Abstract
Wound healing is a complex multiple-phase process for injured tissues repair and any failure in the normal wound healing process results in abnormal scar formation. Although children’s scarring may often be considered trivial, depending on its localisation, it can be aesthetically unpleasant and/or disfiguring, causing anxiety and distress to kids. This study sought to reinforce data on cosmetic outcomes of onion-extract-based Mederma® for Kids™ on kids’ scars.
Thirty subjects (12 female and 18 male) were included in this single-center, open-label study. At screening, mean age was 10.0 years old and 66.7% of subjects were skin phototype III. The Investigator and children’s parents/guardians reported that scar appearance (rated through the evaluation of multiple parameters including dryness, redness, skin pigmentation, color, softness, texture and overall appearance) was improved after the 8 weeks of Mederma® for Kids™ daily application. According to the Investigator, the scar’s dryness and redness were significantly reduced from Day 29 ± 2 days. Likewise, the scar’s color, texture and softness were significantly improved from Day 29 ± 2 days. Parents/guardians’ assessments were comparable, and they also reported a significant reduction of scar discomfort and visibility from Day 29 ± 2 days. Interestingly, the first parent to report the first detectable effect on scar softness, redness and texture (roughness) did so at Day 1 of application and as soon as Day 3 for the first visible effects on discomfort and overall appearance. The product’s perception was very well-appreciated by parents/guardians and their children, and 93.1% of parents/guardians would recommend it to other parents/guardians. Four non-related adverse events were reported during the study. In conclusion, under the study conditions, Mederma® for Kids™ was proved to be effective in improving scar’s appearance after 8 weeks of application 3 times daily.
Keywords
children’s scars, cosmetic outcomes, scar appearance, onion extract, topical skin care
Introduction
Wound healing is a dynamic multiple-phase process for damaged/injured tissues repair through an intricate mechanism involving cellular responses, extracellular matrix remodelling and growth factors [1,2]. Any failure in the normal wound healing process results in abnormal scar formation. Skin injuries or wounds are produced, among others, by trauma, burns, and surgery. Children are particularly vulnerable to injuries due to their low hazard perception, making them more liable to scarring. Although scarring may often be considered trivial, depending on its localisation, it can be aesthetically unpleasant and/or disfiguring, causing anxiety and distress to kids and reduces their quality of life.
Topical botanical agents are commercially available and have visible cosmetic outcomes on scars, such as surgical scars [3-5]. A concentrated onion extract aqueous gel/cream brand of products, including Mederma® for Kids™ (Laboratoire HRA Pharma), first developed and commercialized in USA, have been demonstrated to be effective on old and new scars caused by cuts and scrapes, stitches, burns, insect bites and surgery and to visibly reduce the overall appearance of scars. Mederma® is currently marketed in several countries worldwide.This study was designed to collect additional data on Mederma® for Kids™on cosmetic outcomes.
Materials and Methods
Study Design and subjects
This was a single-center, open-label study. It was conducted in a single investigational center(Centre Pharmacologie Clinique Appliqué à la Dermatologie, CPCAD) located in the Public University Hospital of Nice, France.
Eligible subjects were aged 3 to 17 years old with skin type I to VI according to Fitzpatrick classification [6] with at least one scar formed from wounds (such as cuts and scrapes, stitches, burns, insect bites, surgery) occurring within six months preceding the inclusion. Subjects for whom the parent/guardian: was able to understand the full nature and the purpose of the study, including possible risks and side effects;was able to cooperate with the Investigator and to comply with the requirements of the entire study (including ability to attend all the planned study visits according to the time limits), and signed a written informed consent (both parent/guardian and child).
Subjects excluded from the study were subjects with known allergies or sensitivities to ingredients contained in Mederma® for Kids™ and suffering from a serious or progressive disease that could compromise their participation in the study according to the Investigator (e.g., diabetes, cardiac pathologies, hepatic disorder, renal disorder, pulmonary disease, cancer, neurological or psychological disease, inflammatory/immunosuppressive disease).
The study duration participation per subject was an 8-week product application period and observation with 3 on-site visits.
Investigational product
The investigational product (IP) was Mederma® for Kids™, manufactured, formulated and provided to the Investigator under the responsibility of HRA Pharma. Mederma® for Kids™ has a specific formulation intended for use in the paediatric population. It contains several ingredients like allium cepa, an onion extract and Allantoin, that have been demonstrated to improve the appearance of scars, to reduce complaints about scar appearance and to improve the scarred areas [4,7]. Mederma® for Kids™ has a child-friendly scent and a purple color that disappears upon application and massage into the skin to support compliance.
Ethical aspects
The clinical study protocol was reviewed and approved by the French Ethics Committee (EC) Sud Est I, on September 22nd, 2021, prior to inclusion of subjects. The study was conducted in accordance with the protocol, the Declaration of Helsinki, and in compliance with the applicable local regulatory requirements.
Study procedures
Subjects attended 3 visits at the study center: a screening/baseline visit (Day 1), during which the Investigator collected the informed consent, baseline characteristics (demographics, medical/surgical history, physical examination/vital signs and previous/concomitant therapies), dispensed the IP to each parent/guardian for application at home with an instruction sheet and a diary (compliance, noticeable events and an emergency card); 2 evaluation visits (Week 4/Day 29 ± 2 days and Week 8/Day 57 ± 2 days), during which the Investigator questioned the parent/guardian of the subject regarding compliance, checked that’s questions in the diary were answered and collected any adverse events (AE) and concomitant therapies, performed clinical assessment of scar appearance (dryness, redness, skin pigmentation, color, softness, texture and overall appearance), asked the parent/guardian to assess scar appearance. All AEs were reported on the AE form of the electronic Case Report Form (e-CRF) with complete information as required. At the end of the study (Week 8/Day 57 ± 2 days) the parent/guardian was asked to fill out a self-assessment questionnaire about benefit and product perception (cosmetic acceptability). Subjects were to apply the IP three times a day for 8 weeks as per product instruction.
All collected data (participant outcome reports and Investigator evaluations) were recorded in the e-CRF by the Investigator or designated person.
Study outcomes
The scar appearance was assessed on the following parameters: dryness, redness, skin pigmentation, color, softness, texture and the overall appearance.
The 3 first parameters (dryness, redness and skin pigmentation) were rated by the Investigator and the parent/guardian at Day 29 ± 2 days and 57 ± 2 days, using a 4-point ordinal scale. For dryness and redness parameters, the 4-point ordinal scale was interpreted as 0: none, 1: mild, 2: moderate and 3: significant. For skin pigmentation, the 4-point ordinal scale was interpreted according to the pigmentation characteristics of the scar at Day 1 as follows: 0 being achromic or hypochromic scar, 1: normal skin pigmentation, 2: pigmented scar and 3: marked hyperpigmentation.
Color, softness, texture and overall appearance were assessed by the Investigator and the parent/guardian at each evaluation visit, using the 100-point Visual Analogue Scale (VAS) as follows: color and texture (0: different from normal skin to 100: looks like normal skin), softness (0: not soft at all to 100: maximum softness), overall appearance (0: worst case imaginable to 100: best case imaginable).
As well parent/guardian rated subjects’ reported outcomes on itching and reduction of scar visibility using a 4-point ordinal scale (0: no change, 1: mild change, 2: moderate change, 3: significant change). Discomfort of the scar was evaluated using VAS from 0 to 100 (0: no discomfort at all and 100: maximum discomfort).
The first visible effects of the tested product were rated using parent/guardian daily assessment of overall appearance, redness, softness, roughness and discomfort of the scar.
Finally, the parent/guardian’s evaluated cosmetic acceptability (14 questions on perception/acceptability of the product and 5 modalities of answers: totally agree; somewhat agree; neither agree, nor disagree; somewhat disagree and disagree). AEs were monitored throughout the course of the study.
Statistical analysis
All statistical analyses were performed using R software version 4.0.2. Data were presented as mean ± standard deviation (SD), number (n) and percentage (%), where appropriate. Scar appearance improvement analysis was descriptive. No statistical comparisons were performed. AEs were reported descriptively on an on-going basis. Analysis of cosmetic outcomes was performed as follows: for each cosmetic parameter, statistical comparison versus baseline (Day 1) was performed using a paired sample t-test or a Wilcoxon matched pairs signed-rank test, at all post-baseline visits. Multiplicity was controlled using the Benjamini-Hochberg procedure. The answers to the cosmetic acceptability questionnaire were summarized in a frequency histogram by category. AEs were presented descriptively. Statistical significance was identified where p-value was less than 0.05 with a 2-tailed test.
Results
Subject’s disposition and baseline characteristics
From October 2021 to January 2022, 30 children were screened and completed the study as planned in the protocol. The majority of children were male (n=18; 60.0%) and mean age (± SD) was 10.0 (± 4.5) years. Among included children, 20 (66.7%) had type III skin (Table 1). Physical examination results of children by the Investigator at screening were within normal ranges.
Table 1. Subjects’ baseline characteristics
|
|
N=30 |
Age (years) |
|
10.0±4.5 |
Gender |
|
|
|
Female |
12 (40.0) |
|
Male |
18 (60.0) |
Skin type |
|
|
|
Type II |
5 (16.7) |
|
Type III |
20 (66.7) |
|
Type IV |
5 (16.7) |
Values are presented as mean ± SD or numbers (%).
Cosmetic outcomes
During this study, the Investigator assessed different parameters, using different scales as presented above.
The Investigator rated effect of Mederma® for Kids™ on scar appearance improvement through scar’s dryness, redness assessment and skin pigmentation at Day 1, Day 29 ± 2 days and at Day 57 ± 2 days, after 8 weeks of IP daily application (Figure 1). Mean scores ±CI of dryness were statistically significantly decreased at Day 29 ± 2 days (0.59 ± 0.30; p=0.005) and Day 57 ± 2 days (0.14 ± 0.13; p<0.001), as compared to baseline (1.17 ± 0.30) (Figure 1a). Likewise, scar redness was statistically significantly improved at Day 29 ± 2 days (1.00 ± 0.27; p=0.002) and Day 57 ± 2 days (0.71 ± 0.28; p<0.001), as compared to baseline (1.43 ± 0.32) (Figure 1b). No change in skin pigmentation was reported by the Investigator at Day 29 ± 2 days for 62.1% of children and at Day 57 ± 2 days, repigmentation for 25.0% of children and moderate pigmentation for 21.4% (Figure 1c).
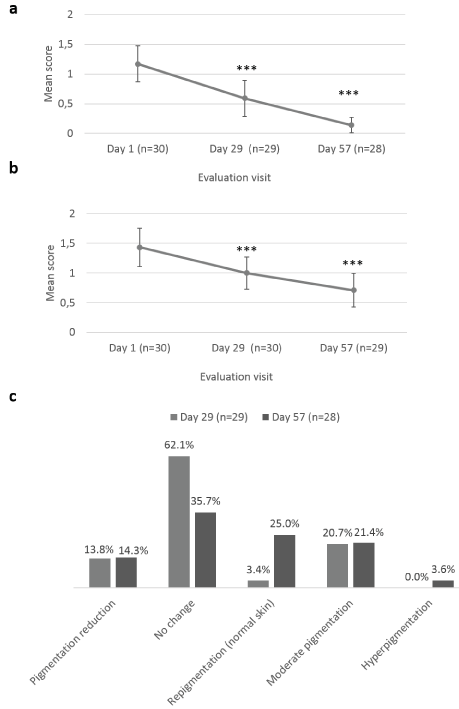
Figure 1. Investigator’s assessment of Mederma®Kids™ scar appearance improvement. Mean scores (± CI, confidence intervals) of (a) dryness and (b) redness; (c) skin pigmentation change from baseline scores distribution, at Day 1, Day 29 ± 2 days and Day 57 ± 2 days. Asterisk indicates statistically significant difference (*, <0.05; **, <0.01; ***, p<0.001) between timepoint (Day 29 ± 2 days, Day 57 ± 2 days) and baseline (Day 1)
The Investigator also assessed scar’s color (compared to normal skin color), texture, softness and overall appearance at Day 1, Day 29 ± 2 days and at Day 57 ± 2 days, after 8 weeks of Mederma® for Kids™application, three times a day (Figure 2 and 3). Scar color was statistically significantly improved at Day 29 ± 2 days (58.52 ± 6.99; p<0.001) and Day 57 ± 2 days (77.11 ± 6.04; p<0.001), as compared to baseline (45.03 ± 7.27) (Figure 2a). Likewise, scar texture was statistically significantly improved at Day 29 ± 2 days (72.93 ± 6.49; p=0.003) and Day 57 ± 2 days (83.07 ± 5.12; p<0.001), as compared to baseline (64.27 ± 8.89) (Figure 2b). In addition, scar softnesswas statistically significantly improved at Day 29 ± 2 days (79.62 ± 6.53; p=0.005) and Day 57 ± 2 days (89.57 ± 5.69; p<0.001), as compared to baseline (75.37 ± 7.63) (Figure 2c). Overall scar appearance as rated by the Investigator was statistically significantly improved from Day 29 ± 2 days (60.03 ± 7.17; p<0.001), and Day 57 ± 2 days (77.36 ± 6.76; p<0.001), as compared to baseline (43.67 ± 6.05) (Figure 3).
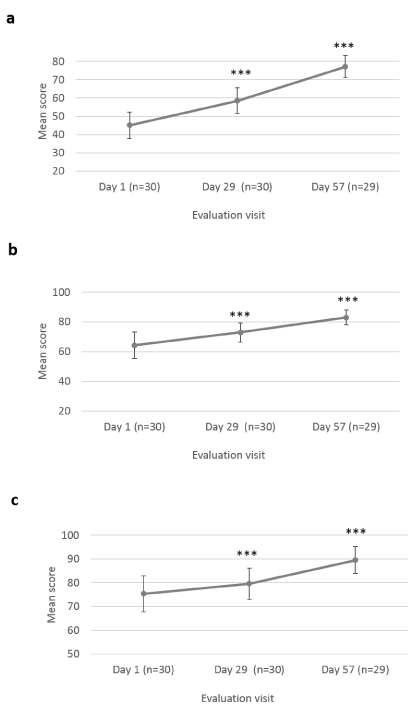
Figure 2. Investigator’s assessment of scar’s color, texture and softness. Mean scores (± CI, confidence intervals) of (a) color and (b) texture, and (c) softness scores distribution, at Day 1, Day 29 ± 2 days and Day 57 ± 2 days. Asterisk indicates statistically significant difference (*, <0.05; **, <0.01; ***, p<0.001) between timepoint (Day 29 ± 2 days, Day 57 ± 2 days) and baseline (Day 1)
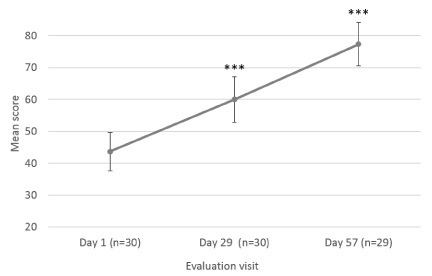
Figure 3. Investigator’s assessment of scar’s overall appearance. Mean scores (± CI, confidence intervals) of overall appearance at Day 1, Day 29 ± 2 days and Day 57 ± 2 days. Asterisk indicates statistically significant difference (***, p<0.001)
The parents/guardians were also asked to evaluate scar appearance improvement according to the same parameters as the Investigator (dryness, redness and skin pigmentation) (Figure 4). Scar dryness was statistically significantly decreased at Day 29 ± 2 days (0.63 ± 0.35; p=0.021) and Day 57 ± 2 days (0.28 ± 0.24; p=0.002), as compared to baseline (1.30 ± 0.44) (Figure 4a). Likewise, scar redness was statistically significantly improved at Day 29 ± 2 days (0.93 ± 0.28; p<0.001) and Day 57 ± 2 days (0.79 ± 0.28; p<0.001), as compared to baseline (1.50 ± 0.36) (Figure 4b). No change in skin pigmentation was reported by parents/guardians at Day 29 ± 2 days for 60.0% of children and at Day 57 ± 2 days, pigmentation reduction was reported for 27.6% of children, no change for 37.9%, and hyperpigmentation for 20.7% (Figure 4c). Parents/guardians also assessed their child’s scar’s color, texture, softness, scar discomfort and overall appearance at Day 1, Day 29 ± 2 days and at Day 57 ± 2 days, after 8 weeks of Mederma® for Kids™application, three times a day (Figure 5 and 6). Scar color was statistically significantly improved at Day 29 ± 2 days (60.67 ± 7.94; p<0.001) and Day 57 ± 2 days (75.41 ± 7.34; p<0.001), as compared to baseline (39.83 ± 9.20) (Figure 5a). Likewise, scar texture was statistically significantly improved at Day 29 ± 2 days (74.57 ± 8.43; p<0.001) and Day 57 ± 2 days (86.93 ± 5.58; p<0.001), as compared to baseline (62.30 ± 10.78) (Figure 5b). In addition, scar softness was statistically significantly improved at Day 29 ± 2 days (79.47 ± 7.82; p=0.001) and Day 57 ± 2 days (89.83 ± 5.12; p=0.001), as compared to baseline (72.73 ± 11.03) (Figure 5c). Markedly, scar discomfort was statistically significantly improved from Day 29 ± 2 days (6.87 ± 6.17; p=0.03), and Day 57 ± 2 days (0.52 ± 1.01; p=0.002), as compared to baseline (13.20 ± 8.43) (Figure 5d). Overall, scar appearance as rated by parents/guardians was statistically significantly improved from Day 29 ± 2 days (56.63 ± 9.82; p<0.001), and Day 57 ± 2 days (76.14 ± 7.97; p<0.001), as compared to baseline (37.30 ± 9.23) (Figure 6).
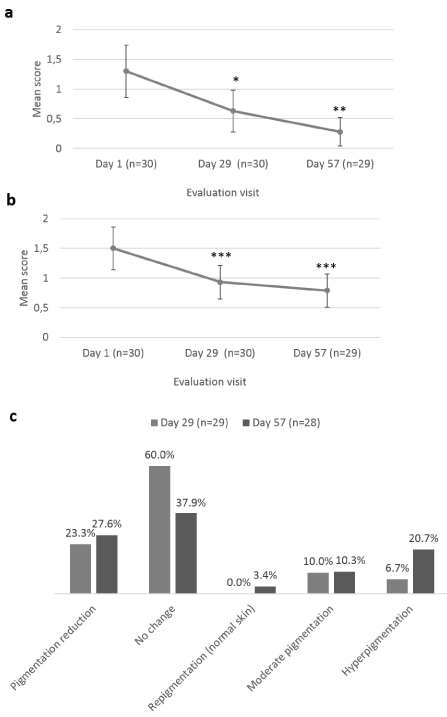
Figure 4. Parent’s assessment of Mederma®Kids™ scar appearance improvement. Mean scores (± CI, confidence intervals) of (a) dryness and (b) redness; (c) skin pigmentation change from baseline scores distribution, at Day 1, Day 29 ± 2 days and Day 57 ± 2 days. Asterisk indicates statistically significant difference (*, <0.05; **, <0.01; ***, p<0.001) between timepoint (Day 29 ± 2 days, Day 57 ± 2 days) and baseline (Day 1)
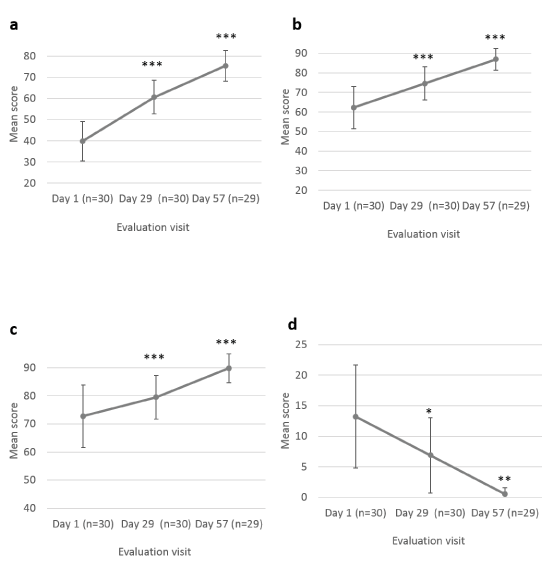
Figure 5. Parent’s assessment of scar’s color, texture, softness and discomfort. Mean scores (± CI, confidence intervals) of (a) color and (b) texture, (c) softness and (d) discomfort scores distribution, at Day 1, Day 29 ± 2 days and Day 57 ± 2 days. Asterisk indicates statistically significant difference (*, <0.05; **, <0.01; ***, p<0.001) between timepoint (Day 29 ± 2 days, Day 57 ± 2 days) and baseline (Day 1)
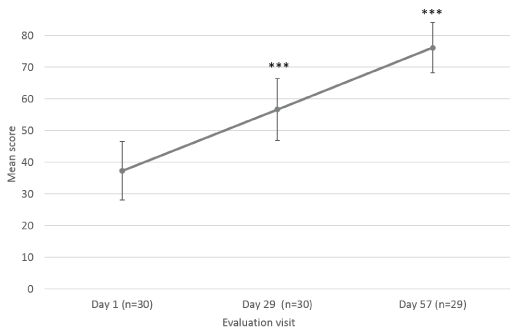
Figure 6. Parent’s assessment of scar’s overall appearance. Mean scores (± CI, confidence intervals) of overall appearance at Day 1, Day 29 ± 2 days and Day 57 ± 2 days. Asterisk indicates statistically significant difference (***, p<0.001)
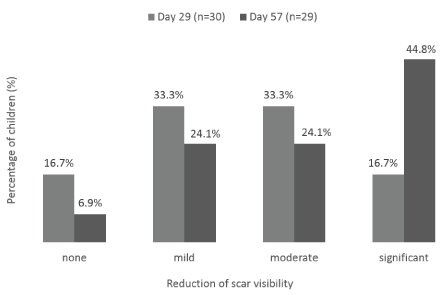
Figure 7. Scar visibility reduction. Percentage of children whose parents/guardians reported visibility reduction (none, mild, moderate and significant) at Day 29 ± 2 days and Day 57 ± 2 days
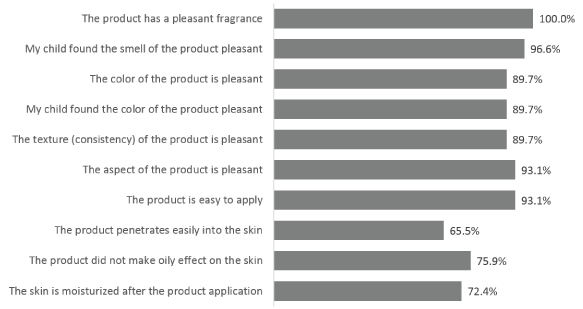
Figure 8. Parent’s positive cosmetic acceptability of Mederma®Kids™.Percentage of parents/guardians who totally and somewhat agreed with the proposed statements at Day 57 ± 2 days (end of study). Only positive answers given by more than 60.0% of subjects are presented
Finally, at each evaluation visit, parents/guardians assessed the scar visibility. On Day 29 ± 2 days, a total of 66.6% of parents/guardians reported a mild to moderate reduction in scar visibility and 16.7% of them reporting significant reduction (Figure 7). By Day 57 ± 2 days, 48.2% of parents/guardians reported a mild to moderate improvement and 44.8% of them reported a significant reduction of scar visibility.
First Positive Visible Effects of the Investigational Product
The first parents/guardians to observe and report the first visible effects of Mederma® for Kids™on their child’s scar did so after the first few applications and quite early during the 8-week application period (Table 2). At Day 14, the first visible signs for overall appearance improvement, scar redness reduction, roughness reduction, scar softness and discomfort improvement were reported by 1 to 11 parents/guardians.
Table 2. Percentage of parents reporting first detectable effect(s) before 14 days
|
Mederma® Kids™
(N=30) |
First detectable effects before 14 days / Number of evaluation (%): |
|
Overall appearance improvement |
11/28 (39.3%) |
Scar redness reduction |
9/24 (37.5%) |
Roughness reduction |
4/17 (23.5%) |
Scar softness |
7/22 (31.8%) |
Discomfort improvement |
2/8 (25.0%) |
Safety and cutaneous tolerability
During the study, 4 out of 30 children (13.3%) experienced a total of 4 adverse events not related to Mederma® for Kids™ (Table 3). One serious adverse event (adenovirus gastroenteritis with fever and dehydration) not related to Mederma® for Kids™, was reported in a 3-year-old child and led to study discontinuation. This event was resolved without sequelae in a few days.
Table 3. Overview of Adverse Events
|
Safety Population
N=30
n (%) [n events] |
All AEs |
4 (13.3) [4] |
Related AEs |
0 (0.0) [0] |
All cutaneous AEs |
0 (0.0) [0] |
Related cutaneous AEs |
0 (0.0) [0] |
All serious AEs |
1 (3.3) [1] |
Related serious AEs |
0 (0.0) [0] |
All severe AEs |
1 (3.3) [1] |
Related severe AEs |
0 (0.0) [0] |
AEs leading to discontinuation |
1 (3.3) [1] |
Related AEs leading to discontinuation |
0 (0.0) [0] |
Values are presented as n (%) of subjects and [n events]. AEs, adverse events; cutaneous AEs, AEs with symptoms expressed on skin.
The itching sensation was also assessed at each evaluation visit by the parents/guardian. Only 1 subject experienced moderate itching at Day 29 ± 2 days and no itching was reported at Day 57 ± 2 days.
Cosmetic acceptability
At the end of the application period, parents/guardians were asked 14 questions concerning their perception of Mederma® for Kids™, to which they could totally or somewhat agree, neither agree nor disagree, somewhat disagree or disagree. Overall, on 12 out of 14 questions, 65.5% - 100.0% of parents/guardians gave positive answers, i.e., totally or somewhat agreed with the proposed statements (Figure 8) and 72.4% of subjects disagreed with the statement that Mederma® for Kids™ stained fabric, like clothes and towels. All parents/guardians and 96.6% of children appreciated its fragrance and over 89.0% of parents/guardians and their children appreciated the color and texture of Mederma® for Kids™. Moreover, 93.1% of parents/guardians found Mederma® for Kids™ to be pleasant and was easy to apply and 65.5% of parents/guardians found it penetrated well into the skin and 75.9% found that it did not make an oily effect. The skin appeared moisturized after application according to 72.4% of parents/guardians. Finally, 65.5% of parents/guardians found that Mederma® for Kids™ could easily fit into the daily skincare routine of their child and 93.1% would recommend it to other parents/guardians.
Discussion
Both the Investigator and parents/guardians rated scar appearance improvement through evaluation of different parameters. The scar’s dryness and redness were improved from Day 29 ± 2 days. The color, softness, texture as well as overall scar appearance were markedly improved over time as compared to baseline as reported by the Investigator and parents/guardians. Likewise, discomfort related to the scar was significantly reduced from Day 29 ± 2 days, as reported by parents/guardians. By Day 57 ± 2 days, 93.0% of parents/guardians also reported mild to significant reduction in scar visibility. Overall scar appearance, assessed by the Investigator and parents/guardians, was significantly improved during the application period.
For all parameters, parents/guardians’ assessments were consistent with the conclusion reached by the Investigator’s assessments. Our results were in line with previously published articles that have shown that onion extract-based formulations significantly improved appearance of several types of scars [3-5].Of course, objectivity of parents/guardians’ and Investigators’ assessments of appearance improvements may be challenged as it was not a comparative study. Moreover, in cosmetic studies such as this one, rating of aesthetic responses in terms of scars’ appearance provides useful “consumer-based” information on acceptability of cosmetic care products. Knowing how scarring can be aesthetically unpleasant and/or disfiguring, causing anxiety and distress to kids (depending on its localisation), cosmetic care products that improve scars’ overall appearance, contribute in providing high subject satisfaction. In our study, this was clearly reflected in the very well-appreciated cosmetic acceptability of Mederma® for Kids™.
In conclusion, under the study conditions, Mederma® for Kids™ applied 3 times daily proved to be effective in improving the appearance of scars in kids.
Acknowledgments
HRA Pharma funded this study. We are thankful to CPCAD and investigational site staff for their collaboration and work.
Conflicts of interest
JZB (Medical Operations Lead), PA (Scientific Affairs Assistant & Medical Affairs Assistant), TJ (Global Category Lead Wound Care) and CAA (Global Head of Medical Affairs) are employees at HRA Pharma. CQR (CPCAD Managing Director) is an employee at CPCAD (Centre Pharmacologie Clinique Appliqué à la Dermatologie), the organization that has conducted the study. MK (Medical writer) is an employee at ICTA, the organization to which the article writing was subcontracted.
References
- Aragona M, Dekoninck S, Rulands S, Lenglez S, Mascré G,et al. (2017) Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat Commun8:14684.
- Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, et al. (2020) Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics12: 735.[Crossref]
- Augusti KT (1996) Therapeutic values of onion (Allium cepa L.) and garlic (Allium sativum L.). Indian J Exp Biol34:634-40.[Crossref]
- Clarke LF, Baker B, Trahan C, Myers L, SE M (1999) A prospective double-blinded study of Mederma skin Care vs. Placebo for post-traumatic scar reduction. Cosmetic Dermatology.
- Zurada JM, Kriegel D, Davis IC (2006) Topical treatments for hypertrophic scars. J Am Acad Dermatol55:1024-31.[Crossref]
- Fitzpatrick TB, Pathak M, Parrish JA (1974) Protection of human skin against the effects of the sunburn ultraviolet (290-320 nm). In: Fitzpatrick TB, al. (Eds) Sunlight and Man, normal and abnormal photobiological responses pp. 751.
- Draelos ZD, Baumann L, Fleischer AB, Plaum S, Avakian EV, et al. (2012) A new proprietary onion extract gel improves the appearance of new scars: a randomized, controlled, blinded-investigator study. J Clin Aesthet Dermatol5:18-24.[Crossref]








