In the future genetic testing to detect high/moderate susceptibility genes for breast cancer (BC) and other cancers will be generalized to the entire population. Until this happens, it is important to improve the current selection criteria for germline testing. We studied the genetic alterations of a total of 133 BC patients (32 women with bilateral BC: total of 165 BCs) in order to determine the molecular alteration responsible for their intense familial aggregation of cancer. We found 44 women with inherit mutations in the BRCA1 gene and 34 in the BRCA2 gene. We compared the series of BRCA1/2-positive BC patients with the rest of the BC patients. Based on the differences found in our series, we propose that the current guidelines for germline BRCA1/2 testing should also include the following criteria: 1) All BC patients with certain histological subtypes: medullary and metaplastic, as well as those that present a specific type of tumor frequently classified as “infiltrating ductal carcinoma of no special type” by pathologists but which we identify as "infiltrating ductal carcinomas with rows and necrosis" (since it has peculiar and easily recognizable histological features: distribution in cords and solid nests of cells with large areas of necrosis, abundant atypical mitoses, and highly pleomorphic nuclei); 2) All families with at least one relative with multiple cancer: especially when the cancer associated with BC is gynecological (BRCA1) or digestive cancer (BRCA2), consider in the presence of any other type of low prevalent cancer (gliomas, leukemias, melanomas); 3) All BC patients whose molecular subtype is triple negative (BRCA1), consider with the luminal B (HER2-negative) if there is familial aggregation of cancer (BRCA2).
breast cancer, hereditary breast and ovarian cancer, BRCA1, BRCA2, selection criteria, germline testing, medullary breast cancer, metaplastic breast cancer, infiltrating ductal breast carcinoma with rows and necrosis, triple negative breast cancer, multiple primary cancers
Most breast cancers (BC) are sporadic, only 5-10% have a mutation inherited from one of their parents (hereditary BC). The germline mutations in the high penetrance BRCA1 and BRCA2 (BRCA1/2) genes are the most frequently associated with hereditary breast and ovarian cancer (HBOC) syndrome. The HBOC syndrome is a syndrome that implies a greater predisposition, mainly to BC and/or ovarian cancer. These mutations are detected in 15-20% of women with a family history of BC and between 60-80% of women with a family history of BC and ovarian cancer [1].
BRCA1/2 genes were discover in the 1990s and are involved in DNA repair, maintaining the integrity of the genome, which is why they are considered caretaker-type tumor suppressor genes. Families with germline mutations in BRCA1/2 (HBOC) present an autosomal dominant hereditary pattern, with early age of cancer onset, bilaterality, and male BC.
Although the association of BRCA1/2 mutations with breast and ovarian cancer risks is well defined, the association with other cancers is inconsistent. Initial studies by the Breast Cancer Linkage Consortium also found an association between these mutations and prostate and pancreatic adenocarcinomas, among others [2, 3]. These associations were subsequently confirmed by others studies [4].
The aim of this paper was to study the characteristics of BRCA1/2-positive BCs and determine if we could detect differences compared to the rest of BCs in order to make a better selection of hereditary BCs among the general population.
This study included the cases of BC with a positive genetic study for BRCA1/2 from several hospitals in the health area of Vigo (Xeral University Hospital of Vigo, Meixoeiro Hospital, Álvaro Cunqueiro University Hospital and Povisa Hospital) during the period between 2000 and 2020.
The variables were studied retrospectively and prospectively during all these years, including: personal data, family history, genetic testing, diagnosis, staging and type of BC. The individualized clinical follow-up of each patient allowed us to collect information on local recurrences and distant metastases, as well as the diagnosis of multiple primary cancers.
The data obtained in this study were entered into a computer database developed in the Microsoft Excel program. Statistical analysis was performed with the statistical program SPSS-PC for Windows.
We studied the genetic alterations of a total of 133 BC patients in order to determine the molecular alteration responsible for their intense familial aggregation of cancer. 32 women had a bilateral BC, so the total number of BCs studied was 165. We found 44 women with inherit mutations in the BRCA1 gene and 34 in the BRCA2 gene (Table 1).
Table 1. Genetic testing in our series (in 133 BC patients)
GENETIC STUDY (n = 133 women with BC) |
MUTATIONS IN HIGH/MODERATE PENETRANCE GENES FOR BC
85 women with BC |
BRCA1 y BRCA2 genes |
BRCA1 |
n = 44 |
33.0% |
BRCA2 |
n = 34 |
25.5% |
BARD1 Gene |
n = 2 |
1.5% |
CHECK2 Gene |
n = 2 |
1.5% |
RAD51D Gene |
n = 1 |
0.7% |
PALB2 Gene |
n = 1 |
0.7% |
Lynch Syndrome |
n = 1 |
0.7% |
CARNEY SYNDROME PHENOTYPE
1 women with BC |
She has no genetic study
(Carney phenotype) |
n = 1 |
0.7% |
NEGATIVE GENETIC STUDY
31 women with BC |
COMPLETE GENETIC PANEL * (n =16) |
n = 31 |
23.3% |
Only BRCA1/2 testing (n = 15) |
MUTATIONS IN GENES WITH UNCERTAIN SIGNIFICANCE
11 women with BC |
BRCA2 variant of uncertain significance |
n = 8 |
6.0% |
PALB2 variant of uncertain significance |
n = 2 |
1.5% |
CHECK2 variant of uncertain significance |
n = 1 |
0.7% |
FAMILY BRCA (+)
WOMEN BRCA (-)
3 women with BC |
BRCA1-Positive Family (BC patient: BRCA -) |
n = 2 |
1.5% |
BRCA2-Positive Family (BC patient: BRCA -) |
n = 1 |
0.7% |
Analysis of large deletions and duplications in BRCA1/2 by the MLPA (Multiplex ligation-dependent probe amplification) technique: Kit P002B was used for the analysis of the BRCA1 gene and P045B for the analysis of BRCA2.
* Complete genetic panel: Search for mutations by NGS (Ion PROTON) sequencing of the entire coding region and flanked intronic regions of the BRCA1 (NM_007294.3), BRCA2 (NM_000059.3), PTEN (NM_00314), TP53 (NM_000546.5), CDH1 (NM_004360.3), RAD51C (NM_058216), RAD51D (NM_001142571), MLH1 (NM_001258274), MSH2 (NM_ 000251), MSH6 (NM_000179), PALB2 (NM_02675), CHEK2 (NM_001142571) (NM_00657194) (NM_00116194) (NM_00116194) (NM_006194) NM_000455) genes.
A total of 78 BC patients (58.5%) BRCA1/2 mutation carriers were identified out of the total of 133 women selected for the genetic study and we focused our research on them. Nineteen of these patients were diagnosed with bilateral BC, so the maximum number of BRCA1/2-positive BCs was 97.
Tables 2 to 4 describe the types of mutations studied in our series: BRCA1, BRCA2 and others associated with increased risk for BC. We highlight the pathogenic variant BRCA1 330A> G, known as the Galician variant because it is the most prevalent in Galicia; it was also the most frequent in our series.
Table 2. Type of BRCA1 mutations found in our series
Age |
BRCA1 MUTATION |
No. of cases
(45 women) |
29 |
BRCA1 positive. We do not know the specific type of mutation |
2 women |
30 |
BRCA1 positive. We do not know the specific type of mutation |
58 y 62 |
BRCA1 c3756_3759del (p.Ser1253Argfs*10) |
1 woman |
27 |
BRCA1 c.4185_4185+3del p.(Gln1395Hisfs*10) |
2 women |
33 |
BRCA1 c.4185_4185+3del p.(Gln1395Hisfs*10) |
33 |
BRCA1 c.951dupA (p.Thr278AsnfsX9) exón 11 |
1 woman |
43 |
BRCA1 Deletion of exons 1 to 13 of the BRCA1 gene |
1 woman |
38 |
BRCA1 4 base pair deletion (GTTC). Exon 11. Codon 264. |
1 woman |
41 |
BRCA1 c.4284delAG (p.Ser1389X) |
1 woman |
69 |
BRCA1 c.4443del (p.Asp1482Ilefs*23 |
1 woman |
57 |
BRCA1 c.3875_3878 delGTCT, p.Ser1253Argfs*10 (exon11) |
3 women |
49 |
BRCA1 c.3875_3878 delGTCT, p.Ser1253Argfs*10 (exon11) |
48 |
BRCA1 c.3875_3878 delGTCT, p.Ser1253Argfs*10 (exon11) |
35 |
BRCA1 c2612delCinsTT (p.P871LfsX32) exon 11 |
1 woman |
71 |
BRCA1 c.5385insC, p.Gln1756ProfsX74. Exon 20 |
2 women |
47 |
BRCA1 c.5385insC, p.Gln1756ProfsX74. Exon 20 |
35 y 45 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
14 women
GALICIAN variant |
42 y 53 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
42 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
37 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
41 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
46 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
63 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
45 y 64 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
37 y 44 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
37 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
27 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
38 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
44 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
56 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
35 y 45 |
BRCA1 c.199G>T;pAsp67Tyr |
2 women |
45 |
BRCA1 c.199G>T;pAsp67Tyr |
67 |
BRCA1 c.3450delCAGG, pGln1111AsnfsX5b exon 11 |
2 women |
28 y 33 |
BRCA1 c.3450delCAGG, pGln1111AsnfsX5b exon 11 |
37 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
11 women |
78 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
66 y 74 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
40 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
54 y 59 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
46 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
53 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
36 y 47 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
45 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
40 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
34 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
Sequence Variant Nomenclature: HGVS (Human Genome Variation Society)
Table 3. Type of BRCA2 mutations found in our series
Age |
BRCA2 MUTATION |
No. of cases
(34 women) |
40 y 47 |
BRCA 2 Positivo. We do not know the specific type of mutation |
2 women |
27 |
BRCA 2 Positivo. We do not know the specific type of mutation |
62 |
BRCA2 c.1156-157insAlu (Alu insertion in exon 3) |
1 woman |
36 y 49 |
BRCA2 c.5192insA (c.4964dupA according HGVS) |
1 woman |
44 |
BRCA2 c.3908_3909delTG; p.(Leu1227GInfs*5) (c.3680_3681delTD, according HGVS) |
1 woman |
44 |
BRCA2 c.8089delT; pTyr2621IlefsX27 (c.7861delT according HGVS) exon 17 |
1 woman |
57 |
BRCA2 c.4233_4234insA, pPhe1336IlefsX2 (c.4005dupA, according HGVS) |
1 woman |
35 |
Deletion of exon 14 of the BRCA2 gene |
2 women |
55 |
Deletion of exon 14 of the BRCA2 gene |
44 |
BRCA2 c.1885delT; p.Cys419TrpfsX11 (c.1257delT, according HGVS) exon 10 |
2 women |
42 |
BRCA2 c.1885delT; p.Cys419TrpfsX11 (c.1257delT, according HGVS) exon 10 |
36 |
BRCA2 c.4876G>T; p.Glu1550X (c.4648G>T HGVS) exón 11 |
1 woman |
44 y 65 |
BRCA2 c.2041insA; pIle605AsnfsX11 (c.1813dup according HGVS) exon10 |
2 women |
40 y 46 |
BRCA2 c.2041insA; pIle605AsnfsX11 (c.1813dup according HGVS) exon10 |
53 |
BRCA2 c.4850_4851delAA (c.4622_4623 according HGVS) p.Lysl541serfcXG |
1 woman |
52 |
BRCA2 c.5164_5167delGAAA; Glu1646GInfs*23(c.4936_4939 according HGVS) exon 11 |
3 women |
61 |
BRCA2 c.5164_5167delGAAA; Glu1646GInfs*23(c.4936_4939 according HGVS) exon 11 |
64 |
BRCA2 c.5164_5167delGAAA; Glu1646GInfs*23(c.4936_4939 according HGVS) exon 11 |
39 |
BRCA2, c.6503delTT; p.Leu2092ProfsX7 |
1 woman |
65 |
BRCA2 c.3296delA, pAsn1023ThrfsX20 |
1 woman |
41 y 41 |
BRCA2 c.9610C>T, p.Arg3128X |
1 woman |
47 |
BRCA2 c.7901delAG; p.(Glu2558ValfsX7) |
1 woman |
42 y 53 |
BRCA2 c.886delTG (c.658del, according HGVS); pVal220IlefsX4. exon 8 |
1 woman |
35 |
BRCA2 c.4088delA, p.Asn1287IlefsX6 exon 11 |
5 women |
50 |
BRCA2 c.4088delA, p.Asn1287IlefsX6 exon 11 |
70 |
BRCA2 c.4088delA, p.Asn1287IlefsX6 exon 11 |
46 |
BRCA2 c.4088delA, p.Asn1287IlefsX6 exon 11 |
51 |
BRCA2 c.4088delA, p.Asn1287IlefsX6 exon 11 |
55 y 58 |
BRCA2 c.5374_5375del; p.Tyr1716LysfX8 exon 11 |
1 woman |
61 |
BRCA2 c.8488-1G>A, intrón 19 |
1 woman |
35 |
BRCA2 c.7786C>T; p.Arg2520X (c.7558C>T, according HGVS) exon15 |
2 women |
56 y 65 |
BRCA2 c.7786C>T; p.Arg2520X (c.7558C>T, according HGVS) exon15 |
38 |
BRCA2 c.598delA |
2 women |
30 |
BRCA2 c.598delA |
Table 4. Other types of BC-associated mutations found in our series
Age |
OTHER TYPES OF MUTATIONS |
No. of cases
(45 women) |
52 |
CHEK2 Gene: c.349A>G p.(Arg117Gly) |
1 woman |
36 y 41 |
CHEK2 Gene: c.349A>G,pArg117Gly y gen PALB2 (exon 4:c.1010T>C) |
1 woman |
54 y 56 |
BARD1 Gene: c.176_177del p.(Glu59Alafs*8) |
1 woman |
58 |
BARD1 Gene: c.176_177del p.(Glu59Alafs*8) |
1 woman |
59 |
PALB2 (deletion of exons 1 to 10 of the PALB2 gene) |
1 woman |
66 |
RAD51C Gene: c.709C>T; p.Arg273X |
1 woman |
77 |
Lynch syndrome. Exon 9 of the EPCAM gene and exon 1 of the MSH2 gene |
1 woman |
|
71 |
Variant of Uncertain Significance in CHEK2 gene: c.320-5T>A (IVS2-5T>A) intron 2 |
1 woman |
44 |
Variant of Uncertain Significance in BRCA2 gene: c.4930G>C (according HGMD); p.Glu1644Gln |
1 woman |
55 |
Variant of Uncertain Significance in BRCA2 gene (c.2612C>T)
and in MSH6 gene (c.3646+16_3646+92del) |
1 woman |
46 |
Carney syndrome phenotype (mandibular myxoma + myxoid fibroadenoma + BC)
No genetic study was done |
1 woman |
39 |
Variant of Uncertain Significance in PALB2 gene |
1 woman |
45 y 45 |
Neutral Variant in BRCA2 gene IVS4+33A>G (c.425+33 according HGVS) intron 4 |
1 woman |
67 |
Variant of Uncertain Significance in BRCA2 gene (c.1184A>C; p.(Asn319Thr) |
4 women |
50 |
Variant of Uncertain Significance in BRCA2 gene (c.1184A>C; p.(Asn319Thr) |
38 |
Variant of Uncertain Significance in BRCA2 gene (c.1184A>C; p.(Asn319Thr) |
59 |
Variant of Uncertain Significance in BRCA2 gene (c.1184A>C; p.(Asn319Thr) |
43 |
Benign Variant in BRCA2 gene (c.4258G>T Class 1) y Possibly benign (c.7008-62A>G Class 2) |
1 woman |
39 y 45 |
Variant of Uncertain Significance in BRCA2 gene (c.7633G>A p.(Val2542Ile) |
1 woman |
69 |
Negative in BRCA1-Positive FAMILY |
1 woman |
34 |
Negative in BRCA1-Positive FAMILY |
1 woman |
54 |
Negative in BRCA2-Positive FAMILY |
1 woman |
The mean age at the time of diagnosis of BRCA1/2-positive BCs was 46.9 years (SD 11.8); significantly lower than that of others BCs: 57.6 years (SD 13.7) (Table 5). Figures 1 and 2 show the age distribution using histograms.
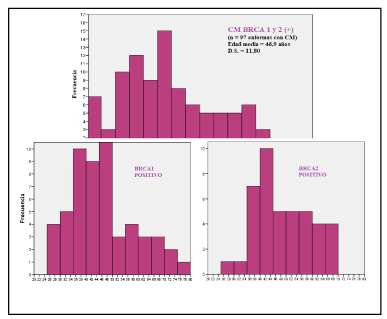
Figure 1. Histograms with age at diagnosis of BRCA1/2-positive BCs
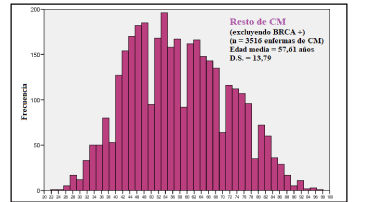
Figure 2. Histogram with age at diagnosis excluding BRCA1/2-positive BCs
Table 5. Age at diagnosis of hereditary BCs (BRCA1/2 +) vs. other BCs
|
No. of cases |
Mean age |
SD |
Significance level |
Hereditary BCs (BRCA1/2 +) |
97 |
46.90 years |
11.80 |
p < 0.001 |
All others BCs |
3,516 |
57.61 years |
13.79 |
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Patients with BRCA1/2-positive BC had a higher percentage of bilaterality: 24.4% versus 5.9% (p <0.001). However, we did not find statistically significant differences when comparing BRCA1 versus BRCA2-positive patients (Table 6).
Table 6. Bilaterality: Hereditary BCs (BRCA1/2 +) vs. others BCs
|
Percentage of BILATERAL BC |
Significance level |
Hereditary BCs (BRCA1/2 +) |
24.4 % (19 of 78) |
p < 0.001 |
All others BCs |
5.9 % (196 of 3,328) |
Hereditary BCs (BRCA1+) |
25.0% (11 of 44 BRCA1+ BCs) |
p > 0.05 |
Hereditary BCs (BRCA2+) |
23.5 % (8 of 34 BRCA2+ BCs) |
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Even if we exclude the patients with bilateral BC, patients with BRCA1/2-positive BC had a higher frequency of multiple cancers (BC and another cancer of different locations) than the rest of the women with BC (21.8% vs. 5, 9% respectively) (Table 7). When we include patients with bilateral BCs, these differences still increase: (41% vs. 14.5% in all others BCs) (Table 8). However, no significant differences were found between BRCA1 versus BRCA2-positive BC patients.
Table 7. Multiple cancers (excluding bilateral BC): Hereditary BCs (BRCA1/2 +) vs. others BCs
|
Percentage of women with multiple cancers (excluding bilateral BC) |
Significance level |
Hereditary BCs (BRCA1/2+) |
21.8 % (17 of 78) |
p = 0.007 |
All others BCs |
5.9 % (196 of 3,328) |
TOTAL |
6,3 % (213 of 3,406) |
|
Hereditary BCs (BRCA1+) |
22.7 % (10 of 44 BRCA1+ BCs) |
p > 0.05 |
Hereditary BCs (BRCA2+) |
20.6 % (7 of 34 BRCA2+ BCs) |
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Table 8. Multiple cancers (including bilateral BC): Hereditary BCs (BRCA1/2 +) vs. others BCs
|
Percentage of women with multiple cancers (including bilateral BC) |
Significance level |
Hereditary BCs (BRCA1/2+) |
41.0 % (32 of 78) |
p < 0.001 |
All others BCs |
14.5 % (483 of 3,321) |
TOTAL |
15.2 % (515 of 3,399) |
|
Hereditary BCs (BRCA1 +) |
40.9 % (18 of 44 BRCA1+ BCs) |
p > 0.05 |
Hereditary BCs (BRCA2 +) |
41.2 % (14 of 34 BRCA2+ BCs) |
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
In a more detailed analysis of BRCA1/2-positive patients with multiple cancers (Table 9) we can observe the high frequency of bilateral BC already mentioned previously, drawing our attention that five of the 44 BRCA1-positive patients were diagnosed with breast and ovarian ancer and 3 other BRCA1-positive patients also presented some type of malignant uterine neoplasia. Among the BRCA2-positive patients with multiple cancers, only one patient was diagnosed with gynecological cancer (endometrial adenocarcinoma).
Table 9. Multiple cancers: type of primary cancers associated with hereditary BCs (BRCA1/2-positive patients)
HEREDITARY BCs (BRCA1-Positive BCs) |
HEREDITARY BCs (BRCA2-Positive BCs) |
|
No. |
% |
|
No. |
% |
Bilateral BC |
10 |
50% |
Bilateral BC |
5 |
41.7% |
Bilateral BC + Colon Lymphoma |
1 |
5% |
Bilateral BC + Basal Cell Carcinoma |
1 |
8.3% |
|
|
|
BC + Basal Cell Carcinoma |
2 |
16.7% |
BC + Ovarian Cancer |
4 |
20% |
|
|
|
BC + Ovarian Cancer and CRC |
1 |
5% |
|
|
|
BC + Endometrial Cancer |
1 |
5% |
BC + Endometrial Cancer |
1 |
8.3% |
BC + Endometrial Cancer + Melanoma |
1 |
5% |
|
|
|
BC + Endometrial Sarcoma |
1 |
5% |
|
|
|
|
|
|
BC + CRC |
1 |
8.3% |
|
|
|
BC + CRC + Papillary Thyroid Carcinoma |
1 |
8.3% |
BC + Gastric adenocarcinoma |
1 |
5% |
|
|
|
|
|
|
BC + Pancreatic adenocarcinoma |
1 |
8.3% |
CRC: colorectal carcinoma
When studying the distribution of BC according to the histological grade, we were struck by the high frequency of poorly differentiated carcinomas among patients with positive BRCA1/2-positive Bs (69.3%) versus 37.8% of all others BCs (p < 0.001). When comparing the histological grade of BRCA1 vs. BRCA2, we found that BRCA1-positive BCs have up to 82% of high-grade carcinomas compared to 52.6% in BRCA2-positive BCs (p = 0.002) (Table 10).
Table 10. Histological grade: BRCA1/2-positive BCs vs. others BCs
|
Grade I
Well-
Differentiated |
Grade II Moderately-
Differentiated |
Grade III
Poorly-Differentiated |
Significance level |
Hereditary BCs
(BRCA1/2+)
(n = 88) |
4.5 %
(4 of 88) |
26.1 %
(23 of 88) |
69.3 %
(61 of 88) |
p <0.001 |
All Others BCs
(n = 2,499) |
22.5 %
(562 of 2,499) |
39.7 %
(996 of 2,499) |
37.8 %
(944 of 2,499) |
BRCA1-
Positive BCs
(n = 50) |
6 %
(3 of 50) |
12 %
(6 of 50) |
82 %
(41 of 50) |
p = 0.002 |
BRCA2-
Positive BCs
(n = 38) |
2.6 %
(1 of 38) |
44.7 %
(17 of 38) |
52.6 %
(20 of 38) |
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
When studying the distribution of the molecular subtypes (Table 11), we found striking and significant differences between the BRCA-positive BCs and all other BCs. Around half (47.9%) of the BRCA-positive BCs were classified as Triple Negative carcinomas compared to only 15% of all the other BCs. In other BCs, the main subtypes were Luminal A (31.8%) and Luminal B-like HER2 Negative (29.7%). Triple Negative BCs were mainly concentrated among BRCA1-positive BCs (60.9% vs. 25.9% of BRCA2-positive BCs). The major subtypes of the BRCA2-positive BCs were Luminal B-like HER2 Negative (48.1%) and Triple Negative (25.9%) (Table 11).
Table 11. Molecular subtypes: Hereditary BCs (BRCA1/2 +) vs. others BCs
|
LUMINAL |
NO LUMINAL |
Significance level |
A |
B-like
HER2-Positive |
B-like
HER2- Negative |
HER2 Positive |
TRIPLE NEGATIVE |
Hereditary BCs
(BRCA1/2 +)
(n = 73) |
9.6 %
(n = 7) |
11.0 %
(n = 8) |
27.4 %
(n = 20) |
4.1 %
(n = 3) |
47.9 %
(n = 35) |
p < 0.001 |
All Others BCs
(n = 1982) |
31.8 %
(n = 631) |
13.8 %
(n = 273) |
29.7 %
(n = 589) |
9.7 %
(n = 193) |
14.9 %
(n = 296) |
BRCA1-positive BCs
(n = 46) |
10.9 %
(n = 5) |
8.7 %
(n = 4) |
15.2 %
(n = 7) |
4.3 %
(n = 2) |
60.9 %
(n = 28) |
p = 0.01 |
BRCA2-positive BCs
(n = 27) |
7.4 %
(n = 2) |
14.8 %
(n = 4) |
48.1 %
(n = 13) |
3.7 %
(n = 1) |
25.9 %
(n = 7) |
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
When studying the distribution of BC according to the Nottingham Prognostic Index, we did not observe statistically significant differences (p> 0.05) (Table 12).
Table 12. Nottingham Prognostic Index: BRCA1/2-positive BCs vs. others BCs
|
BRCA1 Positives
( n = 44 ) |
BRCA2 Positives
( n = 32 ) |
All others BCs
( n = 2396 ) |
Nottingham Prognostic Index |
Good prognosis
( < 3.4 ) |
11.4 % (n = 5) |
25.0 % (n = 8) |
25.2 % (n = 603) |
Intermediate
( 3.4 – 5.4 ) |
54.5 % (n = 24) |
56.3 % (n = 18) |
38.5 % (n = 922) |
Bad prognosis
( > 5.4 ) |
27.3 % (n = 12) |
9.4 % (n = 3) |
19.8 % (n = 475) |
Carcinoma in situ |
4.5 % (n = 2) |
9.4 % (n = 3) |
11.0 % (n = 264) |
Carcinoma in situ + microinfiltration |
0 % (n = 0) |
0 % (n = 0) |
1,6 % (n = 38) |
Stage IV |
2.3 % (n = 1) |
0 % (n = 0) |
3.9 % (n = 94) |
Significance level: p > 0.05
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Nor did we find differences when studying the distribution of carcinomas according to the TNM stage at the time of diagnosis (Table 13).
Table 13. TNM staging: Hereditary BCs (BRCA1/2 +) vs. others BCs
|
BRCA 1-Positive BCs
( n = 51 ) |
BRCA2-Positive BCs
( n = 40 ) |
All others BCs
( n = 2926 ) |
Stage O (pTNM) |
3.9 % (n = 2) |
7.5 % (n = 3) |
9.7 % (n = 276) |
Stage I (pTNM) |
25.5 % (n = 13) |
35.0 % (n = 14) |
28.3 % (n = 803) |
Stage II (pTNM) |
54.9 % (n = 28) |
50.0 % (n = 20) |
37.7 % (n = 1068) |
Stage III (pTNM) |
13.7 % (n = 7) |
7.5 % (n = 3) |
20.9 % (n = 593) |
Stage IV (pTNM) |
2.0 % (n = 1) |
0 % (n = 0) |
3.4 % (n = 94) |
Significance level: p > 0.05
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
When analyzing the distribution of the different BCs according to the histological type (Table 14), we can see that invasive ductal carcinoma NOS (not otherwise specified) also known as invasive carcinoma of no special type (NST) is the most prevalent in both series, representing two thirds of the BCs. However, we found striking and significant differences (p <0.001) in relation to other specific histological types: medullary carcinomas, infiltrating ductal carcinomas with rows and necrosis, and metaplastic carcinomas were associated with BCRA-positive BCs (6.5% vs. 1.4%; 8.6% vs 1.7%; 3.2% vs 0.8%, respectively and comparing them with all others BCs). In contrast, invasive lobular carcinomas (7.9% vs 1.1%) and mucinous carcinomas (2.7% vs 1.1%) were observed with a higher frequency in the series of all others BCs (BRCA-negative BCs). When comparing the distribution of the histological types (Table 14) between the BRCA1-positve and BRCA2-positive BCs, the small differences found did not reach statistical significance.
Table 14. Histological type: BRCA1/2-positive BCs vs. others BCs// BRCA1 vs. BRCA2-positive BCs
HISTOLOGICAL TYPES |
Hereditary BCs
(BRCA1/2 +)
(n = 93) |
All others BCs
(n = 3157) |
BRCA1 +
(n = 53) |
BRCA2 +
(n = 40) |
Ivasive carcinoma (NST) |
74.2 % (n = 69) |
77.0 % (n = 2431) |
73.6 % (n = 39) |
75.0 % (n = 30) |
Invasive lobular carcinoma |
1.1 % (n = 1) |
7.9 % (n = 250) |
0 % (n = 0) |
2.5 % (n = 1) |
Mucinous carcinoma
(typical and mixed) |
1.1 % (n = 1) |
2.7 % (n = 86) |
0 % (n = 0) |
2.5 % (n = 1) |
Medullary carcinoma
(typical and atypical) |
6.5 % (n = 6) |
1.4 % (n = 45) |
7.5 % (n = 4) |
5 % (n = 2) |
Infiltrating ductal carcinoma with rows and necrosis * |
8.6 % (n = 8) |
1.7 % (n = 53) |
11.3 % (n = 6) |
5 % (n = 2) |
Metaplastic carcinoma |
3.2 % (n = 3) |
0.8 % (n = 25) |
5.7 % (n = 3) |
0 % (n = 0) |
Other types |
5.4 % (n = 5) |
8.5 % (n = 267) |
1.9 % (n = 1) |
10 % (n = 4) |
Significance level |
p < 0.001 |
p > 0.05 |
Notes: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
We classify as “infiltrating ductal carcinoma with rows and necrosis” to a special type of carcinoma that shows the following histological appearance: distribution in cords and solid nests of cells, with abundant atypical mitoses and very pleomorphic nuclei. Many of these solid areas show large areas of geographic necrosis (Figures 3 to 6). Usually, invasive carcinomas of the breast that show this appearance (with rows and necrosis) are diagnosed as invasive carcinoma of no special type (NST). When re-evaluating the histological appearance of a randomly chosen consecutive series composed of 431 BCs, we observed that 10% of them showed this specific pattern (Table 15).
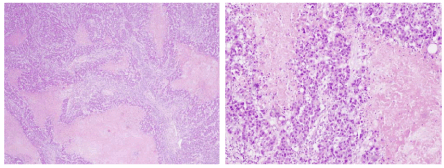
Figures 3 and 4. Histological images of BCs labeled as "Infiltrating ductal carcinomas with rows and necrosis". They show extensive areas of necrotic tissue and bands of neoplastic cells without tubular differentiation
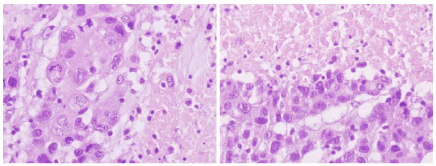
Figures 5 and 6. Histological images of BCs labeled as "Infiltrating ductal carcinomas with rows and necrosis". At higher magnification, neoplastic cells with marked nuclear pleomorphism and numerous atypical mitosis figures are observed, arranged in a syncytial pattern (in rows or bands) and bordering extensive areas of necrosis
Table 15. Reassessment of a consecutive series of 431 BCs chosen at random to recognize BCs with the pattern described: infiltrating ductal carcinoma with rows and necrosis
HISTOLOGICAL TYPE |
SERIES OF 431 BCs |
Invasive Ductal Carcinoma NST |
70.5 % (304) |
Infiltrating Ductal Carcinoma with rows and necrosis |
10.2 % (44) |
Invasive Lobular Carcinoma |
4.4 % (19) |
Invasive Papillary Carcinoma |
3.2% (14) |
Mucinous Carcinoma (Colloid) |
3.0 % (13) |
Other histological types |
8.6 % (37) |
When studying the percentage of locoregional recurrences, we did not observe statistically significant differences (p > 0.05) when establishing comparisons between the hereditary BCs versus all the others BCs (Table 16). Neither were they evident when we compared the series of BRCA1-positive versus BRCA2-positive BCs.
Table 16. Locoregional recurrence: hereditary BCs (BRCA1/2-positive) vs others BCs
|
Percentage of BC that have had a
LOCOREGIONAL RECURRENCE |
Significance level |
Hereditary BCs (BRCA1/2+) |
6.4 % (5 of 78)
In 1 case, local progression was observed after surgery |
p > 0.05 |
All Others BCs |
7.8 % (186 of 2363)
In 5 cases, local progression was observed after surgery |
Notes: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
In our total series of female BCs, we have assessed the percentage of women who met the selection criteria of SEOM (Spanish Society of Medical Oncology) clinical guidelines for germline testing [5]. The SEOM guidelines contain the following recomendations for BRCA1/2 testing: 1) Regardless of family history: Women with synchronous or metachronous breast and ovarian cancer, BC ≤ 40 years; Bilateral BC (the first diagnosed ≤ 50 years), Triple-negative BC ≤ 60 years, High-grade epithelial non-mucinous ovarian cancer (or fallopian tube or primary peritoneal cancer), Male BC, Ancestry with founder mutations, BRCA somatic mutation detected in any tumor type with a allele frequency > 30% (if it is known), Metastatic HER2-negative BC patients eligible to consider PARP (poly ADP–ribose polymerase) inhibitor therapy; 2) Two or more first degree relatives with any combination of the following high-risk features: Bilateral BC + another BC < 60 years, BC < 50 years and prostate or pancreatic cancer < 60 years, Breast and ovarian cancer, Two cases of BC diagnosed before age 50 years; 3) Three or more direct relatives with BC (at least one premenopausal) and/or ovarian cancer and/or, pancreatic cancer or high Gleason (≥ 7) prostate cancer.
In table 17 we show the results analyzing only the criteria of the first of the three sections of SEOM guidelines for germline testing (regardless of family history). When analyzing the total series, we observed that 12.9% of the BC patients (451 of 3,483) fulfilled these criteria. When studying the patients with BRCA1/2-positive BCs this percentage rose to 46.8% compared to the rest of the patients (12.2%), these differences being statistically significant (p < 0.001). However, no differences were found (p > 0.05) when comparing BRCA1-positive versus BRCA2-positive patients.
Table 17. SEOM selection criteria (regardless of family history) for BRCA1/2 testing
REGARDLESS OF FAMILY HISTORY
Women with synchronous or metachronous breast and ovarian cancer, BC ≤ 40 years; Bilateral BC (the first diagnosed ≤ 50 years), Triple-negative BC ≤ 60 years, High-grade epithelial non-mucinous ovarian cancer (or fallopian tube or primary peritoneal cancer), Male BC, Ancestry with founder mutations, BRCA somatic mutation detected in any tumor type with a allele frequency > 30% (if it is known), Metastatic HER2-negative BC patients eligible to consider PARP inhibitor therapy. |
Hereditary BCs
(BRCA1/2 +) |
46.8% meet these criteria (36 of 77) |
BRCA1+:
51.2 % meet these criteria
(22 of 43) |
p > 0.05 |
BRCA2 +:
41.2 % meet these criteria (14 of 34) |
All Others BCs |
12.2 % meet these criteria (415 of 3,406) |
|
Significance level: p < 0.001
Second, we have independently assessed the second section of the SEOM criteria (two or more first degree relatives with any combination of the following high-risk features) in table 18. The percentage of BRCA1/2-positive BC patients who met these criteria was 22.4%, while in the rest of the patients it was 3.7% (p <0.001). These criteria were met by 1 in 3 BRCA1-positive patients compared to only 1 in 10 BRCA2-positive patients (p = 0.04).
Table 18. SEOM selection criteria (two or more first degree relatives with any combination of the following high-risk features) for BRCA1/2 testing
TWO OR MORE FIRST DEGREE RELATIVES WITH ANY COMBINATION OF THE FOLLOWING HIGH-RISK FEATURES:
Bilateral BC + another BC < 60 years, BC < 50 years and prostate or pancreatic cancer < 60 years, Breast and ovarian cancer, Two cases of BC diagnosed before age 50 years |
Hereditary BCs
(BRCA1/2 +) |
22.4 % meet these criteria
(17 of 76) |
BRCA1 +:
31.8 % meet these criteria
(14 of 44) |
p = 0.04 |
BRCA2 +:
9.4 % meet these criteria
(3 of 32) |
All Others BCs |
3.7 % meet these criteria (62 of 1690) |
|
Significance level: p < 0.001
Lastly, we have evaluated the third section of the SEOM criteria (three or more direct relatives with BC and/or ovarian cancer and/or pancreatic cancer or prostate cancer) (Table 19). We observed that BRCA1/2-positive BC patients the percentage who met these criteria was 55.6% compared to 7.2% in the rest of the patients (p < 0.001). However, we did not observe differences when we compared BRCA1 versus BRCA2-positive patients (p > 0.05).
Table 19. SEOM selection criteria (three or more direct relatives with BC and/or ovarian cancer and/or pancreatic cancer or prostate cancer) for BRCA1/2 testing
THREE OR MORE DIRECT RELATIVES WITH BC AND/OR OVARIAN CANCER AND/OR PANCREATIC CANCER OR PROSTATE CANCER: ≥ 3 BC (at least one premenopausal) and/or ovarian cancer and/or, pancreatic cancer or high Gleason (≥ 7) prostate cancer. |
Hereditary BCs
(BRCA 1 y 2 +) |
55.6 % meet these criteria
(40 de 72) |
BRCA1 +:
52.5 % meet these criteria |
p > 0.05 |
BRCA2 +:
59.4 % meet these criteria |
All Others BCs |
7.2 % meet these criteria (122 of 1684) |
|
Significance level: p < 0.001
The selection criteria for the BRCA1/2 genetic testing adopted by the SEOM guide (2020) include the 3 groups of criteria already mentioned and analyzed individually in tables 17, 18 and 19. When we analyze them as a whole; that is, including as “positive” all those who fulfilled at least one of the multiple criteria included among the three mentioned sections (Table 20), we observed that one in four (75%: 57 of 76) BRCA1/2-positive BC patients were positive compared to only 15% (515 of 3348) in all others BC patients (p <0.001). However, when comparing BRCA1-positive versus BRCA2-positive patients (77.3% vs. 73.5%), no significant differences were obtained (p > 0.05).
Table 20. SEOM selection criteria for BRCA1/2 germline testing
AT LEAST ONE oF THE SEOM SELECTION CRITERIA |
HEREDITARY BCs
(BRCA1/2 +) |
75.0 % meet criteria (57 of 76) |
BRCA1 +:
77.3 % meet criteria
(34 of 44) |
p > 0.05 |
BRCA2 +:
73.5 % meet criteria
(25 of 34) |
All OTHERS BCs |
15,4 % meet criteria (515 of 3348) |
|
Significance level: p < 0.001
BRCA1/2 germline mutations are the most frequently associated with HBOC syndrome. They are detected in 15-20% of women with a family history of BC and between 60-80% of women with a family history of breast and ovarian cancer [1, 6].
We know that more genes are been identified, but there are still 50% of hereditary BCs for which we do not know the cause. Due to a greater knowledge of the genetic bases of cancer, the indications for genetic testing have been extended to high-risk families. Currently, the implementation of new genetic diagnosis technologies and the application of multigenic panels associated with hereditary cancer syndromes, allow the simultaneous analysis of patients with several different hereditary syndromes and include the study of other genes of moderate penetrance in the risk of HBOC, being able to identify the genetic cause of 8-10% more families without limiting ourselves only to the BRCA1/2 genes [7].
The alleles of susceptibility to breast and ovarian cancer, according to their frequency in the population and the relative risk (RR) they confer, can be grouped into: high penetrance (rare in the population and with RR> 5), moderate (of low frequency and RR = 2-4) or low (frequent and with RR <1.5) [8]. A) High penetrance genes: BRCA1/2 genes stand out; its pathogenic variants have an estimated population frequency of 1/400 - 1/800 and cause the majority of HBOC, but only 15% of hereditary BC cases [6]. Of the BCs not related to BRCA1/2, a part is associated with rare syndromes due to mutations in other high-penetrance genes, where BC is only one of its components (about 3% of hereditary BCs): TP53 (syndrome of Li-Fraumeni), PTEN (Cowden syndrome), STK11 (Peutz-Jeghers syndrome), CDH1 (hereditary gastric cancer) or BLM (Bloom syndrome) [9]. B) Genes of moderate penetrance: The FANC genes, from Fanconi anemia; within these: genes PALB2 (FANCN), BRIP1 (FANCJ) or RAD51C (FANCO), are associated with an undoubted risk of BC (moderate) and ovarian cancer (high). In addition, variants in ATM and CHECK2 are also included. The list of candidates likely to increase risk contains other genes: components of the MRN complex (genes MRE11, RAD50 and NBN) and genes whose proteins complex with BRCA1 (genes BARD1, RAP80, Abraxas, MERIT4). C) Low penetrance genes: identified by genome-wide association studies of hundreds of thousands of single nucleotide polymorfism (SNPs). They could explain about 14% of the risk of hereditary BC. These SNPs increase the risk little compared to the general population, but the sum of several could explain hereditary BCs without mutations in specific genes.
A recently published international population-based study, including 60,466 women with BC and 53,461 controls, has defined 9 genes with significative evidence in BC risk (ATM, BRCA1, BRCA2, CHEK2, PALB2, BARD1, RAD51C, RAD51D and TP53) [10]. It concludes that these genes are the most useful for inclusion in genetic panels and thus improve genetic counseling to patients and families. The risk of many of them was already well documented, but the risk associated with others (such as RAD51C, RAD51D and BARD1) was not as well established.
BRCA1 and BRCA2 are the most important susceptibility genes with high penetrance, located on chromosome 17 (17q21) and chromosome 13 (13q12) respectively. The BRCA1 and BRCA2 proteins participate in DNA repair, maintaining the integrity of the genome (that is why the BRCA1/2 genes are considered caretaker tumor suppressor genes). Its inactivation by mutation causes genetic instability, which can cause the appearance of the tumor with accumulation of mutations in other genes.
More than 3,500 variants have been recorded in BRCA1/2 genes; including pathogenic mutations, polymorphisms, and variants of uncertain clinical significance. The pathogenic variants are those mutations that can be considered as the main cause of high predisposition to BC. Most are alterations that lead to a premature stop in translation, generating a short (or truncated) and non-functional protein. The Consortium of Investigators of Modifiers of BRCA1/2 has analyzed data from some 30,000 families with approximately 1,700 different pathogenic variants in each gene, finding variations in the type and frequency depending on the region; which can show the existence of founding mutations in certain ethnic groups. For example, 2% of the Ashkenazi Jewish population carries one of the pathogenic variants: 185delAG and 5385insC in BRCA1 or 6174delT in BRCA2. Variant 185delAG has also been described in non-Ashkenazi Jewish populations and is one of the recurrent pathogenic variants in the Spanish population, it comes from the historical presence of Jews in the Iberian Peninsula. Other frequent pathogenic variants in Spanish families are 330A>G (the first to be discovered, Galician) and 243delA (located in Catalonia) in BRCA1; and in BRCA2 the 3036del4 (common in Europe), 6857delAA (Catalan origin) and 9254del5 (in Catalonia and Levante). We highligh the pathogenic variant 330A> G, known as the Galician variant, because it is the most prevalent in Galicia and it was also the most frequent in our series.
BCs associated with a BRCA1/2 mutation tend to develop in younger women. In this study, we corroborated that BRCA-positive BC women were younger at diagnosis (mean age 46.9 years vs. 57.6 years in all others BCs), and this difference was statistically significant. Kuchenbaecker et al., who included 3,886 BRCA1/2 carriers in their study, obtained an even lower mean age at diagnosis (38 years) [11]. In this study, it was observed that the risk of BC in BRCA carrier women increased significantly in early adulthood, reaching a maximum peak at 35-40 years in BRCA1 carriers and 5-10 years later in BRCA2 carriers; stabilizing and maintaining a similar incidence until these women reached 80 years. In our series we also observed this trend: a higher percentage of BC at younger ages for BRCA1, where 70% of the cases were diagnosed before the age of 50; while for BRCA2 this percentage was reached at 55 years of age (5 years later).
BRCA1/2 genes increase the risk of BC (x7) and ovarian cancer (x25). The penetrance of mutations in these genes is not complete (it never reaches 100%) (Table 21).
Table 21. Literature review (national and international): Penetrance for BRCA1 / 2 carriers, compared to the general population
|
BRCA1(+) |
BRCA2 (+) |
General population |
BC risk |
52% * - 65% ** |
47% * - 45% ** |
12% |
Ovarian/tubal cancer risk |
22% * - 40% ** |
18% * - 11% ** |
< 2 %% |
Notes: Based on initial penetrance estimates: * Nationally – Spain (Milne RL et al., Clin Cancer Res 2008) [12] and ** Internationally (Antoniou A et al., Am J Hum Genet 2003) [13].
Penetrance for breast and ovarian cancer has recently been evaluated in the study by Kuchenbaecker et al., a prospective multicenter study that indicates even higher rates for BC: cumulative risk of BC at 80 years of 72% and 69% and cumulative risk of ovarian cancer of 44% and 17% for BRCA1 and BRCA2 carriers, respectively. They showed that family history also influences risk: the higher the risk the more affected relatives exist [11].
BRCA mutation carriers are estimated to have a higher risk of contralateral BC, compared to non-carriers; and BRCA1 carriers are at greater risk than BRCA2 [11,14]. Kuchanbaecker et al. estimated that the cumulative risk of contralateral BC 20 years after diagnosis of the first BC was 40% for BRCA1 and 26% for BRCA2. In our study, we also observed a higher percentage of bilateral BC for BRCA1/2-positive BCs (24.4%) compared to the rest of patients with BC (5.9%) (p <0.001), without finding statistically significant differences when comparing BRCA1 and BRCA2.
Another characteristic associated with hereditary BCs is the association with other tumors. In our series, patients with BRCA1/2-positive BCs had higher frequency of multiple cancers than the rest of BCs (21.8% vs. 5.9%). Among the secondary tumors of other locations associated with the BRCA1/2 genes, the main highlights in the literature are pancreatic cancer and melanoma, for both sexes, and breast and prostate cancer in men (Table 22). In our study, the following cancers stand out: ovarian and endometrial cancer for BRCA1, and basal cell carcinoma of the skin and colorectal carcinoma for BRCA2. In a didactic way, we could simplify it by studying other series and comparing it with ours: BRCA1 has a greater association with gynecological cancers (ovarian and endometrial) and BRCA2 with digestive cancers (pancreatic, colorectal, gastric and bile duct).
Table 22. Relative risk of other tumors in BRCA1/2 carriers according to studies of the Breast Caner Linkage Consortium
|
Páncreas |
Próstata
<65 años |
Endometrio
(seroso) |
Vía biliar |
Estómago |
Melanoma |
BRCA1 |
2,26 |
1,82 |
2,65 |
|
|
|
BRCA2 |
3,51 |
7,33 |
|
4,97 |
2,59 |
2,58 |
Among the responsable genes for familial pancreatic cancer, BRCA1/2 genes account for 1% and 5-10%, respectively [15]. The risk of pancreatic cancer for both genes is moderate (RR 2-8, cumulative risk 2-17%). According to the estimates of the retrospective multicenter study of the Breast Cancer Family Registry, BRCA1 mutation carriers have an increased risk of pancreatic cancer (standardized incidence rate of 4.11), being higher for BRCA2 (5.79). For both genes, the association does not differ with sex, but higher levels of risk are detected in people under 50 years of age [16]. In our study we found a case of pancreatic adenocarcinoma in a BRCA2 carrier woman.
An increased risk of gastric cancer has been described in BRCA1/2 carriers, although it is not generally considered part of the spectrum of cancers because larger studies are needed for its confirmation. Association with gastric cancer was found mainly for BRCA2 (RR 2.5-2.7) [3,17,18].
Although previous studies show conflicting results on the excess risk of endometrial cancer for BRCA1/2 carriers, more recent studies found an increased risk of developing it, especially for BRCA1 and for an aggressive (serous) and high-grade subtype, with reported standardized incidence rates ranging from 14.29 to 32.2 [19-21]. In our series, three cases (two adenocarcinomas and one carcinosarcoma) were found among BRCA1 carriers (15%).
Data on the risk of colorectal cancer in BRCA1/2 carriers are inconsistent. A recent meta-analysis concluded that the risk of colorectal cancer increases in BRCA1 (Odds ratio 1.49), but not in BRCA2 [22]. In our study, we did find an association between BRCA2 and coloretal cancer (diagnosed in 16.6% of BRCA2 carrier women).
The association with melanoma and non-melanoma skin cancer is controversial due to its inconsistency: a clear association between BRCA1/2 and skin cancer has not been established [23]. The current evidence is based on retrospective studies of families at risk, which estimate the increased risk of melanoma in BRCA2 carriers (RR 2.5-2.7) [3,17,24]. Few studies have included non-melanoma skin cancer and generally suggest that BRCA1/2 mutations do not predispose patients to non-melanoma skin cancers. However, one study showed that BRCA1 mutations may be related to squamous cell carcinoma [24] and another study showed that BRCA2 mutation carriers were more likely to develop basal cell carcinoma than BRCA1 carriers [25].
In men, BRCA1/2 mutations have been associated with prostate cancer risk, with a wide range of risk estimates being reported: RR of 1.1-3.8% for BRCA1 and 4.7-8.6 for BRCA2. Prostate cancer is associated with BRCA1/2 mutations in 0.8-5% of cases and approximately 2% of men with early diagnosis of this cancer will carry BRCA2 mutation [26]. Roed Nielsen et al. showed a RR of 3.7 for BRCA2 carriers and 3.1 for their first-degree relatives [27].
BRCA1/2-positive BCs have their own histologic features. They tend to be high-grade histological BCs; BRCA1 tend to be poorly differentiated and BRCA2 with higher grade than sporadic patients of the same age [28]. This fact was verified in our study, where almost 70% of BRCA1/2-positive BCs were grade III (vs. 38% in other BCs); the percentage of poorly differentiated BCs being higher for BRCA1 (80% vs. 53% for BRCA2) (p <0.005).
Triple negative BC (negative for hormone receptors and HER2) occurs in 10-15% of sporadic BCs and in 66-100% of BRCA1-associated BCs. In contrast, 14–35% of triple-negative BCs carry a pathogenic variant in BRCA2, similar to the proportion of sporadic BCs [29]. In our study, we observed statistically significant differences when comparing the molercular subtype between BRCA-positive BCs with the rest of BCs almost half of BRCA-associated BCs were triple negative (vs. almost 15% in the rest of BCs); the percentage of triple negatives was higher for BRCA1-positive BCs (61% vs. 26% for BRCA2).
Regarding the histological type, the literature reports that approximately 75% of BRCA1 positive breast cancers are invasive ductal carcinomas and 10% are atypical medullary cancers; for BRCA2-positive breast cancers, lobular or ductal-lobular types are more common (up to 10% of cases) [30]. In our series, when analyzing the distribution of the different breast carcinomas according to the histological type, we could observe that NST it is the most prevalent in both series, representing two thirds of the BCs. However, we found striking and significant differences (p < 0.001) in relation to other specific histological types that were more associated with BRCA-positive BCs: medullary carcinomas, metaplastic carcinomas and infiltrating ductal carcinoma with rows and necrosis (carcinomas with a basal phenotype). On the contrary, invasive lobular carcinomas and mucinous carcinomas were observed with a higher frequency in all others BCs. When comparing the distribution of the histological types BRCA1- and BRCA2-positive BCs, the small differences found did not reach statistical significance.
We classify as infiltrating ductal carcinoma with rows and necrosis or basal phenotype carcinoma a special type of carcinoma that shows a specific histological appearance: distribution in cords and solid nests of cells, with abundant atypical mitoses and very pleomorphic nuclei and areas. Many of these solid areas show large areas of necrosis within them. Pathologists often classify these tumors as invasive ductal carcinoma NST. We believe that given these microscopic findings the pathologist should call the attention of the clinician to assess the possibility of ruling out the presence of a hereditary BC.
Based in the findings of our study, we propose that the following criteria for germline testing for HBOC should included: 1) All BC patients with certain histological subtypes: medullary and metaplastic, as well as those that present a specific type of tumor frequently classified as NST carcinoma by pathologists but which we identify as "infiltrating ductal carcinomas with rows and necrosis" (since it has peculiar and easily recognizable histological features: distribution in cords and solid nests of cells with large areas of necrosis, abundant atypical mitoses, and highly pleomorphic nuclei); 2) All families with at least one relative with multiple cancer: especially when the cancer associated with BC is gynecological (BRCA1) or digestive cancer (BRCA2), consider in the presence of any other type of low prevalent cancer (gliomas, leukemias, melanomas); 3) All BC patients whose molecular subtype is triple negative (BRCA1), consider with the luminal B (HER2-negative) if there is familial aggregation of cancer (BRCA2).
The authors declare that there is no conflict of interest regarding the publication of this paper.
All authors have no source of funding.
- Nathanson KL, Wooster R, Weber BL (2001) Breast cancer genetics: what we know and what we need. Nat Med 7: 552-556. [Crossref]
- Thompson D, Easton DF (2002) Breast cancer linkage consortium. Cancer incidence in BRCA1 mutation carriers. J Natl Cancer Inst 94: 1358-1365. [Crossref]
- Breast Cancer Linkage Consortium (1999) Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst 91: 1310-1316. [Crossref]
- Mersch J, Jackson MA, Park M, Nebgen D, Peterson SK, et al. (2015) Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer 121: 269-275. [Crossref]
- González-Santiago S, Ramón Y Cajal T, Aguirre E, Alés-Martínez JE, Andrés R, et al. (2019) SEOM clinical guidelines in hereditary breast and ovarian cancer. Clin Transl Oncol 22: 193-200.
- Narod SA, Foulkes WD (2004) BRCA1 and BRCA2: 1994 and beyond. Nat Rev Cancer 4: 665-676. [Crossref]
- Castellanos E, Gel B, Rosas I, Tornero E, Santín S, et al. (2017) A comprehensive custom panel design for routine hereditary cancer testing: preserving control, improving diagnostics and revealing a complex variation landscape. Sci Rep 7: 39348. [Crossref]
- Couch FJ, Nathanson KL, Offit K (2014) Two decades after BRCA: setting paradigms in personalized cancer care and prevention. Science 343: 1466-1470. [Crossref]
- Nielsen FC, van Overeem Hansen T, Sørensen CS (2016) Hereditary breast and ovarian cancer: new genes in confined pathways. Nat Rev Cancer 16: 599-612. [Crossref]
- Breast Cancer Association Consortium, Dorling L, Carvalho S, Allen J, et al. (2021) Breast cancer risk genes - Association analysis in more than 113,000 women. N Engl J Med 384: 428-439. [Crossref]
- Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, et al. (2017) Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317: 2402-2416. [Crossref]
- Milne RL, Osorio A, Cajal TR, Vega A, Llort G, et al. (2008) The average cumulative risks of breast and ovarian cancer for carriers of mutations in BRCA1 and BRCA2 attending genetic counseling units in Spain. Clin Cancer Res 14: 2861-2869. [Crossref]
- Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, et al. (2003) Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet 72: 1117-1130. [Crossref]
- van den Broek AJ, van 't Veer LJ, Hooning MJ, Cornelissen S, Broeks A, et al. (2016) Impact of age at primary breast cancer on contralateral breast cancer risk in BRCA1/2 mutation carriers. J Clin Oncol 34: 409-418. [Crossref]
- Pilarski R (2019) The role of BRCA testing in hereditary pancreatic and prostate cancer families. Am Soc Clin Oncol Educ Book 39: 79-86. [Crossref]
- Mocci E, Milne RL, Méndez-Villamil EY, Hopper JL, John EM, et al. (2013) Risk of pancreatic cancer in breast cancer families from the breast cancer family registry. Cancer Epidemiol Biomarkers Prev 22: 803-811.
- Moran A, O'Hara C, Khan S, Shack L, Woodward E, et al. (2012) Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer 11: 235-242. [Crossref]
- Figer A, Irmin L, Geva R, Flex D, Sulkes J, et al. (2001) The rate of the 6174delT founder Jewish mutation in BRCA2 in patients with non-colonic gastrointestinal tract tumours in Israel. Br J Cancer 84: 478-481. [Crossref]
- Shu CA, Pike MC, Jotwani AR, Friebel TM, Soslow RA, et al. (2016) Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol 2: 1434-1440. [Crossref]
- Saule C, Mouret-Fourme E, Briaux A, Becette V, Rouzier R, et al. (2018) Risk of serous endometrial carcinoma in women with pathogenic BRCA1/2 variant after risk-reducing Salpingo-Oophorectomy. J Natl Cancer Inst 110. [Crossref]
- Laitman Y, Michaelson-Cohen R, Levi E, Chen-Shtoyerman R, Reish O, et al. (2019) Uterine cancer in Jewish Israeli BRCA1/2 mutation carriers. Cancer 125: 698-703. [Crossref]
- Oh M, McBride A, Yun S, Bhattacharjee S, Slack M, et al. (2018) BRCA1 and BRCA2 gene mutations and colorectal cancer risk: Systematic review and meta-analysis. J Natl Cancer Inst 110: 1178-1189. [Crossref]
- Gumaste PV, Penn LA, Cymerman RM, Kirchhoff T, Polsky D, et al. (2015) Skin cancer risk in BRCA1/2 mutation carriers. Br J Dermatol 172: 1498-1506.
- Johannsson O, Loman N, Möller T, Kristoffersson U, Borg A, et al. (1999) Incidence of malignant tumours in relatives of BRCA1 and BRCA2 germline mutation carriers. Eur J Cancer 35: 1248-1257. [Crossref]
- Ginsburg OM, Kim-Sing C, Foulkes WD, Ghadirian P, Lynch HT, et al. (2010) BRCA1 and BRCA2 families and the risk of skin cancer. Fam Cancer 9: 489-493.
- Edwards SM, Kote-Jarai Z, Meitz J, Hamoudi R, Hope Q, et al. (2003) Two percent of men with early-onset prostate cancer harbour germline mutations in the BRCA2 gene. Am J Hum Genet 72: 1-12. [Crossref]
- Roed Nielsen H, Petersen J, Therkildsen C, Skytte AB, Nilbert M (2016) Increased risk of malecancer and identification of a potential prostate cancer cluster region in BRCA2. Acta Oncol 55: 38-44.
- Honrado E, Benítez J, Palacios J (2006) Histopathology of BRCA1- and BRCA2-associated breast cancer. Crit Rev Oncol Hematol 59: 27-39. [Crossref]
- Lee A, Moon BI, Kim TH (2020) BRCA1/BRCA2 pathogenic variant breast cancer: Treatment and prevention strategies. Ann Lab Med 40: 114-121. [Crossref]
- Han SA, Park SK, Ahn SH, Lee MH, Noh DY, et al. (2011) The Korean Hereditary Breast Cancer (KOHBRA) study: protocols and interim report. Clin Oncol (R Coll Radiol) 23: 434-441. [Crossref]
Editorial Information
Editor-in-Chief
John Livingston Powell
University of North Carolina School of Medicine
USA
Article Type
Research Article
Publication history
Received date: March 16, 2021
Accepted date: April 02, 2021
Published date: April 08, 2021
Copyright
©2021 Rodríguez-Fernández V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Rodríguez-Fernández V, Cameselle-Cortizo L, Valdés-Pons J, Castro-Parga GJD, Figueiredo-Alonso E, et al. (2021) New criteria to select patients with breast cancer to perform germline BRCA1/2 testing. Clin Obstet Gynecol Reprod Med 7: DOI: 10.15761/COGRM.1000326

Figure 1. Histograms with age at diagnosis of BRCA1/2-positive BCs

Figure 2. Histogram with age at diagnosis excluding BRCA1/2-positive BCs

Figures 3 and 4. Histological images of BCs labeled as "Infiltrating ductal carcinomas with rows and necrosis". They show extensive areas of necrotic tissue and bands of neoplastic cells without tubular differentiation

Figures 5 and 6. Histological images of BCs labeled as "Infiltrating ductal carcinomas with rows and necrosis". At higher magnification, neoplastic cells with marked nuclear pleomorphism and numerous atypical mitosis figures are observed, arranged in a syncytial pattern (in rows or bands) and bordering extensive areas of necrosis
Table 1. Genetic testing in our series (in 133 BC patients)
GENETIC STUDY (n = 133 women with BC) |
MUTATIONS IN HIGH/MODERATE PENETRANCE GENES FOR BC
85 women with BC |
BRCA1 y BRCA2 genes |
BRCA1 |
n = 44 |
33.0% |
BRCA2 |
n = 34 |
25.5% |
BARD1 Gene |
n = 2 |
1.5% |
CHECK2 Gene |
n = 2 |
1.5% |
RAD51D Gene |
n = 1 |
0.7% |
PALB2 Gene |
n = 1 |
0.7% |
Lynch Syndrome |
n = 1 |
0.7% |
CARNEY SYNDROME PHENOTYPE
1 women with BC |
She has no genetic study
(Carney phenotype) |
n = 1 |
0.7% |
NEGATIVE GENETIC STUDY
31 women with BC |
COMPLETE GENETIC PANEL * (n =16) |
n = 31 |
23.3% |
Only BRCA1/2 testing (n = 15) |
MUTATIONS IN GENES WITH UNCERTAIN SIGNIFICANCE
11 women with BC |
BRCA2 variant of uncertain significance |
n = 8 |
6.0% |
PALB2 variant of uncertain significance |
n = 2 |
1.5% |
CHECK2 variant of uncertain significance |
n = 1 |
0.7% |
FAMILY BRCA (+)
WOMEN BRCA (-)
3 women with BC |
BRCA1-Positive Family (BC patient: BRCA -) |
n = 2 |
1.5% |
BRCA2-Positive Family (BC patient: BRCA -) |
n = 1 |
0.7% |
Analysis of large deletions and duplications in BRCA1/2 by the MLPA (Multiplex ligation-dependent probe amplification) technique: Kit P002B was used for the analysis of the BRCA1 gene and P045B for the analysis of BRCA2.
* Complete genetic panel: Search for mutations by NGS (Ion PROTON) sequencing of the entire coding region and flanked intronic regions of the BRCA1 (NM_007294.3), BRCA2 (NM_000059.3), PTEN (NM_00314), TP53 (NM_000546.5), CDH1 (NM_004360.3), RAD51C (NM_058216), RAD51D (NM_001142571), MLH1 (NM_001258274), MSH2 (NM_ 000251), MSH6 (NM_000179), PALB2 (NM_02675), CHEK2 (NM_001142571) (NM_00657194) (NM_00116194) (NM_00116194) (NM_006194) NM_000455) genes.
Table 2. Type of BRCA1 mutations found in our series
Age |
BRCA1 MUTATION |
No. of cases
(45 women) |
29 |
BRCA1 positive. We do not know the specific type of mutation |
2 women |
30 |
BRCA1 positive. We do not know the specific type of mutation |
58 y 62 |
BRCA1 c3756_3759del (p.Ser1253Argfs*10) |
1 woman |
27 |
BRCA1 c.4185_4185+3del p.(Gln1395Hisfs*10) |
2 women |
33 |
BRCA1 c.4185_4185+3del p.(Gln1395Hisfs*10) |
33 |
BRCA1 c.951dupA (p.Thr278AsnfsX9) exón 11 |
1 woman |
43 |
BRCA1 Deletion of exons 1 to 13 of the BRCA1 gene |
1 woman |
38 |
BRCA1 4 base pair deletion (GTTC). Exon 11. Codon 264. |
1 woman |
41 |
BRCA1 c.4284delAG (p.Ser1389X) |
1 woman |
69 |
BRCA1 c.4443del (p.Asp1482Ilefs*23 |
1 woman |
57 |
BRCA1 c.3875_3878 delGTCT, p.Ser1253Argfs*10 (exon11) |
3 women |
49 |
BRCA1 c.3875_3878 delGTCT, p.Ser1253Argfs*10 (exon11) |
48 |
BRCA1 c.3875_3878 delGTCT, p.Ser1253Argfs*10 (exon11) |
35 |
BRCA1 c2612delCinsTT (p.P871LfsX32) exon 11 |
1 woman |
71 |
BRCA1 c.5385insC, p.Gln1756ProfsX74. Exon 20 |
2 women |
47 |
BRCA1 c.5385insC, p.Gln1756ProfsX74. Exon 20 |
35 y 45 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
14 women
GALICIAN variant |
42 y 53 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
42 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
37 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
41 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
46 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
63 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
45 y 64 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
37 y 44 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
37 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
27 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
38 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
44 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
56 |
BRCA1 c.330A>G(p.R71G) exón5 c.211A>G (p.Arg71Gly) according HGVS |
35 y 45 |
BRCA1 c.199G>T;pAsp67Tyr |
2 women |
45 |
BRCA1 c.199G>T;pAsp67Tyr |
67 |
BRCA1 c.3450delCAGG, pGln1111AsnfsX5b exon 11 |
2 women |
28 y 33 |
BRCA1 c.3450delCAGG, pGln1111AsnfsX5b exon 11 |
37 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
11 women |
78 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
66 y 74 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
40 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
54 y 59 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
46 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
53 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
36 y 47 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
45 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
40 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
34 |
BRCA1 c.3808 T>G (c.3689 according HGVS), p.Leu1230X exon 11 |
Sequence Variant Nomenclature: HGVS (Human Genome Variation Society)
Table 3. Type of BRCA2 mutations found in our series
Age |
BRCA2 MUTATION |
No. of cases
(34 women) |
40 y 47 |
BRCA 2 Positivo. We do not know the specific type of mutation |
2 women |
27 |
BRCA 2 Positivo. We do not know the specific type of mutation |
62 |
BRCA2 c.1156-157insAlu (Alu insertion in exon 3) |
1 woman |
36 y 49 |
BRCA2 c.5192insA (c.4964dupA according HGVS) |
1 woman |
44 |
BRCA2 c.3908_3909delTG; p.(Leu1227GInfs*5) (c.3680_3681delTD, according HGVS) |
1 woman |
44 |
BRCA2 c.8089delT; pTyr2621IlefsX27 (c.7861delT according HGVS) exon 17 |
1 woman |
57 |
BRCA2 c.4233_4234insA, pPhe1336IlefsX2 (c.4005dupA, according HGVS) |
1 woman |
35 |
Deletion of exon 14 of the BRCA2 gene |
2 women |
55 |
Deletion of exon 14 of the BRCA2 gene |
44 |
BRCA2 c.1885delT; p.Cys419TrpfsX11 (c.1257delT, according HGVS) exon 10 |
2 women |
42 |
BRCA2 c.1885delT; p.Cys419TrpfsX11 (c.1257delT, according HGVS) exon 10 |
36 |
BRCA2 c.4876G>T; p.Glu1550X (c.4648G>T HGVS) exón 11 |
1 woman |
44 y 65 |
BRCA2 c.2041insA; pIle605AsnfsX11 (c.1813dup according HGVS) exon10 |
2 women |
40 y 46 |
BRCA2 c.2041insA; pIle605AsnfsX11 (c.1813dup according HGVS) exon10 |
53 |
BRCA2 c.4850_4851delAA (c.4622_4623 according HGVS) p.Lysl541serfcXG |
1 woman |
52 |
BRCA2 c.5164_5167delGAAA; Glu1646GInfs*23(c.4936_4939 according HGVS) exon 11 |
3 women |
61 |
BRCA2 c.5164_5167delGAAA; Glu1646GInfs*23(c.4936_4939 according HGVS) exon 11 |
64 |
BRCA2 c.5164_5167delGAAA; Glu1646GInfs*23(c.4936_4939 according HGVS) exon 11 |
39 |
BRCA2, c.6503delTT; p.Leu2092ProfsX7 |
1 woman |
65 |
BRCA2 c.3296delA, pAsn1023ThrfsX20 |
1 woman |
41 y 41 |
BRCA2 c.9610C>T, p.Arg3128X |
1 woman |
47 |
BRCA2 c.7901delAG; p.(Glu2558ValfsX7) |
1 woman |
42 y 53 |
BRCA2 c.886delTG (c.658del, according HGVS); pVal220IlefsX4. exon 8 |
1 woman |
35 |
BRCA2 c.4088delA, p.Asn1287IlefsX6 exon 11 |
5 women |
50 |
BRCA2 c.4088delA, p.Asn1287IlefsX6 exon 11 |
70 |
BRCA2 c.4088delA, p.Asn1287IlefsX6 exon 11 |
46 |
BRCA2 c.4088delA, p.Asn1287IlefsX6 exon 11 |
51 |
BRCA2 c.4088delA, p.Asn1287IlefsX6 exon 11 |
55 y 58 |
BRCA2 c.5374_5375del; p.Tyr1716LysfX8 exon 11 |
1 woman |
61 |
BRCA2 c.8488-1G>A, intrón 19 |
1 woman |
35 |
BRCA2 c.7786C>T; p.Arg2520X (c.7558C>T, according HGVS) exon15 |
2 women |
56 y 65 |
BRCA2 c.7786C>T; p.Arg2520X (c.7558C>T, according HGVS) exon15 |
38 |
BRCA2 c.598delA |
2 women |
30 |
BRCA2 c.598delA |
Table 4. Other types of BC-associated mutations found in our series
Age |
OTHER TYPES OF MUTATIONS |
No. of cases
(45 women) |
52 |
CHEK2 Gene: c.349A>G p.(Arg117Gly) |
1 woman |
36 y 41 |
CHEK2 Gene: c.349A>G,pArg117Gly y gen PALB2 (exon 4:c.1010T>C) |
1 woman |
54 y 56 |
BARD1 Gene: c.176_177del p.(Glu59Alafs*8) |
1 woman |
58 |
BARD1 Gene: c.176_177del p.(Glu59Alafs*8) |
1 woman |
59 |
PALB2 (deletion of exons 1 to 10 of the PALB2 gene) |
1 woman |
66 |
RAD51C Gene: c.709C>T; p.Arg273X |
1 woman |
77 |
Lynch syndrome. Exon 9 of the EPCAM gene and exon 1 of the MSH2 gene |
1 woman |
|
71 |
Variant of Uncertain Significance in CHEK2 gene: c.320-5T>A (IVS2-5T>A) intron 2 |
1 woman |
44 |
Variant of Uncertain Significance in BRCA2 gene: c.4930G>C (according HGMD); p.Glu1644Gln |
1 woman |
55 |
Variant of Uncertain Significance in BRCA2 gene (c.2612C>T)
and in MSH6 gene (c.3646+16_3646+92del) |
1 woman |
46 |
Carney syndrome phenotype (mandibular myxoma + myxoid fibroadenoma + BC)
No genetic study was done |
1 woman |
39 |
Variant of Uncertain Significance in PALB2 gene |
1 woman |
45 y 45 |
Neutral Variant in BRCA2 gene IVS4+33A>G (c.425+33 according HGVS) intron 4 |
1 woman |
67 |
Variant of Uncertain Significance in BRCA2 gene (c.1184A>C; p.(Asn319Thr) |
4 women |
50 |
Variant of Uncertain Significance in BRCA2 gene (c.1184A>C; p.(Asn319Thr) |
38 |
Variant of Uncertain Significance in BRCA2 gene (c.1184A>C; p.(Asn319Thr) |
59 |
Variant of Uncertain Significance in BRCA2 gene (c.1184A>C; p.(Asn319Thr) |
43 |
Benign Variant in BRCA2 gene (c.4258G>T Class 1) y Possibly benign (c.7008-62A>G Class 2) |
1 woman |
39 y 45 |
Variant of Uncertain Significance in BRCA2 gene (c.7633G>A p.(Val2542Ile) |
1 woman |
69 |
Negative in BRCA1-Positive FAMILY |
1 woman |
34 |
Negative in BRCA1-Positive FAMILY |
1 woman |
54 |
Negative in BRCA2-Positive FAMILY |
1 woman |
Table 5. Age at diagnosis of hereditary BCs (BRCA1/2 +) vs. other BCs
|
No. of cases |
Mean age |
SD |
Significance level |
Hereditary BCs (BRCA1/2 +) |
97 |
46.90 years |
11.80 |
p < 0.001 |
All others BCs |
3,516 |
57.61 years |
13.79 |
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Table 6. Bilaterality: Hereditary BCs (BRCA1/2 +) vs. others BCs
|
Percentage of BILATERAL BC |
Significance level |
Hereditary BCs (BRCA1/2 +) |
24.4 % (19 of 78) |
p < 0.001 |
All others BCs |
5.9 % (196 of 3,328) |
Hereditary BCs (BRCA1+) |
25.0% (11 of 44 BRCA1+ BCs) |
p > 0.05 |
Hereditary BCs (BRCA2+) |
23.5 % (8 of 34 BRCA2+ BCs) |
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Table 7. Multiple cancers (excluding bilateral BC): Hereditary BCs (BRCA1/2 +) vs. others BCs
|
Percentage of women with multiple cancers (excluding bilateral BC) |
Significance level |
Hereditary BCs (BRCA1/2+) |
21.8 % (17 of 78) |
p = 0.007 |
All others BCs |
5.9 % (196 of 3,328) |
TOTAL |
6,3 % (213 of 3,406) |
|
Hereditary BCs (BRCA1+) |
22.7 % (10 of 44 BRCA1+ BCs) |
p > 0.05 |
Hereditary BCs (BRCA2+) |
20.6 % (7 of 34 BRCA2+ BCs) |
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Table 8. Multiple cancers (including bilateral BC): Hereditary BCs (BRCA1/2 +) vs. others BCs
|
Percentage of women with multiple cancers (including bilateral BC) |
Significance level |
Hereditary BCs (BRCA1/2+) |
41.0 % (32 of 78) |
p < 0.001 |
All others BCs |
14.5 % (483 of 3,321) |
TOTAL |
15.2 % (515 of 3,399) |
|
Hereditary BCs (BRCA1 +) |
40.9 % (18 of 44 BRCA1+ BCs) |
p > 0.05 |
Hereditary BCs (BRCA2 +) |
41.2 % (14 of 34 BRCA2+ BCs) |
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Table 9. Multiple cancers: type of primary cancers associated with hereditary BCs (BRCA1/2-positive patients)
HEREDITARY BCs (BRCA1-Positive BCs) |
HEREDITARY BCs (BRCA2-Positive BCs) |
|
No. |
% |
|
No. |
% |
Bilateral BC |
10 |
50% |
Bilateral BC |
5 |
41.7% |
Bilateral BC + Colon Lymphoma |
1 |
5% |
Bilateral BC + Basal Cell Carcinoma |
1 |
8.3% |
|
|
|
BC + Basal Cell Carcinoma |
2 |
16.7% |
BC + Ovarian Cancer |
4 |
20% |
|
|
|
BC + Ovarian Cancer and CRC |
1 |
5% |
|
|
|
BC + Endometrial Cancer |
1 |
5% |
BC + Endometrial Cancer |
1 |
8.3% |
BC + Endometrial Cancer + Melanoma |
1 |
5% |
|
|
|
BC + Endometrial Sarcoma |
1 |
5% |
|
|
|
|
|
|
BC + CRC |
1 |
8.3% |
|
|
|
BC + CRC + Papillary Thyroid Carcinoma |
1 |
8.3% |
BC + Gastric adenocarcinoma |
1 |
5% |
|
|
|
|
|
|
BC + Pancreatic adenocarcinoma |
1 |
8.3% |
CRC: colorectal carcinoma
Table 10. Histological grade: BRCA1/2-positive BCs vs. others BCs
|
Grade I
Well-
Differentiated |
Grade II Moderately-
Differentiated |
Grade III
Poorly-Differentiated |
Significance level |
Hereditary BCs
(BRCA1/2+)
(n = 88) |
4.5 %
(4 of 88) |
26.1 %
(23 of 88) |
69.3 %
(61 of 88) |
p <0.001 |
All Others BCs
(n = 2,499) |
22.5 %
(562 of 2,499) |
39.7 %
(996 of 2,499) |
37.8 %
(944 of 2,499) |
BRCA1-
Positive BCs
(n = 50) |
6 %
(3 of 50) |
12 %
(6 of 50) |
82 %
(41 of 50) |
p = 0.002 |
BRCA2-
Positive BCs
(n = 38) |
2.6 %
(1 of 38) |
44.7 %
(17 of 38) |
52.6 %
(20 of 38) |
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Table 11. Molecular subtypes: Hereditary BCs (BRCA1/2 +) vs. others BCs
|
LUMINAL |
NO LUMINAL |
Significance level |
A |
B-like
HER2-Positive |
B-like
HER2- Negative |
HER2 Positive |
TRIPLE NEGATIVE |
Hereditary BCs
(BRCA1/2 +)
(n = 73) |
9.6 %
(n = 7) |
11.0 %
(n = 8) |
27.4 %
(n = 20) |
4.1 %
(n = 3) |
47.9 %
(n = 35) |
p < 0.001 |
All Others BCs
(n = 1982) |
31.8 %
(n = 631) |
13.8 %
(n = 273) |
29.7 %
(n = 589) |
9.7 %
(n = 193) |
14.9 %
(n = 296) |
BRCA1-positive BCs
(n = 46) |
10.9 %
(n = 5) |
8.7 %
(n = 4) |
15.2 %
(n = 7) |
4.3 %
(n = 2) |
60.9 %
(n = 28) |
p = 0.01 |
BRCA2-positive BCs
(n = 27) |
7.4 %
(n = 2) |
14.8 %
(n = 4) |
48.1 %
(n = 13) |
3.7 %
(n = 1) |
25.9 %
(n = 7) |
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Table 12. Nottingham Prognostic Index: BRCA1/2-positive BCs vs. others BCs
|
BRCA1 Positives
( n = 44 ) |
BRCA2 Positives
( n = 32 ) |
All others BCs
( n = 2396 ) |
Nottingham Prognostic Index |
Good prognosis
( < 3.4 ) |
11.4 % (n = 5) |
25.0 % (n = 8) |
25.2 % (n = 603) |
Intermediate
( 3.4 – 5.4 ) |
54.5 % (n = 24) |
56.3 % (n = 18) |
38.5 % (n = 922) |
Bad prognosis
( > 5.4 ) |
27.3 % (n = 12) |
9.4 % (n = 3) |
19.8 % (n = 475) |
Carcinoma in situ |
4.5 % (n = 2) |
9.4 % (n = 3) |
11.0 % (n = 264) |
Carcinoma in situ + microinfiltration |
0 % (n = 0) |
0 % (n = 0) |
1,6 % (n = 38) |
Stage IV |
2.3 % (n = 1) |
0 % (n = 0) |
3.9 % (n = 94) |
Significance level: p > 0.05
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Table 13. TNM staging: Hereditary BCs (BRCA1/2 +) vs. others BCs
|
BRCA 1-Positive BCs
( n = 51 ) |
BRCA2-Positive BCs
( n = 40 ) |
All others BCs
( n = 2926 ) |
Stage O (pTNM) |
3.9 % (n = 2) |
7.5 % (n = 3) |
9.7 % (n = 276) |
Stage I (pTNM) |
25.5 % (n = 13) |
35.0 % (n = 14) |
28.3 % (n = 803) |
Stage II (pTNM) |
54.9 % (n = 28) |
50.0 % (n = 20) |
37.7 % (n = 1068) |
Stage III (pTNM) |
13.7 % (n = 7) |
7.5 % (n = 3) |
20.9 % (n = 593) |
Stage IV (pTNM) |
2.0 % (n = 1) |
0 % (n = 0) |
3.4 % (n = 94) |
Significance level: p > 0.05
Note: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Table 14. Histological type: BRCA1/2-positive BCs vs. others BCs// BRCA1 vs. BRCA2-positive BCs
HISTOLOGICAL TYPES |
Hereditary BCs
(BRCA1/2 +)
(n = 93) |
All others BCs
(n = 3157) |
BRCA1 +
(n = 53) |
BRCA2 +
(n = 40) |
Ivasive carcinoma (NST) |
74.2 % (n = 69) |
77.0 % (n = 2431) |
73.6 % (n = 39) |
75.0 % (n = 30) |
Invasive lobular carcinoma |
1.1 % (n = 1) |
7.9 % (n = 250) |
0 % (n = 0) |
2.5 % (n = 1) |
Mucinous carcinoma
(typical and mixed) |
1.1 % (n = 1) |
2.7 % (n = 86) |
0 % (n = 0) |
2.5 % (n = 1) |
Medullary carcinoma
(typical and atypical) |
6.5 % (n = 6) |
1.4 % (n = 45) |
7.5 % (n = 4) |
5 % (n = 2) |
Infiltrating ductal carcinoma with rows and necrosis * |
8.6 % (n = 8) |
1.7 % (n = 53) |
11.3 % (n = 6) |
5 % (n = 2) |
Metaplastic carcinoma |
3.2 % (n = 3) |
0.8 % (n = 25) |
5.7 % (n = 3) |
0 % (n = 0) |
Other types |
5.4 % (n = 5) |
8.5 % (n = 267) |
1.9 % (n = 1) |
10 % (n = 4) |
Significance level |
p < 0.001 |
p > 0.05 |
Notes: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Table 15. Reassessment of a consecutive series of 431 BCs chosen at random to recognize BCs with the pattern described: infiltrating ductal carcinoma with rows and necrosis
HISTOLOGICAL TYPE |
SERIES OF 431 BCs |
Invasive Ductal Carcinoma NST |
70.5 % (304) |
Infiltrating Ductal Carcinoma with rows and necrosis |
10.2 % (44) |
Invasive Lobular Carcinoma |
4.4 % (19) |
Invasive Papillary Carcinoma |
3.2% (14) |
Mucinous Carcinoma (Colloid) |
3.0 % (13) |
Other histological types |
8.6 % (37) |
Table 16. Locoregional recurrence: hereditary BCs (BRCA1/2-positive) vs others BCs
|
Percentage of BC that have had a
LOCOREGIONAL RECURRENCE |
Significance level |
Hereditary BCs (BRCA1/2+) |
6.4 % (5 of 78)
In 1 case, local progression was observed after surgery |
p > 0.05 |
All Others BCs |
7.8 % (186 of 2363)
In 5 cases, local progression was observed after surgery |
Notes: We exclude from hereditary BC mutations in other genes and variants of uncertain significance.
Table 17. SEOM selection criteria (regardless of family history) for BRCA1/2 testing
REGARDLESS OF FAMILY HISTORY
Women with synchronous or metachronous breast and ovarian cancer, BC ≤ 40 years; Bilateral BC (the first diagnosed ≤ 50 years), Triple-negative BC ≤ 60 years, High-grade epithelial non-mucinous ovarian cancer (or fallopian tube or primary peritoneal cancer), Male BC, Ancestry with founder mutations, BRCA somatic mutation detected in any tumor type with a allele frequency > 30% (if it is known), Metastatic HER2-negative BC patients eligible to consider PARP inhibitor therapy. |
Hereditary BCs
(BRCA1/2 +) |
46.8% meet these criteria (36 of 77) |
BRCA1+:
51.2 % meet these criteria
(22 of 43) |
p > 0.05 |
BRCA2 +:
41.2 % meet these criteria (14 of 34) |
All Others BCs |
12.2 % meet these criteria (415 of 3,406) |
|
Significance level: p < 0.001
Table 18. SEOM selection criteria (two or more first degree relatives with any combination of the following high-risk features) for BRCA1/2 testing
TWO OR MORE FIRST DEGREE RELATIVES WITH ANY COMBINATION OF THE FOLLOWING HIGH-RISK FEATURES:
Bilateral BC + another BC < 60 years, BC < 50 years and prostate or pancreatic cancer < 60 years, Breast and ovarian cancer, Two cases of BC diagnosed before age 50 years |
Hereditary BCs
(BRCA1/2 +) |
22.4 % meet these criteria
(17 of 76) |
BRCA1 +:
31.8 % meet these criteria
(14 of 44) |
p = 0.04 |
BRCA2 +:
9.4 % meet these criteria
(3 of 32) |
All Others BCs |
3.7 % meet these criteria (62 of 1690) |
|
Significance level: p < 0.001
Table 19. SEOM selection criteria (three or more direct relatives with BC and/or ovarian cancer and/or pancreatic cancer or prostate cancer) for BRCA1/2 testing
THREE OR MORE DIRECT RELATIVES WITH BC AND/OR OVARIAN CANCER AND/OR PANCREATIC CANCER OR PROSTATE CANCER: ≥ 3 BC (at least one premenopausal) and/or ovarian cancer and/or, pancreatic cancer or high Gleason (≥ 7) prostate cancer. |
Hereditary BCs
(BRCA 1 y 2 +) |
55.6 % meet these criteria
(40 de 72) |
BRCA1 +:
52.5 % meet these criteria |
p > 0.05 |
BRCA2 +:
59.4 % meet these criteria |
All Others BCs |
7.2 % meet these criteria (122 of 1684) |
|
Significance level: p < 0.001
Table 20. SEOM selection criteria for BRCA1/2 germline testing
AT LEAST ONE oF THE SEOM SELECTION CRITERIA |
HEREDITARY BCs
(BRCA1/2 +) |
75.0 % meet criteria (57 of 76) |
BRCA1 +:
77.3 % meet criteria
(34 of 44) |
p > 0.05 |
BRCA2 +:
73.5 % meet criteria
(25 of 34) |
All OTHERS BCs |
15,4 % meet criteria (515 of 3348) |
|
Significance level: p < 0.001
Table 21. Literature review (national and international): Penetrance for BRCA1 / 2 carriers, compared to the general population
|
BRCA1(+) |
BRCA2 (+) |
General population |
BC risk |
52% * - 65% ** |
47% * - 45% ** |
12% |
Ovarian/tubal cancer risk |
22% * - 40% ** |
18% * - 11% ** |
< 2 %% |
Notes: Based on initial penetrance estimates: * Nationally – Spain (Milne RL et al., Clin Cancer Res 2008) [12] and ** Internationally (Antoniou A et al., Am J Hum Genet 2003) [13].
Table 22. Relative risk of other tumors in BRCA1/2 carriers according to studies of the Breast Caner Linkage Consortium
|
Páncreas |
Próstata
<65 años |
Endometrio
(seroso) |
Vía biliar |
Estómago |
Melanoma |
BRCA1 |
2,26 |
1,82 |
2,65 |
|
|
|
BRCA2 |
3,51 |
7,33 |
|
4,97 |
2,59 |
2,58 |




