The internal root resorption can be associated with external granuloma. If there is a connection between the two pathological process through a lateral accessory canal is possible that some cells to be recruited from external granuloma. The possible mechanisms involved in osteoclasts recruitment from external granuloma and various dentin resorption patterns in internal root resorption will be discussed based on a case in which the upper lateral incisor with a history of pulp necrosis treated endodontically, developed an internal root resorption in association with external granuloma. Also, a review of literature was performed
internal root resorption, external granuloma, osteoclasts, odontoclasts
Bone resorption is initiated by osteoclasts and resorption of hard dental structures by odontoclasts. Osteoclasts and odontoclasts are multinucleated cells derived from hematogenous bone marrow, from the monocyte/macrophage cell lineage, and are formed by the fusion of these cells under the action of colony stimulating factor 1 (CSF-1), macrophage colony stimulating factor (M-CSF), monocyte chemotactic protein-1 (MCP-1) and RANKL (receptor activator of nuclear factor kappa-B ligand), which also have a role in chemotaxis and cell differentiation [1]. The osteoclastic potential of bone marrow stromal monocytes (BMSCs) in maxillary/mandibular bones differs from that of BMSCs in long bones. The study conducted by Chaichanasakul T et al. showed that the osteoclastic potential of mandibular bone marrow stromal cells and the number of osteoclasts are higher compared to long-bone marrow stromal cells [2]. This translates into a larger resorption area, a higher RANKL expression and a lower OPG mRNA expression, with an increased RANKL/OPG ratio. OPG (osteoprotegerin) is a soluble TNF receptor-like molecule and the natural inhibitor of osteoclastogenesis [3]. The differentiation of the osteoclast precursor into a mature osteoclast requires RANKL binding to its receptor RANK (receptor activator of nuclear factor kappa-B). However, OPG binding to RANKL will block RANKL binding to RANK, which will result in inhibition of osteoclastogenesis (Figure 1) [3]. The balance of the RANKL/RANK/OPG triad contributes to bone remodeling.
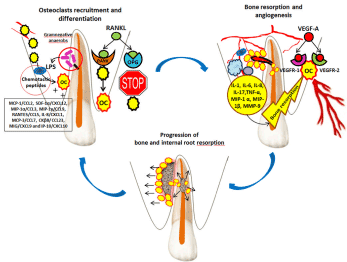
Figure 1. Osteoclasts recruitment and activation, and angiogenesis in maxillary alveolar processes in internal root resorption associated with external granuloma.
 - osteoclast precursors;
- osteoclast precursors;  - osteoclasts;
- osteoclasts;  - polymorphonuclear leukocytes;
- polymorphonuclear leukocytes;  - macrophages;
- macrophages;  - T helper cells;
- T helper cells;  - gram-negative anaerobes; LPS –lipopolysaccharides; RANKL (receptor activator of nuclear factor kappa-B ligand); RANK (receptor activator of nuclear factor kappa-B); OPG (osteoprotegerin); VEGF (vascular endothelial growth factor); VEGFR (vascular endothelial growth factor receptor); MCP-1/CCL2 (monocyte chemoattractant protein-1), SDF-1α/CXCL12 (stromal derived factor-1α), MIP-1α/CCL3 (macrophage inflammatory protein-1α), MIP-1γ/CCL9 (macrophage inflammatory protein-1γ), RANTES/CCL5 (regulated on activation, normal T cell expressed and secreted), IL-8/CXCL1 (interleukin-8), MCP-3/CCL7 (monocyte chemoattractant protein-3), CKβ8/ CCL23 (chemokine β8), MIG/CXCL9 (monokine induced by IFN-γ) and IP-10/CXCL10 (IFN-γ-inducible protein 10)
- gram-negative anaerobes; LPS –lipopolysaccharides; RANKL (receptor activator of nuclear factor kappa-B ligand); RANK (receptor activator of nuclear factor kappa-B); OPG (osteoprotegerin); VEGF (vascular endothelial growth factor); VEGFR (vascular endothelial growth factor receptor); MCP-1/CCL2 (monocyte chemoattractant protein-1), SDF-1α/CXCL12 (stromal derived factor-1α), MIP-1α/CCL3 (macrophage inflammatory protein-1α), MIP-1γ/CCL9 (macrophage inflammatory protein-1γ), RANTES/CCL5 (regulated on activation, normal T cell expressed and secreted), IL-8/CXCL1 (interleukin-8), MCP-3/CCL7 (monocyte chemoattractant protein-3), CKβ8/ CCL23 (chemokine β8), MIG/CXCL9 (monokine induced by IFN-γ) and IP-10/CXCL10 (IFN-γ-inducible protein 10)
Osteoclast recruitment and activation in maxillary or mandibular alveolar processes can be triggered by pathological processes such as the presence of pathogens (gram-negative anaerobes) in the root canal and dentinal canaliculi. The presence of a lateral accessory canal infected with gram-negative bacteria will induce the release of endotoxins (lipopolysaccharides - LPS). Thus, the alternative pathway of the complement system will be activated, with the formation of chemotactic peptides [4] and the attraction of defensive phagocytic cells (PMNs - polymorphonuclear leukocytes and macrophages). PMNs have a limited life span, they rapidly invade the infection area in high numbers, unlike macrophages, which invade the infection area more slowly and are fewer in numbers (between 4 and 50% of inflammatory cell infiltrate) but have a longer life span [4]. The chemotactic and differentiation factors for osteoclasts are: MCP-1/CCL2, SDF-1α/CXCL12, MIP-1α/CCL3, MIP-1γ/CCL9, RANTES/CCL5, IL-8/CXCL1, MCP-3/CCL7, CKβ8/ CCL23, MIG/CXCL9 and IP-10/CXCL10. Of these, it seems that in infectious processes such as granulomas, the osteoclast chemotactic and differentiation factor is MCP-1/CCL2 [5].
The cytokines involved in bone resorption and immune response in lateral granuloma are: IL-1, IL-6, IL-8, TNF-α, MIP-1 α and MIP-1β. They are secreted by cells participating in the inflammatory and immune process (PMNs, macrophages and T helper cells), as well as by osteoclasts (which secrete IL-1) [6]. This complex process also involves matrix metalloproteinases such as collagenases (MMP-1, MMP-8 and MMP-13) and gelatinases (MMP-2 and MMP-9), which degrade the extracellular matrix [7,8]. An increase of MMP-9 activity in osteoclasts has been observed, which leads to an increase of bone resorption capacity [9].
For osteoclast recruitment to be possible, blood supply is required. The formation of new blood vessels in the maxillary alveolar process and their penetration through the lateral accessory canal into the root canal might explain the dentinoclastic action of osteoclasts reaching this site and the presence of blood vessels in the internal granuloma.
However, angiogenesis might have occurred subsequently, under the action of VEGFs (vascular endothelial growth factors), more precisely VEGF-A, whose receptors are VEGFR-1 and VEGFR-2 [10,11]. In granuloma, VEGFs and its receptors have been evidenced in blood vessels, PMNs, macrophages and fibroblast-like cells [12]. The presence of VEGFs and its receptors in both osteoclasts and odontoblasts shows the interconnection between bone remodeling and angiogenesis [13,14]. It is possible that immune cells involved in the inflammatory process in lateral granuloma communicate with one another, as well as with endothelial cells through VEGFs, as signal molecules [15].
Although osteoclasts and odontoclasts have the same origins [16], a similar morphology, similar enzyme characteristics (such as cathepsin K, cathepsin D, tartrate-resistant acid phosphatase - TRAP, MMP-9, H+-ATPase, membrane type 1 - MT1-MMP) [17,18] and the same resorption pattern [19], there are differences between them, given that they degrade different structures. Odontoclasts are cells residing in dental pulp and periodontal ligament, they are smaller in size, have fewer nuclei, smaller sealing zones, and form smaller lacunar resorption zones [20,21]. It is not yet clear whether osteoclasts are the same type of cells as clastic cells that degrade dental structures (such as odontoclasts, dentinoclasts and cementoclasts). Like osteoclasts, odontoclasts have a ruffled border but they have a smaller or no clear zone, and a small number of nuclei (less than 10 nuclei or even fewer). Odontoclasts with a small number of nuclei (less than 5) resorb more dentin/nucleus than odontoblasts with more nuclei. The delineation limit between pulp tissue and dentin is represented by the two protective layers: the odontoblast layer and the predentin layer. Their presence is a barrier to dentin resorption because odontoclasts need to be in contact with and adhere to a mineralized matrix, not a non-mineralized matrix, such as predentin. However, under pathological conditions, when this barrier is destroyed, odontoclasts initiate dentin resorption (Figure 2). Nonetheless, not all teeth that have lost this barrier and have a local infectious triggering factor develop internal root resorption over time (a condition quite rarely found in practice). A possible explanation is the high OPG expression in dental structures, which would block odontoclast activation and differentiation. Another explanation could be the presence of non-collagen components in dentin, which would inhibit the resorption process [22].
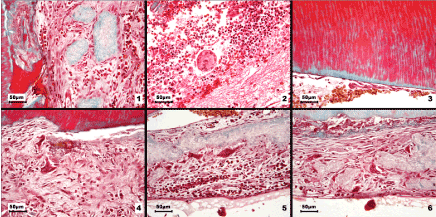
Figure 2. Light microscopy images of odontoclasts and various dentin resorption patterns in internal root resorption
1. Numerous odontoclast precursors were recruited and formed a continuous layer covering the fragments of necrotic dentin. 2. Granulation tissue containing numerous polymorphonuclear neutrophils, extravasated erythrocytes, tissue debris and a multinucleated giant cell. 3. The sites where predentin was present showed no signs of resorption. 4. Initial phase of odontoclast formation: massive recruitment of odontoclast precursors with a pronounced tendency to fuse; two forming odontoclasts could be identified. 5. Active odontoclasts on necrotic dentin fragments, accompanied by mononuclear inflammatory cells in the adjacent connective tissue. 6. Intense resorptive activity associated with the dentin surface and detachment of necrotic tissue; odontoclasts on the dentin fragments and numerous activated precursors. Goldner’s trichrome stain
Internal root resorption remains an incompletely understood process, all the more so as the pathogenic mechanisms presented in this study raise new dilemmas and new hypotheses that will require further research in the future.
The authors declare no conflicts of interest with respect to the authorship and/or publication of this article.
This study was supported partially by the COFUND-ERA-HDHL ERANET Project, European and International Cooperation - Subprogram 3.2 - Horizon 2020, PNCDI III Program - Biomarkers for Nutrition and Health – “Innovative technological approaches for validation of salivary AGEs as novel biomarkers in evaluation of risk factors in diet-related diseases”, no 25/1.09.2017.
2021 Copyright OAT. All rights reserv
- Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423: 337-342. [Crossref]
- Chaichanasakul T, Kang B, Bezouglaia O, Aghaloo TL, Tetradis S (2014) Diverse osteoclastogenesis of bone marrow from mandible versus long bone. J Periodontol 85: 829-836. [Crossref]
- Menezes R, Bramante CM, da Silva Paiva KB, Letra A, Carneiro E, et al. (2006) Receptor activator NFkappaB-ligand and osteoprotegerin protein expression in human periapical cysts and granulomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102: 404-409.
- Márton IJ, Kiss C (2000) Protective and destructive immune reactions in apical periodontitis. Oral Microbiol Immunol 15: 139-150. [Crossref]
- Márton IJ, Rot A, Schwarzinger E, Szakáll S, Radics T, et al. (2000) Differential in situ distribution of interleukin-8, monocyte chemoattractant protein-1 and Rantes in human chronic periapical granuloma. Oral Microbiol Immunol 15: 63-65.
- Graunaite I, Lodiene G, Maciulskiene V (2012) Pathogenesis of apical periodontitis: a literature review. J Oral Maxillofac Res 2: e1. [Crossref]
- Tsuji M, Yamasaki M, Amano K, Matsui H, Morimoto T, et al. (2009) Histochemical localization of neutral proteases released during development of rat periradicular lesion. Arch Oral Biol 54: 1128-1135.
- Lin SK, Chiang CP, Hong CY, Lin CP, Lan WH, et al. (1997) Immunolocalization of interstitial collagenase (MMP-1) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in radicular cysts. J Oral Pathol Med 26: 458-463. [Crossref]
- Silva TA, Garlet GP, Fukada SY, Silva JS, Cunha FQ (2007) Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J Dent Res 86: 306-319. [Crossref]
- Halin C, Detmar M (2008) Chapter 1. Inflammation, angiogenesis, and lymphangiogenesis. Methods Enzymol 445: 1-25. [Crossref]
- Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF (2008) Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 11:109-119.
- Bletsa A, Virtej A, Berggreen E (2012) Vascular endothelial growth factors and receptors are up-regulated during development of apical periodontitis. J Endod 38: 628-635.
- Aldridge SE, Lennard TW, Williams JR, Birch MA (2005) Vascular endothelial growth factor receptors in osteoclast differentiation and function. Biochem Biophys Res Commun 335: 793-798.
- Hah YS, Jun JS, Lee SG, Park BW, Kim DR, et al. (2011) Vascular endothelial growth factor stimulates osteoblastic differentiation of cultured human periosteal-derived cells expressing vascular endothelial growth factor receptors. Mol Biol Rep 38: 1443-1450.
- Virtej A, Løes SS, Berggreen E, Bletsa A (2013) Localization and signaling patterns of vascular endothelial growth factors and receptors in human periapical lesions. J Endod 39: 605-611.
- Sahara N, Toyoki A, Ashizawa Y, Deguchi T, Suzuki K (1996) Cytodifferentiation of the odontoclast prior to the shedding of human deciduous teeth: an ultrastructural and cytochemical study. Anat Rec 244: 33-49. [Crossref]
- Gotz W, Quondamatteo F, Ragotzki S, Affeldt J, Jager A (2000) Localization of cathepsin D in human odontoclasts, a light and electron microscopical immunocytochemical study. Connect Tissue Res 41: 185-194.
- Linsuwanont Santiwong B, Takagi Y, Ohya K, Shimokawa H (2006) Expression of MT1-MMP during deciduous tooth resorption in odontoclasts. J Bone Miner Metab 24: 447-453.
- Nilsson E, Bonte E, Bayet F, Lasfargues JJ (2013) Management of internal root resorption on permanent teeth. Int J Dent 929486: 1-7.
- Lindskog S, Blomlöf L, Hammarström L (1983) Repair of periodontal tissues in vivo and in vitro. J Clin Periodontol 10: 188-205. [Crossref]
- Fernandes M, de Ataide I, Wagle R (2013) Tooth resorption part I - pathogenesis and case series of internal resorption. J Conserv Dent 16: 4-8. [Crossref]
- Haapasalo M, Endal U (2006) Internal inflammatory root resorption: the unknown resorption of the tooth. Endodontic Topics 14: 60-79.


 - osteoclast precursors;
- osteoclast precursors;  - osteoclasts;
- osteoclasts;  - polymorphonuclear leukocytes;
- polymorphonuclear leukocytes;  - macrophages;
- macrophages;  - T helper cells;
- T helper cells;  - gram-negative anaerobes; LPS –lipopolysaccharides; RANKL (receptor activator of nuclear factor kappa-B ligand); RANK (receptor activator of nuclear factor kappa-B); OPG (osteoprotegerin); VEGF (vascular endothelial growth factor); VEGFR (vascular endothelial growth factor receptor); MCP-1/CCL2 (monocyte chemoattractant protein-1), SDF-1α/CXCL12 (stromal derived factor-1α), MIP-1α/CCL3 (macrophage inflammatory protein-1α), MIP-1γ/CCL9 (macrophage inflammatory protein-1γ), RANTES/CCL5 (regulated on activation, normal T cell expressed and secreted), IL-8/CXCL1 (interleukin-8), MCP-3/CCL7 (monocyte chemoattractant protein-3), CKβ8/ CCL23 (chemokine β8), MIG/CXCL9 (monokine induced by IFN-γ) and IP-10/CXCL10 (IFN-γ-inducible protein 10)
- gram-negative anaerobes; LPS –lipopolysaccharides; RANKL (receptor activator of nuclear factor kappa-B ligand); RANK (receptor activator of nuclear factor kappa-B); OPG (osteoprotegerin); VEGF (vascular endothelial growth factor); VEGFR (vascular endothelial growth factor receptor); MCP-1/CCL2 (monocyte chemoattractant protein-1), SDF-1α/CXCL12 (stromal derived factor-1α), MIP-1α/CCL3 (macrophage inflammatory protein-1α), MIP-1γ/CCL9 (macrophage inflammatory protein-1γ), RANTES/CCL5 (regulated on activation, normal T cell expressed and secreted), IL-8/CXCL1 (interleukin-8), MCP-3/CCL7 (monocyte chemoattractant protein-3), CKβ8/ CCL23 (chemokine β8), MIG/CXCL9 (monokine induced by IFN-γ) and IP-10/CXCL10 (IFN-γ-inducible protein 10)