HPV association usually confers a survival benefit for oropharyngeal SCC, it does not appear to positively influence prognosis for the more aggressive oropharyngeal NEC.
Unfortunately, HPV status was not determined in our reported cases. Having reviewed the literature, we would advocate that HPV status should be checked in all cases of oropharyngeal NEC as although yet to be confirmed, this may be associated with a poorer prognosis and increased propensity for the development of distant metastases. Moreover, if HPV status is routinely checked in cases of oropharyngeal NEC, this will increase the body of evidence and data required to draw statistically significant conclusions on the relationship between HPV status and oropharyngeal NEC.
In conclusion, NEC of the oropharynx is a rare condition which is known to be aggressive and associated with a poor prognosis. Diagnosis is based on characteristic histopathological and immunohistochemical features described above. Here, we have reported a further two cases. HPV status should also be checked routinely in confirmed cases to help better understand the association between HPV and oropharyngeal NEC with regard to prognosis. With no prospective data, recommended treatment modalities are based on retrospective information. The optimal treatment protocol, whilst uncertain, appears to favour radiotherapy combined with chemotherapy.
- Wang H-Y, Zou J, Zhou G-Y, Yan J-Q, Liu S-X (2014) Primary small cell neuroendocrine carcinoma of the tonsil: a case report and review of the literature. Int J Clin Exper Path 7: 2678-2682. [Crossref]
- Renner G (2007) Small cell carcinoma of the head and neck: a review. Semin Oncol 34: 3-14. [Crossref]
- Koss LG, Spiro RH, Hajdu S (1972) Small cell (oat cell) carcinoma of minor salivary gland origin. Cancer 30: 737-741. [Crossref]
- Misawa K, Kawasaki H, Matsuo R, Sugiyama K, Mochizuki D, et al. (2016) Human papillomavirus-associated small cell carcinoma/neuroendocrine carcinoma of the oropharynx: a report of two cases. SpringerPlus 5: 1847. [Crossref]
- Sehdev A, Zhao Y, Singh AK, Sharma N (2012) Primary small cell carcinoma of the tonsil: a case report and review of the literature. Case Rep Oncol 5: 537-541. [Crossref]
- Pointer KB, Ko HC, Brower JV, Witek M, Kimple RJ, et al. (2017) Small cell carcinoma of the head and neck: An analysis of the National Cancer Database. Oral Oncol 69: 92-98. [Crossref]
- Bates T, McQueen A, Iqbal MS, Kelly C, Robinson M (2013) Small cell neuroendocrine carcinoma of the oropharynx harbouring oncogenic HPV-infection. Head Neck Pathol 8: 127-131. [Crossref]
- Kraft S, Faquin W, Krane J (2012) HPV-associated neuroendocrine carcinoma of the oropharynx: a rare new entity with potentially aggressive clinical behavior. Am J Surg Pathol 36: 321-330. [Crossref]





