Introduction: An insulinoma is a rare pancreatic endocrine tumor that is typically sporadic, solitary, and less than 2 cm in diameter. Fewer than 5% of insulinomas are larger than 3 cm. Ninety percent or more of all insulinomas are benign.
Case presentation: This study reports on a 42-year-old woman was admitted with lightheadedness, chills and shakiness. The onset of the symptoms was from 1 year ago. This symptom improved after eating of candy or fruit juice. Initial loboratory testing revealed blood glucose level in 30-40 mg/dl. The patient was admitted for management for hypoglycemia. Blood tests evidenced high insulin and c-peptide levels despite low plasma glucose level. Endoscopic ultrasonography (EUS) revealed pancreatic mass. She underwent enucleation of mass, and the surgical pathology was consistent with insulinoma.
Conclusion: Proper management for timely treatment of a patient with insulinoma involves complex medical teamwork consisting of physicians from various specialties: endocrinology, internal medicine, surgery, pathology, medical imaging, and oncology.
insulinoma, endocrine tumor, hypoglycemia, pancreas
Among pancreatic endocrine tumors, the most common type is insulinoma. This tumor was reported in 1–4 people per one million person years [1]. Usually insulinoma is benign tumor, with a solitary and small size (<2 cm in diameter). Most are sporadic, however, 10 % of insulinomas are multiple and occur as part of multiple endocrine neoplasia syndrome type I (MEN–I). Because of nonspecific symptoms, insulinoma may be misdiagnosed with other disorders. Patients often present with hypoglycemia symptoms resulting from inappropriate insulin secretion [2,3]. It can be seen at any age and occurs slightly more frequently in woman than man [2]. After biochemical confirmation of hyperinsulinism, preoperative localization of the tumor in the pancreas may be difficult. Surgical removal, often curative, continues to be the treatment of choice.
In this manuscript, we report case of pancreatic insulinoma, and we discuss diagnosis, localization and management of this uncommon disease.
A 43-yr-old woman was seen in the endocrinologic clinic with the acute onset of lightheadedness, chills and shakiness. A glucose level detected with glucometer was 32 mg/dl. She was eaten candy and fruit juice, and her symptoms resolved. The patient reported that she had experienced similar symptoms, but of lesser severity, for approximately 1-yr duration. As per patient’s information most episodes were in the early morning between 3 to 7 am, and were associated with prolonged fasting and over exertion. She also reported a recent 15 kg weight gain. For further evaluation and management patient was advised hospitalization. She denied having seizures and diabetes but he noticed increased appetite over the past year. She had no family history of diabetes, thyroid or pituitary disease.
This physical examination revealed a well-nourished woman with weight 69 Kg and BMI 26.9 Kg/M2. On the basis of history, keeping insulinoma a possibility; patient was subjected to laboratory investigations for which she was hospitalized. After dinner around to midnight, patient developed symptoms of hypoglycemia. At that time on examination his pulse was 120/min, blood pressure 130/80 mmHg, respiratory rate was 20/min.
At the same time laboratory investigation were sent. Blood sample taken at the time of hypoglycaemic episode showed low plasma glucose of 36 mg/ dl, elevated insulin of 94.8 mu/l (normal range, 1.7 - 31 mu/l), elevated C-peptide level of 10.6 ng/ml (normal range 0.9-4 ng/ml. The serum cortisol level was normal suggesting intact pituitary adrenal axis. Thyroid function tests were within normal range.
In view of the clinical picture and laboratory data, the clinical impression was that of an insulinoma.
Abdominal ultrasound (US) was normal. A computed tomography of the abdomen and pelvis with contrast using pancreas protocol was normal. Thus, endoscopic ultrasound (EUS) was performed showing a hypoechoic mass of pancreatic body with diameter 10 mm. FNA of detected mass was performed. The histopathological appearance pleaded for Insulinoma (Figure 1). We transferred the patient to the surgery department. The pathology findings were supporting the diagnosis of pancreatic endocrine neoplasm low grade. Immediately after surgical treatment, the glucose level increase to the diabetic range.
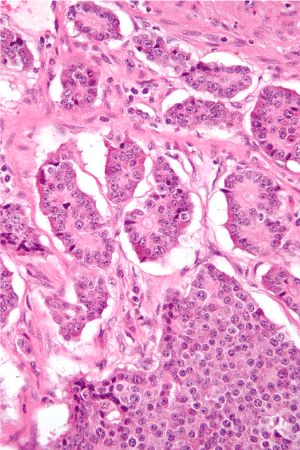
Figure 1. Histopathological appearance of Insulinoma.
Sporadic distribution, small size and high benignity rate are known insulinoma features, however etiopathogenesis remains still unclear. This rare tumor may have variable and nonspecific presentations all referable to the hypoglycemic state. Hypoglycemic symptoms can be divided into neuroglycopenic signs, most common, including confusion, behavioral changes, visual disturbances, weakness, dizziness, seizures and loss of consciousness, and neurogenic signs, such as anxiety, sweating, palpitations, tremors and feeling of warmth [4,5]. These symptoms become typically evident after fasting and are often precipitated by physical exercises. However, the median duration of symptoms before diagnosis remains variable and can reach 12–18 months on average or even years in rare cases [2].
Insulinoma is still suggested by the Whipple’s triad including: symptoms of hypoglycemia induced by fasting or exercise, plasma glucose level below than 45 mg/dl and relief of symptoms following the administration of glucose. The supervised 72 h fasting test remains to be the gold standard for biochemical diagnosis with measurement of plasma glucose, insulin, C-peptide, and proinsulin during the onset of hypoglycemic symptoms.
Various preoperative procedures can be used to localize the tumor in order to plan therapeutic strategy. The choice of procedure depends upon which tests are available and local radiologic skills. In our context, multiple noninvasive and invasive options are used including trans-abdominal ultrasonography, CT scan, MRI and EUS. The reported sensitivity of conventional CT and MRI for detection of pancreatic insulinoma ranges respectively from 33 to 64 and 40 to 90 %. However, the advent of helical CT scan has enabled detection of about 94% of insulinomas [1,4].
Consequently, it is currently accepted that CT scan is the first-line and MRI is the second-line investigation. These modalities can identify the exact size and location of an insulinoma, describe its anatomic relationship to surrounding structures and detect the presence of metastatic lesions suggestive of malignancy [6].
Some authors consider EUS as the best exam for preoperative localization of insulinoma, with a sensitivity of up to 94 %. It can detect even small tumors of 5 mm, and reveal important relation to the bile duct and adjacent blood vessels. Also, EUS allows performing fine-needle aspiration cytology of suspicious lesions and preoperative marking of tumors to facilitate surgical excision particularly with laparoscopic approach. However, EUS findings depend largely on the examiner’s experience [1,2]. In our institution, we generally use this technique when the tumor is not detected with the previously mentioned imaging modalities.
Bimanual palpation combined with intraoperative ultrasonography (IOUS) is the most effective method to detect more than 95 % of tumors, but requires complete mobilization of the pancreas [7,8]. Because of this operative success particularly in the hands of experienced surgeons, some authors suggest that preoperative localization studies are not necessary [9,10]. However, with recent advances in imaging techniques, preoperative topographic assessment is considered useful in avoiding blind resection and planning for rapid, accurate and safe surgery. Additionally, there is absolutely no question that positive localization is required prior to re-operative insulinoma [4].
Most insulinomas can be cured with surgery. Surgical procedure choice depends on the size and location of the mass. Tumor enucleation is the procedure of choice especially in case of small and solitary nodule that is not encroaching on the pancreatic or bile ducts [11]. The lesions are typically reddish-brown, firm, and encapsulated with a clear plane of dissection between the tumor and surrounding soft pancreatic parenchyma [12]. In addition, recent guidelines suggest that enucleation is enough in front of a well-circumscribed lesion, clearly localized before surgery, near or at the pancreatic surface, and easily defined intra-operatively [4]. Moreover, pancreatic resection is indicated for lesions invading or in close proximity to the pancreatic duct or major vessels, or suspicious for malignancy with a hard, infiltrating tumor and puckering of the surrounding soft tissue, pancreatic duct dilatation or lymph node involvement [4,13]. Resection options include distal pancreatectomy (with or without splenectomy), Whipple procedure (pancreaticoduodenectomy), or median pancreatectomy, depending on the site of insulinoma.
If the tumor is not identified despite a careful surgical exploration with bimanual palpation and IOUS, termination of the surgical procedure without blind resection is recommended. In such cases, the patient should be evaluated and re-operated at a referral center. Blind distal pancreatectomy is currently not appropriate and must be avoided [10,14]. Consequently, more extensive localization procedures must be applied before reoperation, often including the intra-arterial calcium stimulation test with hepatic venous sampling (IACS-test). This test helps to regionalize the lesion preoperatively with a high detection rate ranging from 94 to 100 %. [15]. Therefore, the use of IACS-test allows for a more accurate surgical approach and can minimize the likelihood of re-operation. It may be appropriate when an insulinoma is strongly suspected but all previously described tests are negative. However, high cost and exclusive availability in some specialized centers lead to reserve this modality for patients with persistent or recurrent hyperinsulinism after initial surgery [16].
Furthermore, laparoscopic approach is currently feasible and becomes increasingly reported with good results in selected patients [17,18]. Tumor location should be confirmed intra-operatively by laparoscopic ultrasonography [19,20]. Both of our patients underwent tumor enucleation using open surgical approach. The lesions were easily identified thanks to only bimanual palpation of the pancreatic parenchyma.
Histologically, insulinomas are epithelial neoplasms associated with strong and diffuse immunohistochemical expression of neuroendocrine markers such as synaptophysin and chromogranin. Mitotic rate (number of mitoses per 10 HPF) and proliferation index (Ki-67 labeling index) are particularly helpful to separate well-differentiated from poorly differentiated tumors [21]. Conversely, malignant insulinomas are difficult to distinguish histologically and often the diagnosis of malignancy is only made when metastases occur [10].
Medical management of insulinoma, used to treat and prevent hypoglycemia, is generally restricted to unresectable metastatic tumors, unsuccessful operation with persistent symptoms, inoperable patients, and patients awaiting or refusing surgery [1,4]. Moreover, other recent techniques for the management of insulinoma have been reported, including injection of octreotide, EUS guided alcohol ablation, radiofrequency ablation, or embolization of an insulinoma [1].
The diagnosis of insulinoma often comes from a hunch, from the impossibility of highlighting the cause of repeated hypoglycemia in an apparently healthy patient. But for diagnostic certainty, patient follow-up and repeated analysis are necessary. Proper management for timely treatment of a patient with insulinoma involves complex medical teamwork consisting of physicians from various specialties: endocrinology, internal medicine, surgery, pathology, medical imaging, and oncology.
The author like to thanks of surgery and pathology team for helping in management this patient.
- Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, et al. (2013) Diagnosis and management of insulinoma. World J Gastroenterol 19: 829–837. [Crossref]
- Bouslama K, Maghrebi H, Bedioui H, Bouslama K (2014) Pancreatic insulinoma: diagnostic approach and therapeutic modalities. J Afr Hépatol Gastroentérol 8: 11–15. [Crossref]
- Patel S, Narwari M, Parekh D, Shah V (2013) Insulinoma: case report and review of diagnostic and treatment modalities. J Assoc Physicians India 61: 423–426. [Crossref]
- Abboud B, Boujaoude J (2008) Occult sporadic insulinoma: localization and surgical strategy. World J Gastroenterol 14: 657–665. [Crossref]
- Cryer PE (1999) Symptoms of hypoglycemia, thresholds for their occurrence, and hypoglycemia unawareness. Endocrinol Metab Clin North Am 28: 495–500, v–vi. [Crossref]
2021 Copyright OAT. All rights reserv
- McAuley G, Delaney H, Colville J, Lyburn I, Worsley D, et al. (2005) Multimodality preoperative imaging of pancreatic insulinomas. Clin Radiol 60: 1039–1050. [Crossref]
- Correnti S, Liverani A, Antonini G, Paganelli MT, Mercati U (1996) Intraoperative ultrasonography for pancreatic insulinomas. Hepatogastroenterology 43: 207–211. [Crossref]
- Norton JA (1999) Intraoperative methods to stage and localize pancreatic and duodenal tumors. Ann Oncol 10: 182–184. [Crossref]
- Hashimoto LA, Walsh RM (1999) Preoperative localization of insulinomas is not necessary. J Am Coll Surg 189: 368–373. [Crossref]
- Shin JJ, Gorden P, Libutti SK (2010) Insulinoma: pathophysiology, localization and management. Future Oncol 6: 229–237. [Crossref]
- Finlayson E, Clark OH (2004) Surgical treatment of insulinomas. Surg Clin North Am 84: 775–785. [Crossref]
- Vanderveen K, Grant C (2010) Insulinoma. Cancer Treat Res 153: 235–252. [Crossref]
- Grant CS (2005) Insulinoma. Best Pract Res Clin Gastroenterol 19: 783–798. [Crossref]
- Hirshberg B, Libutti SK, Alexander HR, Bartlett DL, Cochran C, et al. (2002) Blind distal pancreatectomy for occult insulinoma, an inadvisable procedure. J Am Coll Surg 194: 761–764. [Crossref]
- Morita S, Machida H, Kuwatsuru R, Saito N, Suzuki K, et al. (2007) Preoperative localization of pancreatic insulinoma by super selective arterial stimulation with venous sampling. Abdom Imaging 32: 126–128. [Crossref]
- Tseng LM, Chen JY, Won JG, Tseng HS, Yang AH, et al. (2007) The role of intra-arterial calcium stimulation test with hepatic venous sampling (IACS) in the management of occult insulinomas. Ann Surg Oncol 14: 2121–2127. [Crossref]
- Isla A, Arbuckle JD, Kekis PB, Lim A, Jackson JE, et al. (2009) Laparoscopic management of insulinomas. Br J Surg 96: 185–190. [Crossref]
- Jaroszewski DE, Schlinkert RT, Thompson GB, Schlinkert DK (2004) Laparoscopic localization and resection of insulinomas. Arch Surg 139: 270–274. [Crossref]
- Iihara M, Kanbe M, Okamoto T, Ito Y, Obara T (2001) Laparoscopic ultrasonography for resection of insulinomas. Surgery 130: 1086–1091. [Crossref]
- Lo CY, Lo CM, Fan ST (2000) Role of laparoscopic ultrasonography in intraoperative localization of pancreatic insulinoma. Surg Endosc 14: 1131–1135. [Crossref]
- Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S (2010) The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 39: 707–712. [Crossref]

