In this mini-Review was presented some of the most important and high impact developments within Neurophotonics. In this way, it was discussed about non-invasive and invasive techniques, and methods highlighting their different levels of information recorded. Thus, Magnetic Resonance Imaging, Positron emission Tomography, and other non-invasive techniques were mentioned. As well, varied Optical Set-ups, Advanced Microscopy techniques, miniaturized instrumentation, Microdevices, and portable approaches were showed. In addition, with the perspective to develop Nano-tools to incorporate in these Optical Set-ups as well new ones, it was afforded to the discussion about the design and development of Nanophotonics and Nanomedicine approaches. Therefore, the generation of non-classical light combined with Multifunctional properties such as Drug delivery, and tissue regenerative therapies could be proposed. In this manner, as for example it was presented some examples developed by us as potential exalted smart Opto-active Nanoplatforms, Ultraluminescence Bioimaging, and targeted Light delivery. In the same way, it was showed different labelling techniques, using small Organic molecules, and Laser dyes; towards new reduced sized Nano-emitters and Meta-emitters. Thus, with a point of view from the Nano-scale it was discussed the Neuro-signaling tracking by Neuro-transmitters, Ions, as well as Neuro-peptides, and Neuro-proteins correlated with healthy/unhealthy status, and behaviours associated. In this manner, it was revised the main topics within the Nano-scale towards Nanophotonics and Neurophotonics approaches.
optical approaches, miniaturized instrumentation, nano-optics, nano-chemistry, nano-pharmacology, neuro-medicine, nanomedicine, neurophotonics, nanophotonics
Actually, Neuroscience is very well developed at different levels by varied experimental approaches, strategies, and Instrumentation [1]. Thus, from behavioural studies to brain signaling, and single Neuron signaling, are currently in progress [2]. In order to afford these types of studies, it was applied different technologies to generate brain imaging [3]. Therefore, it could be registered faster data collection, analysis and diagnosis [4]. By a general overview of the techniques, it could be highlighted differentiated non-invasive and invasive techniques and methodologies. The non-invasive technologies were developed based on variable radiation-matter interaction with tissues. For example, it could be mentioned different techniques with varied data generation and power of information; as for example, i) Magnetic Resonance Imaging [5]; ii) Photoacoustic tomography [6]; iii) Positrons emission tomography (PET) imaging [7]; iv) varied advanced Microscopy techniques and methods [8]. These techniques were developed with minimal invasive procedures; however, many of them are not being used in humans, but they provide a very important data recording In Vivo from small animals mainly [9]. It should be noted that for human there are many approaches in progress by Magnetic Resonance Imaging and IR based approaches [10].
In the previous recent mentioned references, it was highlighted the high impact data recording, with capability to do it by a dairy basis due to their minimal invasive approaches. However, the invasive approaches could target different objectives looking for other types of particular needs within varied Research studies, and analysis. Moreover, other developments of new treatments as well requires other more invasive strategies where it should be applied from direct contact of molecules, pharmacophores and varied materials with brain tissues such as for cell recovery after injury [11] towards other ways of administrations related with micro-needles [12,13], devices [14], and technological approaches [15]. In this perspective, these types of developments are related as for example with design and synthesis of small molecules [16] and Nanomaterials for Neurophotonics applications [17]. By this manner, the Nanomaterial could act by transferring their properties and Energy-matter interactions for varied targeted uses as sensitive as for example for image guided surgery [18]. Therefore, from molecular, biomolecular levels, it should be highlighted as for example tracking of varied Neuro-transmitters [19] based on Organic chemical structures [20], Neuro-peptides [21], proteins [22], vesicles, exosomes [23], and Ions [24]. So, in this context it is possible to design targeted Biosensing, Neuro-signaling, and Neuro-imaging based on the control of; i) Molecules as probes, ii) the Nano-scale for Nanoplatform design, and iii) confined Optical properties. Thus, varied Nanophotonics systems could be developed for targeted interactions, labelling, non-classical light generation; drug, and Light delivery, Bioimaging and Neuro-imaging, chemical sensing, etc. In these perspectives, in this communication it was afforded as well to i) Optical-set ups, ii) miniaturized Instrumentation, and iii) varied spectroscopical and Laser based techniques for Nano-Neurophotonics approaches. In addition, it was discussed the potential of functional and Multi-functional Nanoarchitectures design that could lead to new treatments, Neuro-generation, and tissue repairing. By this manner it was introduced and updated a wide view of topics within Nanophotonics for Neurophotonics, and Nanomedicine applications accompanied with Optics, Photonics, and Instrumentation basis too.
In this section, it was afforded to the discussion about the detection and tracking of the most mentioned and recently reported Neurotransmitters [25]. These were chosen for varied reasons due to their participation within different functions and illness. In this perspective it could be of high interest; Acetylcholine [26], Cortisol, Dopamine [27,28], Glutamate [29], and Neurotransmitter-derived lipoids [30] as synaptic vesicles [31], Neuroactive steroids [32,33] and related hormones such as cortisol [34]. In similar manner, other types of Neuro-stimulators [35] should be mentioned due to their close relation with Neuro-signaling.
These Neurotransmitters, related hormones, and external chemical structures with variable intrinsic chemical constitution could be of interest in many cases with the detection of their metabolites directly In-Situ, In-Vivo and indirectly from processed real samples by different Optical approaches and Spectroscopical techniques [36]. It should be highlighted that many of these potential interests are currently studied. So, there are a high impact the development of new approaches based on the combination of Nano-tools and new Optical approaches, miniaturized instrumentation, and related Optical approaches. For example, it could be mentioned the Dopamine tracking within Amygdala that showed Signal Salient Somato sensory Events during Fear Learning effect [37]. In this Research work it was injected Fluorescent labellers into the amygdale region of mouse brain (Figure 1a) that permitted to detect varied levels of Dopamine (Figure 1b) when it was applied cycles of fear stimulation (Figure 1c). As it is known, Amygdala, region of the brain is primarily associated with emotional processes; so, it is highly sensitive to stress application.
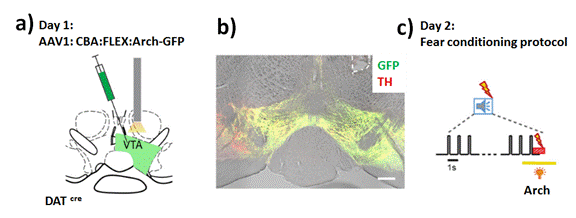
Figure 1. a) Schematic of the experimental approach for E-H. A stereotactic injection of the Cre-dependent Arch-eGFP vector into the VTA of DATCre mice was followed by unilateral optic fiber implantation above the injectionsite. b) Posthoc histologic verification of Arch-GFP expression and optic fiber placement (white dashed line) in the VTA of one example mouse. Red represents TH immunohistochemistry signal. Green represents fluorescence of Arch-eGFP. Scale bar, 200mm. c) Schematic of yellow laser light illumination during the CS-US pairing protocol. The tone block (n=30 tone beeps of 0.1 s at 1Hz) is followed by a 1 s footshock. Yellow laser light (l = 561nm) is applied for 3 s starting 1 s before the footshock to activate Arch. Reprinted with permissions of W. Tang-R. Schneggenburger et al. (cite 37) Journal of Neuroscience 2020
Moreover, it should be noted the participation of ions for Neuro-communication and signaling, such as Calcium [38], well know element participating in open gated channels between inter-neuronal contact and signaling against varied neuronal stimulation (Figure 2). In this context, it could be highlighted the use of different Fluorescent probes to detect calcium delivery. However there still exist needs for new improved probes [39] and approaches of detections due to the non-Optical activity of ions [40].
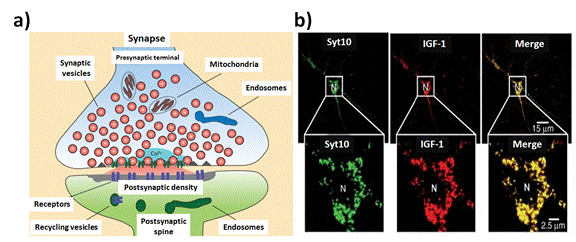
Figure 2. Tracking time course of Ca2+-triggered synaptic transmission: a) Schematic diagram of a synapse illustrating the localized influx of Ca2+ at the active zone (red = secreted neurotransmitters). b) Syt10 colocalizes with IGF-1 containing vesicles in the somatodendritic regions of cultured mitral neurons. Images show double immunofluorescence labeling of tagged Syt10 and either IGF-1, synapsin, or MAP2 as indicated (Syt10: synaptotagmins binds Ca2+). Reprinted with permission from, C. Sudhof et al. (cite 38), Cold Spring Harb Perspect Biol; respectively (2012)
It should be noted the participation of different peptides and proteins in close relation with Neuro-signaling associated with different pathways, phenomena, and illness. For example, the Tau protein as Neurophysiological signature in Alzheimer’s disease and cognitive decline [41]. Moreover, the implication of amyloid-Beta Peptides in the Triggered aggregation of Alpha-Synuclein In Vitro as well implicated as possible factors in the development of Alzheimer [42]. Moreover, other types of interactions with consequent effects on neurons such as the increase of the synaptic NMDA receptor abundance by enhancing the local translation of Pyk2 in cultured hippocampal neurons from BDNF interactions [43].
For a complete understanding of the implications of all the mentioned Biostructures and for clinical diagnoses; it should be developed further studies and Bioassays. In this perspective, to study the production of the TAU protein, it was afforded to computational modeling of tau pathology spread and experimentally by immune-fluorescent labelling within mouse brains [44]. By this manner, it was revealed the inter-connectomes and variability along the pass by Neuroimaging (Figure 3).
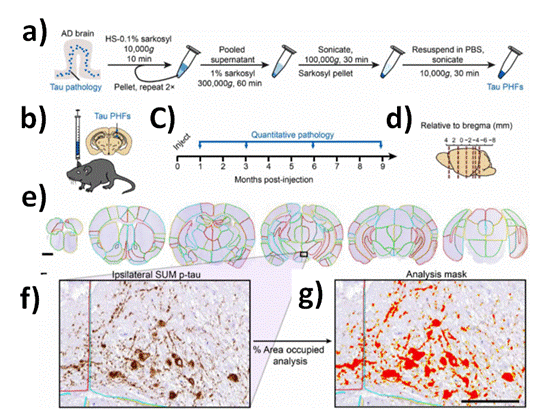
Figure 3. Quantitative immunohistochemistry to evaluate pathological tau spread. a) AD brain with a high burden of tau pathology was chosen for extraction of pathological tau. Brains went through sequential extraction of tau PHFs as noted in the schematic. Final tau PHF preparations were used for all subsequent steps. PBS, phosphate-buffered saline. HS, high salt. b) NTG mice were injected unilaterally with AD PHF tau in the hippocampus and overlaying cortex as shown at 3 to 4 months of age. c) Mice were euthanized 1 (n = 4), 3 (n = 8), 6 (n = 6), or 9 (n = 6) months following injection. d) Mouse brain was sectioned, and the sections representing the regions shown were stained for pathological tau. e) Representative sections were selected, and 194 regions were annotated for each brain. A second set of nearby sections was similarly annotated to reduce selection bias. Scale bar, 1 mm. Annotation colors are arbitrary. f) An enlarged image of the annotated supramammillary nucleus (SUM) is shown with the inclusions stained for pS202/T205 tau. g) Annotations allow automated quantification of percentage of area occupied with pathology in specific regions of the brain. An analysis mask is overlaid on the image in (f) to demonstrate this quantification of pathology. Scale bar, 100 µm. Reprinted with permissions of E. J. Cornblath-M. X. Henderson et al. (cite 46) Sci. Adv 2021
In addition, peptides released by different types of stimulations with consequent cascade signaling, such as the Oxcytocine hormone associated with varied biological events [45]. Its plays a role in social bonding, reproduction, childbirth and the period after childbirth as well. So, small Biostructures showed very important roles and functions in the human health that still being a challenge their study. Other example that it could be mentioned is related with neuropeptide secretion by organizing dense-core vesicle fusion sites in neurons activated by Dynamin’s yeast Vps1 orthologs [46]. These complex Neuro-Biological events showed a wide and large number of biological implications that should be mentioned and highlighted to propose new queries and approaches of study from the control of targeted Molecules towards the Nano-scale with impact on the micro-, and macro-scale.
The generation of Neuro-imaging with high level of resolution, accuracy and precision is still being needed it depending of the Research interest and application. Thus, Multimodal approaches are in progress being developed with different approaches of data acquisition. In particular in this section, it was leaded to the discussion on chemical species levels and Biostructures information. In this perspective there were many approaches of labelling techniques that offered an open window for divers complex studies, such as retrograde labelling techniques to track Neurodevelopment and inter-connectomes [47], by incorporating Modified virus wilt Fluorescent molecular tracers [48] or by Injection of Fluorescent labelled microbeads [49].
But it is important to achieve lower resolutions in order to manage inter-neuronal connections and deeper levels towards molecular detection too. Thus, it could be mentioned Neurotransmitters tracking, Calcium, exosomes release, protein transport, membrane modifications, etc. [50]. Therefore, it could be evaluated targeted functional responses of Neurons In Vitro and In Vivo [51]. So, to develop new strategies it could be highlighted Advances in Nanophotonics, and Nano-Optics; accompanied with varied Optical Set-ups; from where it was achieved reduced sized focusing on molecular detection events [52]. And, in this aspect it is of high interest the generation of imaging by design of specific chemical reagents and new Nano-tools to manage targeted interactions on tissues In Vivo; and recognize molecules from the media such as on smart responsive particles [53]. These concepts and ideas are not all developed; for this reason and the impact associated it is an invitation to propose other approaches. For example, the concept of controlled light delivery [54] from reduced sizes at the molecular level and from confined Nanoplatforms [55] could show a high potential use for these types of studies and applications.
Then, depending on the properties developed in these specific reagents and Nano-tools, it will be the Optical Set-up chosen. As well, it should be taken into account particular physical and chemical properties of targeted molecules, concentrations, media, interferents, and accessibility to measure or contemplate other possibilities as non-invasive techniques that all and remote sensing modes.
Therefore, metabolites of serotonin (5-hydroxy-triptamine) could be detected in urine by its metabolite [56] however in order to track In Vivo, it should be developed strategies to detect in place the molecules of interest. Thus, for small animals and rodents it could be applied varied Microscopy techniques [57,58] and new Nano-Optical approaches [59]. Some of these studies were permitted by In Vivo with open skull procedures [60]. In this perspective, miniaturized instrumentation such as mini microscopes [61], endoscopes [62,63], and photonics Optical Fibers [64] were reported with high impact on Neuroimaging and data collection. By this manner, it could be focused on reduced sized spots for molecular spectroscopic by targeted light collection by the right excitation, applying different non-classical light generation such as Fluorescence Resonance Energy Transfer (FRET) [65], and Metal Enhanced Fluorescence (MEF) [66-69]. All those mechanisms could be involucrated to the specific FRET targeted Biomolecular detection [70-72], for the generation of new synthetic Enhanced Luminescence approaches based on Nano-Bio-FRET [73], MEF Bioassays [74], and FRET-MEF coupled phenomena [75]. Thus, the design, synthesis, and application of Nano-tools could be the next generation of Nanotechnology within Neurophotonics. These Nanoarchitectures by a proper design could be proposed as smart responsive Nanoplatforms that ideally would detect at Single Molecular Detection (SMD) level [76]; and even ions by Enhanced Nano-Optics [77]. And, with these perspectives, it should be highlighted that not so many were developed; but some proofs of concepts were reported. In this manner, there is a huge potential in this Research field in the next years. In the same way, the capability to the design of miniaturized instrumentation and devices, it could be proposed the tracking of SMD detections in real time externally and remotely controlled by varied stimulation sources such as Multi-photons Lasers [78]. For example, it could be noted that by the use of small Fluorescent molecular labellers, it was leaded to obtain Neuroimaging with a high potential of data analysis [79]. However, there are still being needs to incorporate other types of new emitters, Meta-emitters, and Nano-emitters to tune more stable, less photobleached, and higher resolved Neuroimages. In this manner the non-classical Light is generated from confined Nano-volumes affording by this way the highlighting of their environment with a consequent generation of pattern of variable degree of resolution. In this context, it could be mentioned he Bioimaging generation by Fluorescence Lifetime Imaging (FLIM) [80] (Figure 4). This technique based on controlled Fluorescence emission by incorporating specific Fluorescent dyes to interact with varied biological media produced different resolution within the Nanoscale. In these scales of lengths, the concept of light delivery could be developed from tiny Nanoparticles towards higher ones in similar manner as it was previously showed; and by using other approaches such as by Optrodes [81,82].
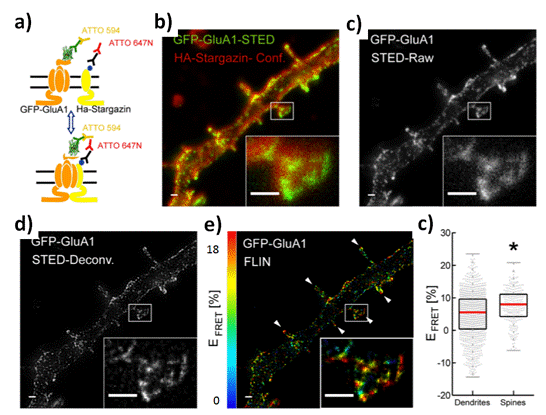
Figure 4. Levels of association between AMPAreceptors and stargazing in spines and dendrites: a) Double immunolabeling configuration of GFP-GluA1 with mouse anti-GFP/GaM-ATTO 594 (Donor) and HAstargazinwithratanti-HA/GaR-ATTO647N (Acceptor). b) STED image of GFP-GluA1 (Donor) and confocal(Conf.) imageofHA-Stargazin(Acceptor)onatransfecteddendriteandaninsetshowingadendritic spine. c) STED raw intensity image of the donor showing GluA1 nanoclusters in spines and dendrites. d) Corresponding deconvolved image of that showed in (c) (Richardson–Lucy deconvolution, simulated PSF of 60 nm FWHM). e) Intensity-weighted FLIN image of the deconvolved image in (d) depicting higher FRET level in spines (white arrows) compared to dendrites. Inset: crop of one spine showing nanoclusters of fluorescently labeled AMPARs exhibiting different levels of FRET with fluorescently labeled stargazin. f) The median FRET efficiencies per nanocluster on the membrane surface was significantly higher in spines (8.0%, IQR¼6.9%, n ¼271 clusters, 10 neurons) compared with dendrites (5.5%, IQR¼8.0%, n ¼1058 clusters, 10 neurons) (p ¼9.45×10−10). Scale bars 500 nm, inset: 1.56×1.18 μm.. Reprinted with permissions of P. De Koninck et al. (cite30), Neurophoton, SPIE 2019
In addition to these strategies, and others such staining, classical labelling techniques, and new ones by the use of new emitters, Meta-emitters [83], Enhanced emitters [84] and Quantum emitters [85,86], it should be added the application and development of probes for sensing uses. For example, targeted highly conjugated organic molecules with proper chemical groups functionalization could lead to targeted ions sensing as Calcium [87]. These organic moieties modify their electronic properties in presence just only of the targeted ion. Therefore, it is possible to register Calcium delivery by an increase of Fluorescence signaling. Thus, it was achieved Neuro-stimulation studies against varied types of stress factors applied on small rodents. By this manner it could be sensed varied types of stimulations such as light, fear, nutrition, variable activity, etc. By this manner it was studied behaviours In Vivo [88,89], And with this perspective, as well it should be noted the importance of the design of Chips, devices as Micro-fluidics, Nano-devices with potential applications for Drug Delivery and Bio-sensing too [90,91]. These approaches could be perfectly coupled with previous Optical approaches mentioned in order to track Neuro-Biological events (Figure 5). In this perspective it could be designed varied experimental approaches such as In Vitro, In Vivo, within Microfluidics approaches [92,93], Organelle on Chips Bioassays [94,95], targeted Single Neurons and related cells too [96].
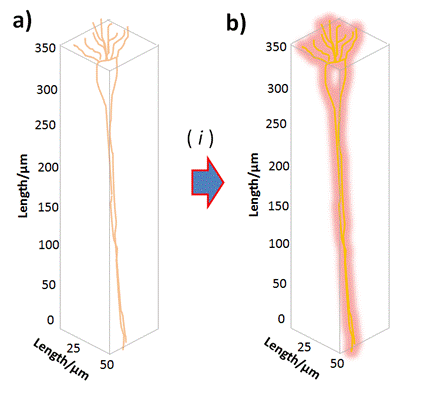
Figure 5. Schema of Single Neuro-labelling assays: a) Single Neuron constitution inserted within an average confined volume; b) Labelled Single Neuron by varied controlled and tuneable Neuro-Imaging modes nominated as ( i ). Reprinted with permissions of A. G. Bracamonte et al. 2022
Therefore, as concluding remarks, it could be mentioned that it was afforded to an overview about the capabilities of different modes of Neuro-imaging generation based on the analysis of the State of the Art of Multidisciplinary Research fields focused on Neurophotonics. In this manner, it was discussed from molecular detections, molecular labelling, to Nano-labellers and Nano-rulers for Imaging leading to couple Optical approaches for their signal tracking. Thus, in brief it was opened and showed potential future developments.
There are many important and high impact studies in progress as well as required within Neuroscience to dilucidate varied developments of illness, mechanism associated with normal brain function, and its stimulation by a controlled manner. In this context, it should be highlighted the current high level of technology achieved for data recording by collaborative Research and development works from Academia and Industry in many cases. Therefore, from the different Optical approaches, techniques and methodologies it could be achieved a given degree of precision and accuracy when it is look for a targeted application. For example, there are many particular interests to specific signaling from the whole brain tissue, varied cells involucrated, Biomolecules, and ions. For the different studies and applications, within the different levels mentioned, it is required improvements in many aspects depending on the variables involucrated. And there, it is the challenge to afford to new developments related with variable sized focused hot spots associated with confined Neuro-Biological events where the resolution is highly required. The design of new probes for Biosensing from the molecular control towards the Nano-scale Engineering could provide tools to manage the different challenges to overcome. These Molecular-, and Nano-tools will be incorporated in varied Optical Set ups to record targeted signaling depending of the application. So, from this wide point of view, it was showed and discussed in this communication many high impact Research works reported that could be involucrated in Future developments as well. In this way, it should be highlighted other developments not necessarily related within Neurophotonics that could lead to new studies. As for example, the recent Nobel in Chemistry awarded in 2017 related with a Super-resolved Fluorescence Microscopy technique based on Laser molecular switch on/off activation at Single Molecule Detection (SMD) level [97]. In the same direction the study and developments on Neurons by varied Optical approaches, such as Optrodes and new Microscopy methods could afford to resolution below the Nanoscale within In Vivo Neurons by incorporation targeted new highly efficient Nano-emitters [98] and by Optical tagging [99] of intrinsic Optical active Neuronal biomarkers [100], or by labelling and Bioconjugation techniques [101] (Figure 6).
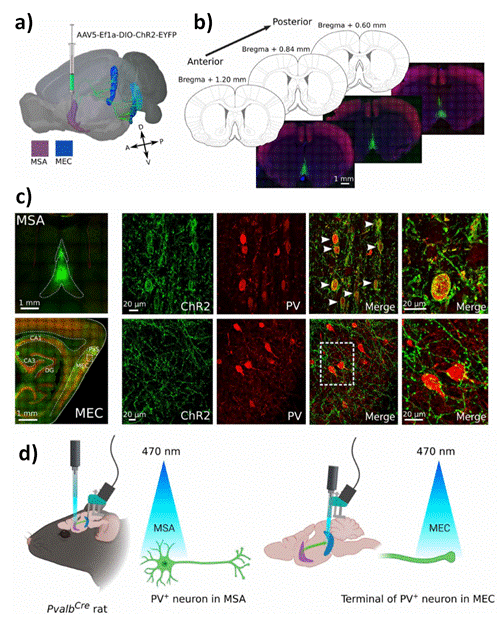
Figure 6. PV+ cells in MSA selectively express channel rhodopsin (ChR2) after injection of virus in PvalbCre rats. a) Illustration of a rat brain seen slightly from above on the left side highlights the MSA in purple and MEC in blue/cyan, with long-range projections from MSA to MEC (green). These projections target all layers of MEC, from the dorsal to the ventral area. A viral construct carrying ChR2 was injected in MSA. b) Three coronal sections from a representative animal show the extent of the virus expression (green) in MSA at three different anterior-posterior positions. Expression covered large parts of MSA. c) Virus expression in MSA was restricted to PV+ cells (red). White arrowheads mark overlap between virus (ChR2) and PV+ cells (PV) (top row). Virus-expressing projections were found in MEC, parasubiculum (PaS), and all regions of the hippocampus [CA1, CA3, and dentate gyrus (DG)] (bottom row). The area of MEC chosen for the magnified images is indicated by a small square. ChR2- labeled septal projections target PV+ cells in MEC (small outline). d) Illustration of experimental setup. PvalbCre rats were implanted with optic fiber in MSA and recording electrodes in MEC (two animals also had optic fibers together with recording electrodes in MEC). Blue laser light (470 nm) was used to activate PV+ cells in MSA at two different frequencies, 11 and 30 Hz. Reprinted with permissions of T. Haftinget al. (cite100), Science Advances, Science 2021
It is gratefully acknowledge the different Grants received in order to accomplish the Research work in progress of the authors related with developments of Luminescent Nanoplatforms and Biodetection; such as “CONICET, Consejo Nacional de Investigaciones Científicas y Técnicas (National Research Council of Argentine); ANPCyT, Agencia Nacional de Promoción Científica y Tecnológica (National Agency of Scientific and Technology Promotion of Argentine)”; and to “SECyT (Secretary of Science and Technology from the National University of Cordoba (UNC)”, Argentina, for awarding us the extension of the Grant for Young Researchers to the author A. G. B. at INFIQC. As well, especially thanks to FONCYT, National Agency of Scientific and Technology promotion, PICT A 2020, for the Research proposal acceptation for the Group in formation (Call 2020). Moreover, It is gratefully acknowledge to Professor Denis Boudreau, and Daniel Côté from “Département de chimie and Centre d’optique, photonique et laser (COPL)”, Québec, Canada, for Research Collaboration; and inspiring discussions afforded respectively; as well as to all the Canadian Grants that permitted it. In similar manner, thanks to Professor Jesse Greener from Laval University, Quebec, Canada, for let visit his lab to A.G.B., and discuss about Microfluidic insides.
- Tuchscherer R (2020) Brain scanning backpack brings neuroscience into the real world. Science News Brain and Behaviour 1-2.
- Rudolph U (2019) Modulating anxiety and activity. Perspective in Neurscience Science 11: 185-186. [Crossref]
- Allen WE (2020) Brain mapping, from molecules to networks. Science 370: 1-2.
- Mello-Thoms C, Abbey CK, Krupinski EA (2020) Introducing the Special Series on 2D and 3D Imaging: Perspectives in Human and Model Observer Performance. Journal of Medical Imaging 1: 1-2.
- Ren W, Skulason H, Schlegel F, Rudin M, Klohs J (2019) Automated registration of magnetic resonance imaging and optoacoustic tomography data for experimental studies. Neurophoton 2: 1-11.
- Wang X (2003) Non-invasive laser-induced photoacoustic tomography for structural and functional in vivo imaging of the brain. Nat. Biotechnol 21: 803-806.
- Becker G, Dammicco S, Ali Bahri M, Salmon E (2020) The Rise of Synaptic Density PET Imaging. Molecules 25: 1-20. [Crossref]
- Steffens H, Mott AC, Li S, Wegner W (2021) Stable but not rigid: Chronic in vivo STED nanoscopy reveals extensive remodeling of spines, indicating multiple drivers of plasticity. Sci. Adv 7: 1-12.
- Matsumoto K, Mitchell JB, Krishna MC (2021) Multimodal Functional Imaging for Cancer/Tumor Microenvironments Based on MRI, EPRI, and PET. Molecules 26: 1-27.
- Irani F, Platek SM (2007) Functional near infrared spectroscopy (fNIRS): an emerging neuroimaging technology with important applications for the study of brain disorders.
Clin Neuropsychol 1: 9-37.
- Li Z (2021) M-CSF, IL-6, and TGF-promote generation of a new subset of tissue repair macrophage for traumatic brain injury recovery. Sci Adv 7: 1-17.
- Jiang B (2020) Injectable, photoresponsive hydrogels for delivering neuroprotective proteins enabled by metal-directed protein assembly, Sci Adv 6: 1-9.
- Caffarel-Salvador E (2021) A microneedle platform for buccal macromolecule delivery. Sci Adv 7: 1-11.
- Takehara H, Nagaoka A (2014) Lab-on-a-brain:Implantablemicro-optical fluidic devices for neural cell analysis in vivo Scientific Reports. Nature 1: 1-6.
- Hatcher M (2020) Wearables get wet as physiology meets Photonics, SPIE Photonics West. Technology 1-2.
- Li B, Zhang D, An R (2019) A 7-Hydroxybenzoxazinone-Containing Fluorescence Turn-On Probe for Biothiols and Its Bioimaging Applications. Molecules 24: 1-9.
- Soo Choi H, Frangioni JV (2010) Nanoparticles for Biomedical Imaging: Fundamentals of Clinical Translation. Mol Imaging 6: 291-310.
- Colombé C, Le Guével X, Martin-Serrano A (2019) Gold Nanoclusters as a contrast agent for image-guided surgery of head and neck tumors, Nanomedicine: Nanotechnology, Biology, and Medicine 20: 1-26.
- Salinas-Hernandez XI (2018) Dopamine neurons drive fear extinction learning by signaling the omission of expected aversive outcomes. eLife 7: 1-25.
- Beyene AG (2019) Imaging striatal dopamine release using a nongenetically encoded near infrared fluorescent catecholamine nanosensor. Sci Adv 5: 1-11. [Crossref]
- Lotze J, Wolf P (2018) Time-Resolved Tracking of Separately Internalized Neuropeptide Y2 Receptors by Two-Color Pulse-Chase. ACS Chem Biol 3: 618-627.
- Nakamura A, Kaneko N, Villemagne VL (2018) High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 554: 249-254.
- Ivanova D (2021) Control of synaptic vesicle release probability via VAMP4 targeting to endolysosomes. Sci Adv 7: 1-21.
- Ludewig S, Herrmann U (2021) APPsα rescues impaired Ca homeostasis in APPand APLP2deficient hippocampal neurons. PNAS 26: 1-10.
- Varela J (2016) Targeting neurotransmitter receptors with nanoparticles in vivo allows single-molecule tracking in acute brain slices. Nat Commun 7: 1-10. [Crossref]
- Baig AM (2018) Traced on the Timeline: Discovery of Acetylcholine and the Components of the Human Cholinergic System in a Primitive Unicellular Eukaryote Acanthamoeba spp. ACS Chem Neurosci 3: 494-504.
- Stern P (2020) Dopamine circuits facilitate fear learning. Science 6494: 962-963.
- Moon JM (2021) Non-invasive sweat based tracking of L-Dopa Pharmacokinetics profiles following an oral Tablet administration. Angew Chem Int Ed 60: 19074-19078.
- Zhou Y, Danbolt NC (2014) Glutamate as a neurotransmitter in the healthy brain. J Neural Transm (Vienna) 8: 799-817.
- Ma F, Yang L, Sun Z, Chen J (2020) Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci Adv 6: 1-10.
- Ivanova D, Dobson KL (2021) Control of synaptic vesicle release probability via VAMP4 targeting to endolysosomes. Sci Adv 7: 1-21.
- Paul SM, Purdy RH (1992) Neuroactive steroids. FASEB Journal 6: 2311-2322.
- Lan NC, Gee KW (1994) Neuroactive steroid actions at the GABAA receptor. Hormones and Behavior 4: 537-454.
- Torrente-Rodriguez RM (2020) Investigation of cortisol dynamics in human sweat using Graphene based wireless mHealth System. Matter 2: 921-937. [Crossref]
- Kosowski M, Smolarczyk-Kosowska J, Hachula M, Maliglówka M, Basiak M (2021) The Effects of Statins on Neurotransmission and Their Neuroprotective Role in Neurological and Psychiatric Disorders. Molecules 26: 1-19.
- Su Y, Bian S, Sawan M (2020) Real-time in vivo detection techniques for neurotransmitters: a review, Analyst 145: 6193-6210.
- Tang W, Kochubey O, Kintscher M, Schneggenburger R (2020) AVTA to Basal Amygdala Dopamine Projection Contributes to Signal Salient Somato sensory Events during Fear Learning. The Journal of Neuroscience 20: 3969-3980
- Sudhof TC (2012) Calcium Control of Neurotransmitter Release. Cold Spring Harb Perspect Biol 4: 1-15.
- Kim EH, Chin G (2018) Optical Probes for Neurobiological Sensing and Imaging. Acc Chem Res 5: 1023-1032.
- Hee Lee M, Seung Kim J, Sessle JL (2015) Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem Soc Rev 13: 4185-4191.
- Ranasinghe KG, Cha J, Iaccarino L, Hinkley LB (2020) Neurophysiological signatures in Alzheimer’s disease are distinctly associated with TAU, amyloid-β accumulation, and cognitive decline. Science Translational Medicine 12 :1-10.
- Köppen J, Schulze A, Machner L, Wermann M (2020) Amyloid-Beta Peptides Trigger Aggregation of Alpha-Synuclein In Vitro. Molecules 25: 1-18. [Crossref]
- Afonso P, De Luca P, Carvalho RS, Cortes L, Pinheiro P (2019) BDNF increases synaptic NMDA receptor abundance by enhancing the local translation of Pyk2 in cultured hippocampal neurons. Sci Signal 12: 1-20.
- Cornblath EJ, Li HL Changolkar L (2021) Computational modeling of tau pathology spread reveals patterns of regional vulnerability and the impact of a genetic risk factor. Sci Adv 7: 1-15.
- Ananthanarayanan VS, Brimble KS (1997) Interaction of Oxcytocin with Ca2+:1. CD and Fluorescence Spectral Characterization and Comparison with Vasopressin. Peptidomimetics & Small Molecule Design CCC 11: 433-443.
- Moro A, van Nifterick A, Toonen RF, Verhage M (2021) Dynamin controls neuropeptide secretion by organizing dense-core vesicle fusion sites. Sci Adv 7: 1-17. [Crossref]
- O'Donnell P, Lavín A, Enquist LW, Grace AA, Card JP (1997) Interconnected Parallel Circuits between Rat Nucleus Accumbens and Thalamus Revealed by Retrograde Transynaptic Transport of Pseudorabies Virus. Journal of Neuroscience 6: 2143-2167.
- Benjamin D, Glenn RF, Schnell MJ (2015) Everything You Always Wanted to Know About Rabies Virus (But Were Afraid to Ask). Annual Review of Virology 2: 451-471.
- Katz LC, Burkhalter A, Dreyer WJ (1984) Fluorescent latex microspheres as a retrograde neuronal marker for in vivo and in vitro studies of visual cortex. Nature 310: 498-500.
- Ping Chien M, Brinks D, Guilherme Testa-Silva, Tian H, Phil Brooks F, et al. (2020) Photoactivated voltage imaging in tissue with an archaerhodopsin-derived reporter. Sci Adv 7: 1-17.
- Garcia-Alloza M, Bacskai BJ (2004) Techniques for brain imaging in vivo. Neuromol Med 6: 65-78.
- Aswendt M, Schwarz M, Abdelmoula WM (2017) Whole-Brain Microscopy Meets In VivoNeuroimaging: Techniques, Benefits, and Limitations. Mol Imaging Biol 19: 1-9.
- Ame M (2021) Detection of Viruses and Development of New Treatments: Insights into Antibody-Antigen Interactions and Multifunctional Lab-On-Particle for SARS CoV-2. J Nanotechnol Nanomaterials, Scientific Archives 2: 67-75.
- Dufour S, De Koninck Y (2015) Optrodes for combined optogenetics and electrophysiology in live animals. Neurophotonics 2: 1-15.
- Salinas C, Amé M, Bracamonte AG (2020) Tuning silica nanophotonics based on fluorescence resonance energy transfer for targeted non-classical light delivery applications. J Nanophoton 4: 1-19.
- Bracamonte AG, Veglia AV (2011) Spectrofluorimetric determination of Serotonin and 5-hydroxyindoleacetic acid in urine with different cyclodextrin media. Talanta 83: 1006-1013. [Crossref]
- Ji N, Freeman J, Smith SL (2016)Technologies for imaging neural activity in large volumes. Nat Neurosci 19: 1154-1164.
- Streich L, Boffi JC, Wang L (2021) High-resolution structural and functional deep brain imaging using adaptive optics three-photon microscopy. Nat Methods 18: 1253-1258.
- Veglia AV, Bracamonte AG (2019) β-Cyclodextrin grafted gold nanoparticles with short molecular spacers applied for nanosensors based on plasmonic effects. Microchem J 148: 277-284.
- Zhao YJ, Yu TT, Zhang C (2018) Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution. Light Sci Appl 7: 1-9.
- Senarathna J, Yu H, Deng C, Zou AL, Issa JB, et al. (2019) A miniature multi-contrast microscope for functional imaging in freely behaving animals. Nature Communication 10: 1-13.
- Heidari A (2016) Future Prospects of Point Fluorescence Spectroscopy, Fluorescence Imaging and Fluorescence Endoscopy in Photodynamic Therapy (PDT) for Cancer Cells. J Bioanal Biomed 8: 1-2.
- Shin J, Tran DN, Stroud JR, Chin S (2019) A minimally invasive lens free computational microendoscope. Sci Adv 5: 1-6. [Crossref]
- Thrapp AD, Hughes MR (2020) Automatic motion compensation for structured illumination endomicroscopy using a flexible fiber bundle, J Biomed Opt 25: 1-14.
- Dacres H, Wang J, Dumancic MM, Trowell SC (2010) Experimental Determination of the Forster distance for two commonly used Bioluminescent Resonance Energy Transfer pairs. Anal Chem 82: 432-435.
- JR Lackowicz (2005) Radiative decay engineering: Metal enhanced fluorescence and plasmon emission. Analytical Biochemistry 337: 171-194.
- Viger ML (2008) Reduction of Self-Quenching in Fluorescent Silica-Coated Silver Nanoparticles, Plasmonics 3: 33-40.
- Asselin J, Legros P, Grégoire A, Boudreau D (2016) Correlating metal-enhanced fluorescence and structural properties in Ag@ SiO2 Core-shell nanoparticles. Plasmonics 5: 1369-1376.
- Rioux M, Gontero D, Veglia AV, Bracamonte AG, Boudreau D (2017) Synthesis of Ultraluminiscent gold core-shell Nanoparticles as NanoImaging Platforms for Biosensing applications based on Metal enhanced fluorescence. RSC Adv 7: 10252-10258.
- Gong Y (2014) Imaging neural spiking in brain tissue using FRETopsin protein voltage sensors. Nat Commun 5: 1-10. [Crossref]
- Wan R, Wu J, Ouyang M, Lei L (2019) Biophysical basis underlying dynamic Lck activation visualized by Zaplack FRET biosensor. Sci Adv 5: 1-14.
- Nuhn L, Van Herck S, Best A, Deswarte K, Kokkinopoulou M, et al. (2018) FRET Monitoring of Intracellular Ketal Hydrolysis in Synthetic Nanoparticles. Angewandte Chemie International Edition 130: 10760-10764.
- Salinas C, Ame MV, Bracamonte AG (2020) Synthetic non-classical luminescence generation by Enhanced Silica Nanophotonics based on Nano-Bio-FRET. RSC Advances 10: 20620-20637.
- Golberg K, Elbaz A, McNeil R, Kushmaro A, Geddes CD (2014) Increased bioassay sensitivity of bioactive molecule discovery using metal-enhanced bioluminescence., J Nanopart Res 16: 1-14.
- Brouard D, Lessard Viger M, Bracamonte AG, Boudreau D (2011) Label-free biosensing based on multilayer fluorescent nanocomposites and a cationic polymeric transducer. ACS Nano 5: 1888-1896.
- Schuler B (2013) Single-molecule FRET of protein structure and dynamics - a primer. Journal of Nanobiotechnology 11: 1-17.
- Bondre N, Zhang Y, Geddes CD (2011) Metal Enhanced Fluorescence based calcium detection greater than 100 fold increase in signal/noise using Fluo-3 or Fluo-4 and silver nanostructures. Sensors and Actuators, Sensors and Actuators B: Chemical 1: 82-87.
- Klioutchnikov A, Wallace DJ (2020) Three photon head mounted microscope for imaging dep cortical layers in freely moving rats. Nature Methods 17: 509-513.
- Liu YZ, Renteria C, Courtney CD, Ibrahim B (2020) Simultaneous two-photon activation and imaging of neural activity based on spectral-temporal modulation of supercontinuum light. Neurophotonics 4: 1-15.
- Tardif C, Nadeau G, Labrecque S, Côté D, Lavoie-Cardinal F (2019) Fluorescence lifetime imaging nanoscopy for measuring Förster resonance energy transfer in cellular nanodomains. Neurophoton 1: 1-17. [Crossref]
- V Gradinaru (2007) Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci 52: 14231-14238.
- Zalocusky K, Deisseroth K (2013) Optogenetics in the behaving rat: integration of diverse new technologies in a vital animal model. Optogenetics 1: 1-17.
- Woehrstein JB, Strauss MT, Ong LL, Wei B, Zhang DY (2017) Sub-100 nm metafluorophores with digitally tunable optical properties self-assembled from DNA. Sci Adv 3: 1-12. [Crossref]
- Ye S, Tian T, Christofferson AJ, Erikson S, Jagleski J (2021) Continous color tuning of single fluorophore emission via polymerization mediated through space charge transfer. Sci Adv 7: 1-13.
- Salam M (2020) Superior Properties and Biomedical Applications of Microorganism-Derived Fluorescent Quantum Dots. Molecules 25: 1-27.
- Bracamonte AG (2020) New Matter Properties and Applications based on Hybrid Graphene-based Metamaterials, Special Issue: Design and Synthesis of Graphene based Metamaterials. Current Graphene Science, Bentham Sci Pub 4: 1-4.
- Locka JT, Parker I, Smith IF (2015) A comparison of fluorescent Ca2+ indicators for imaging local Ca2+ signals in cultured cells. Cell Calcium 6: 638-648.
- Wulansar N, Handoko W, Darsono W, Woo HJ (2021) Neurodevelopmental defects and neurodegenerative phenotypes in human brain organoids carrying Parkinson’s disease linked DNAJC6 mutations. Sci Adv 7: 1-18.
- Kragel PA, Reddan MC, LaBar KS, Wager TD (2019) Emotion schemas are embedded in the human visual system. Sci Adv 5: 1-15. [Crossref]
- Ma F, Yang L, Sun Z, Chen J, Rui X, et al. (2020) Neurotransmitter-derived lipidoids (NT-lipidoids) for enhanced brain delivery through intravenous injection. Sci Adv 6: 1-10.
- O’Cearbhaill E (2019) 3D bioprinting chips away at glioblastomal resistance. Science Translational Medicine 4: 1-10.
- Ma MC (2021) Suppression of dendrite growth by cross-flow in microfluidics. Sci Adv 7: 1-8. [Crossref]
- Tsai HF, IJspeert C (2020) Voltage-gated ion channels mediate the electrotaxis of glioblastoma cells in a hybrid PMMA/PDMS microdevice. APL Bioeng 4: 1-4.
- Pousti M, Pouyan Zarabadi M, Amirdehi MA (2019) Microfluidic bioanalytical flow cells for biofilm studies: a review. Analyst 144: 68-86.
- Ramadan Q, Zourob M (2020) Organ-on-a-chip engineering: Toward bridging the gap between lab and industry. Biomicrofluidics 14: 1-24.
- Botvinik-Nezer R, Holzmeister F, Camerer CF (2020) Variability in the analysis of a single neuroimaging dataset by many teams. Nature 582: 84-88.
- Betzig E, Hell SW, Moerner WE (2014) The Nobel Prize in Chemistry 2014: “for the development of super-resolved fluorescence microscopy”, Press Release. The Royal Swedish Academy of Sciences Oct. 8: 1.
- Labrecque S, Sylvestre JP, Marcet S, Mangiarini F (2016) Hyperspectral multiplex single-particle tracking of different receptor subtypes labeled with quantum dots in live neurons. Journal of Biomedical Optics 21: 1-12. [Crossref]
- Lee D, Kume M, Holy TE (2019) Sensory coding mechanisms revealed by optical tagging of physiologically dened neuronal types. Science 647: 1384-1389.
- Lepperød ME, Charlotte Christensen A, Kinden Lensjø K, Buccino AP, Yu J (2021) Optogenetic pacing of medial septum parvalbumin-positive cells disrupts temporal but not spatial firing in grid cells. Sci Adv 7: 1-18.
- Gralle M, Labrecque S, Salesse C, De Koninck P (2021) Spatial dynamics of the insulin receptor in living neurons. J Neurochem 1:88-105.






