Pulmonologists frequently encounter indeterminate pulmonary nodules. Predicting the risk of developing lung cancer is a difficult task as evidenced by the high rate of overdiagnosis and overtreatment of indolent disease. There is an unmet clinical need for a non-invasive, easy to administer, diagnostic assay to help prevent the potentially serious consequences of overdiagnosis. Here we report on the clinical work-up of a high-risk patient with an indeterminate pulmonary nodule. This case suggests that applying a new blood test in a point of care setting in a community-based practice can accurately characterize scan identified, or incidentally found, lung nodules. The novel assay may potentially minimize aggressive interventions in patients with benign disease.
With cigarette smoking as the acknowledged root cause, lung cancer remains a leading cause of cancer deaths worldwide, with high mortality largely attributed to its diagnosis late in the disease process when cure is not possible [1]. The National Lung Screening Trial (NLST) brought hope that screening high-risk patients with a yearly chest low-dose CT scan could lead to a 20% relative risk reduction in lung cancer deaths [2]. This decrease in mortality was paralleled by an increase in the diagnosis of stage I non-small cell lung cancer, implying that this screening paradigm leads to decreased mortality by shifting the stage at diagnosis to an earlier, curative stage.
Coupled with the increase in chest CT scans performed for lung cancer screening, CT imaging is increasingly used for the diagnosis and evaluation of thoracic and extra-thoracic disease, all leading to increased identification of pulmonary nodules [3]. In the National Lung Screening Trial (NLST), 24% of screened patients were found to have a concerning pulmonary nodule with only 4% of those ultimately determined to be malignant, even in this high-risk population [2]. The current paradigm for management of pulmonary nodules > 8 mm diameter is centered on estimates of a pretest probability for malignancy. Those nodules with a high pretest probability (> 65%) are aggressively managed (typically surgical resection), whereas those at low risk (0-5%) are managed conservatively. Intermediate-risk nodules (5%-65%), which constitute almost one-half of the pulmonary nodules identified by chest CT scan, require further diagnostic evaluation, including other imaging, bronchoscopy, percutaneous biopsy, or surgical biopsy [4]. Even minimally invasive procedures carry significant risks and anxiety to patients, and the cost of diagnostic evaluation increases 28-fold when biopsy is performed [5,6]. Patients with intermediate-risk nodules would therefore benefit from additional risk stratification tools to determine those truly in need of more aggressive evaluation, and those for whom a less risky approach is warranted. These desires have led to considerable interest in identifying blood-based biomarkers that can differentiate lung cancer from benign disease nodules [7].
A recent publication highlights the clinical validation and performance of a novel, multiplexed, plasma protein signature as a risk assessment tool [8]. The authors established the assay’s ability to aid in correctly identifying the risk of malignancy for a pulmonary nodule that falls into the inconclusive intermediate risk for lung cancer as calculated by the VA Clinical Factors Model [9]. The assay was evaluated with a set of 277 samples, all from current smokers with an indeterminate pulmonary nodule 4-30 mm in diameter from which an algorithm was defined for risk classification. The assay and algorithm were then evaluated in an independent validation cohort of 97 subjects [8].
Among the 97 validation study subjects, 68 were grouped as having intermediate risk by the VA model. The biomarker algorithm-based test correctly identified 44 (65%) of these intermediate-risk samples as lower risk (n = 16) or higher risk (n = 28). The test showed a sensitivity of 94% and a negative predictive value (NPV) of 94% [8] in the intended use population having a cancer prevalence rate of 25% [10]. Almost all subjects (98%) had early stage disease as defined by lung cancer Stage I or II [8].
The novel blood test demonstrated efficacy in accurately identifying patients at low risk of lung cancer to rule-out the need for risky aggressive actions. Thus, the plasma protein assay has the potential to aid clinicians to more accurately characterize radiologically-indeterminate pulmonary nodules in current smokers and help provide additional insight to support a more informed clinical decision about performing an invasive evaluation of the patient’s lung nodule [8].
The REVEAL test aids decision making and reduces the psychologic toll of uncertainty in a patient with indeterminate lung nodules and prevents unnecessary risky interventions.
A 73-year-old male from the Dominican Republic presented on August 2, 2018 complaining of a dry cough and shortness of breath on exertion as well as hypertension, back and neck pain and leg cramps. He was diagnosed with COPD/Emphysema in the Dominican Republic 5 years before presenting to the clinic. He brought with him a thoracic CT (performed on 4/17/18) from his country demonstrating combined emphysema and interstitial lung disease (ILD) with reticulonodular densities in the posterior lateral right mid lung, exhibiting ground glass airspace opacities, fibrosis, and bullous disease concentrated primarily in the upper lobes.
The patient had a 25-year history of smoking two packs of cigarettes per day, and over 50 years of occupational exposure to wheat flour in a factory. He had a history of gout but denied any active symptoms. The patient reported home oxygen saturations in the 80s and poor compliance with supplemental oxygen and prescribed inhalers including LABA/IC and SABA. He denied chest tightness or wheezing. His surgical history is remarkable for right shoulder rotator cuff repair, a procedure on his right wrist, and hammertoe repair. Previous blood work indicated renal insufficiency and hyponatremia, which the patient confirmed are chronic. He denied any allergies, asbestos exposure, or recreational history with lung irritants. He stated he has five domesticated dogs at his home.
The patient is divorced. His social history includes daily alcohol use. He is currently retired. His family history includes a grandfather with diabetes and a brother with hypertension, obstructive sleep apnea, and psoriasis. The patient also reported several relatives with a history of colon cancer which prompts him to undergo a colonoscopy every 2 years.
His physical exam was significant for slight prolonged expiration and 1+ pitting edema in both lower extremities. No rhonchus, wheezing or crackles were evident. Spirometry performed exposed small airway disease and restrictive lung disease, but not a pattern of COPD. Pulmonary function tests were ordered which revealed restrictive pattern, small airway disease and decreased DLCO. A review of symptoms was positive for acid reflux treated with Prilosec. No collagen vascular disease was evident except for mild elevation of CRP and ESR. A baseline polysomnograph was positive for severe obstructive sleep apnea syndrome.
A thoracic CT was performed on August 3, 2018 per chest ILD protocol and showed bullous disease and ground glass opacification. Additionally, a dominant 7x5 mm, spiculated, LUL nodule was described for the first time.
The patient was referred to the Mayo Clinic in Jacksonville, Florida for an open lung biopsy to characterize his lung pathology. However, due to his increased risk of bleeding from pulmonary hypertension, it was deferred. A repeat chest CT at Mayo Clinic in September 2018 was consistent with the findings previously described, including the lung nodule. The patient opted for surveillance of the nodule and medical management with a course of prednisone for the ILD.
On October 15, 2018 a novel multiplexed, plasma-protein assay – REVEAL -- was ordered to better characterize the patient’s indeterminate lung nodule found on CT. His pre-test probability, calculated by the VA Clinical Factors Model, indicted 53%, placing the patient’s risk of malignancy in the intermediate risk range. A simple, venous blood draw in the office was conducted and the plasma sent to the CLIA certified testing laboratory. The assay result was provided within three days as a score of 34 (Figure 1) indicating a 94% probability the patient’s nodule was lower-risk, benign disease. Based on this new information, the patient agreed with us to proceed with a serial surveillance strategy.
Three months later, a follow up thoracic CT was performed indicating resolution of the ILD and reduction of the size of the LUL nodule to 6.2 × 2.8 mm. The prednisone was reduced and CellCept was added.
From a pulmonary standpoint, the patient continues to do well. He is scheduled to return to the clinic in four months for a full PFT and a chest CT.
Millions of indeterminate pulmonary nodules (IPN) are found annually, either incidentally or through the proliferation of CT screening programs targeting high-risk individuals for lung cancer following the encouraging results of the National Lung Screening Trial and the USPSTF recommendations. Although the large majority (96.4%) of IPN are benign, current predictive tools to discriminate benign from malignant nodules are suboptimal, leading to a larger number of more frequent follow-up CT scans, unnecessary invasive biopsies with attendant morbidity and rare mortality, anxiety, and wasted healthcare spending -- up to $28 billion/year in the United States. Incorrect evaluation of an IPN causes risks that range from anxiety to a high rate of unnecessary thoracotomies for benign nodules and missed chances for cure during follow-up resulting in death. Chest CT is not capable of providing the diagnostic accuracy needed [11].
To address the large numbers of false positive findings, an effective rule-out test would be a valuable aid in the management of an IPN. The performance reported for the novel plasma protein assay by Trivedi, et al. [8] indicates it could guide the clinician toward reassurance, watchful waiting, or sooner biopsy or resection, and thus decrease the anxiety, cost, and uncertainty of lung cancer screening. It may also reduce the problem of overdiagnosis in lung cancer screening.
For most patients with a small pulmonary nodule, clinical practice guidelines recommend a conservative approach of radiographic surveillance to avoid the potential harms of biopsy. If the nodule does not grow during 2-3 years of surveillance, it is assumed to be benign. Guidelines calling for surveillance of low-risk nodules offer an effective strategy for doctors to manage the uncertainly that these nodules present with a routine approach. Yet patients may not accept surveillance as ‘routine’. For patients, the often-unexpected discovery of a ‘spot’ on the lung may be alarming and the uncertainty ominous. Research indicates that some individuals experience substantial distress and reduced health-related quality of life. Moreover, many patients with an indeterminate pulmonary nodule must live through years of uncertainty [12].
Trivedi et al. [8] articulated the false negative rate for the plasma-protein biomarker assay was 3%. It is doubtful patients with a lower risk result would be discharged completely from the practice. Rather, they will likely be asked to return for a follow up CT scan at a longer interval than might have been done in the absence of the test results. A blood-based assay, such as the one they described, is attractive due to the ease of acquisition. It is an accurate, time-saving, and cost-effective way to help evaluate and characterize indeterminate pulmonary nodules, so we clinicians and our patients can make more informed decisions about possible next steps.
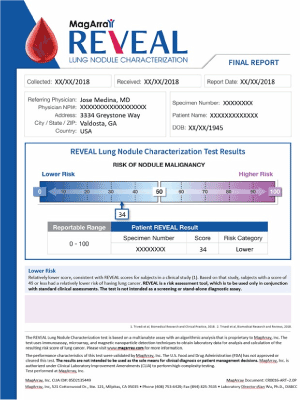
In this clinical case, the assay provided important, additional information that modified this patient’s management. Although the patient was an anxious current smoker, and had a 7 mm nodule, the multiplexed plasma protein assay test score of 34 (out of 100) indicated a lower risk that the nodule was malignant. This finding helped the patient and us make a better-informed decision to adopt a serial surveillance approach. It also significantly reduced the psychologic toll of uncertainty [12].
Although the REVEAL biomarker assay has shown promising results in differentiating malignant from benign lesions, further research is needed to more broadly assess the impact of the test on clinical decision making [13]. Ideally, long-term follow up including the rate of lung cancer deaths prevented using this test is desired to further verify this an effective risk assessment of lung cancer.
The plasma-protein signature should also be more directly assessed in all races, as well as specific conditions such as obesity and its pro-inflammatory state, steroid use, etc., that may affect the test performance. Finally, future clinical studies are warranted to further define the value of the test in accurately identify patients who are most likely to benefit from serial surveillance or early treatment, while reducing the rate of false-positive results, unnecessary interventions, and their associated morbidity and healthcare costs.
Here we report on a patient case illustrating the benefit of a novel, lung cancer-specific biomarker assay. The assay can be used as a non-invasive risk assessment tool for clinicians in characterizing indeterminate pulmonary nodules. When the results of this assay are combined with the traditional clinical risk factors, risk stratification for indeterminate pulmonary nodules is improved compared to current methods in clinical practice. We hypothesize the assay will significantly reduce costs to the healthcare system while further improving a patient’s quality of care. Providers and their patients may consider using this novel assay prior to proceeding with an invasive evaluation of their patient’s indeterminate pulmonary nodule.
Informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
- Siegel RL, Miller KD, Jemal A (2017) Cancer statistics 2017. CA Cancer J Clin 67: 7–30. [Crossref]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, et al. (2011) Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365: 395–409 [Crossref]
- Gould MK, Tang T, Liu IL, Lee J, Zheng C, et al. (2015) Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med 192: 1208-1214. [Crossref]
- Gould MK, Donington J, Lynch WR, Mazzone PJ, Midthun DE, et al. (2013) Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143: e93S–e120S [Crossref]
- Lokhandwala T, Bittoni MA, Dann RA, D'Souza AO, Johnson M, et al. (2017) Costs of diagnostic assessment for lung cancer: A Medicare claims analysis. Clin Lung Cancer 18: e27-e34. [Crossref]
- Gareen IF, Duan F, Greco EM, Snyder BS, Boiselle PM, et al. (2014) Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer 120: 3401–3409. [Crossref]
- Codreanu SG, Hoeksema MD, Slebos RJC, Zimmerman LJ, Rahman SMJ, et al. (2017) Identification of Proteomic Features to Distinguish Benign Pulmonary Nodules from Lung Adenocarcinoma. J Proteome Res 16: 3266-3276. [Crossref]
- Trivedi NN, Arjomandi M, Brown JK, Rubenstein T, Rostykus AD, et al. (2018) Risk assessment for indeterminate pulmonary nodules using a novel, plasma-protein based biomarker assay. Biomed Res Clin Prac 3: 1-8.
- Gould MK, Ananth L, Barnett PG, Veterans Affairs SNAP Cooperative Study Group (2007) A Clinical Model to Estimate the Pretest Probability of Lung Cancer in Patients with Solitary Pulmonary Nodules. Chest 131: 383–388. [Crossref]
- Tanner NT, Aggarwal J, Gould MK, Kearney P, Diette G, et al. (2015) Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest 148: 1405–1414. [Crossref]
- Massion PP, Walker RC (2014) Indeterminate pulmonary nodules: risk for having or for developing lung cancer? Cancer Prev Res (Phila) 7: 1173-1178. [Crossref]
- Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA (2012) The Thing is Not Knowing: Patients Perspectives on Surveillance of an Indeterminate Pulmonary Nodule. Health Expect 18: 355-365. [Crossref]
- Mazzone PJ, Sears CR, Arenberg DA, Gaga M, Gould MK, et al. (2017) Evaluating molecular biomarkers for the early detection of lung cancer: When is a biomarker ready for clinical use? An Official American Thoracic Society Policy Statement. Am J Respir Crit Care Med 196: e15-e29. [Crossref]

