Abstract
Aim: This study was designed to evaluate the potential therapeutic effects of synthesized nano-hydroxyapatite (nHAp) on acute renal failure induced by cisplatin (CP) in rat model.
Materials and Methods: The rats (n=40) were divided into equal four groups. Negative control group (C) was always received 1 mg/kg body weight (b.w.) of 9% saline. Positive control group (nHAp) was injected intravenously (i.v.) with 100 mg/kg b.w. nHAp weekly for three weeks. Third group (CP) was received CP as a single dose of 5 mg/kg b.w. while, the last group (CP+nHAp) was received cisplatin and nHAp as previous manner.
Results and Discussion: DNA fragmentation and kidney injury molecule-1 (Kim-1) were estimated in kidney tissue. While, the activity of Neutrophil Gelatinase-Associated Lipocalin (NGAL), tumor necrosis factor-α (TNF- α), interleukin-10 (IL-10) and monocytes chemoattractant protein-1 (MCP-1) were measured in rats' sera. The efficiency of kidney function was evaluated by analyzing the levels of urea and creatinine as well as the concentrations of sodium (Na) and potassium (K). Histopathological examination of kidney tissues was also performed. The obtained results revealed that CP administration induced significant injury in kidney represented by DNA fragmentation besides increases in the level of Kim-1 and kidney functions figure as well as disturbance in the cytokines activities. The potent therapeutic efficacy of nHAp was manifested in repairing the fragmented DNA, amelioration of kidney function biomarkers and activities of cytokines as compared to CP treated group. The most important finding that, the treatment with nHAp was accompanied with complete recovery of histopathological symptoms associated with CP administration.
Conclusion: In conclusion, with nHAp treatment, the symptoms of acute renal failure associated with CP have begun to recover in a short time. Such findings represented a new hope for patients suffering from renal failure whether from exposure to chemotherapy or any other hazardous factors to have safe, fast, and effective remedy.
Introduction
Nano-hydroxyapatite with a chemical formula (Ca10 (PO4)6(OH)2), is one of the most important nano-materials concerned by nanotechnology progresses. Because nHAp has a good issue compatibility inside the body, it intentionally added into oral hygiene products and used as the biological material for bone damage, simultaneously used in body medicine [1-3] as genetic carrier [4] and drug delivery [5]. HAp nanoparticles have also an obvious strong inhibitory effect on growth of some kinds of tumor cells [6]. Of the advantages of nHAp are, the thermodynamic property which is the most stable phase in physiological conditions and it has also a very small excitation effect on blood vessel in case of intravenous injection [7]. Moreover, nHAp was considered a good chelator agent in vitro and in vivo as reported by the studies of Aoki, et al., Hu, et al., and Abdel-Gawad and Awwad [2,6,8]. On contrary, there is a discrepancy of opinions about nHAp bio-toxicity, but the most studies eventually congregated upon that the toxicity of nHAp depended on diameter of the particles, morphology of the particles, exposure dose and contact way as well as the composition of it [9-11].
Cisplatin, a platinum-based inorganic compound, is one of the most potent and widely used chemotherapeutic agent for various forms of cancers [12]. Despite its effectiveness, the use of cisplatin in high-dose therapy has been reported to be limited by renal and cardiac toxicities, sharing similar cellular and molecular mechanisms. Other side effects of CP include ototoxicity, neurotoxicity, gastric toxicity, myelosuppression, severe nausea and emetic effects have been reported [13-15]. The mechanism for cisplatin-induced acute renal failure appeared to involve several endogenous mediators correspondingly, enhanced oxidative stress, inflammation, increased endoplasmic reticulum stress, cytokines and renal cell apoptosis [16,17].
Owing to potent and wide range of therapeutic benefit of cisplatin against various malignancies, establishment of a new adjunct therapeutic strategy which ameliorates the severity of cisplatin toxicity in the field of cancer research is, therefore, warranted. On the other hand, with improved the biological properties of the prepared nHAp, the current study was conducted to evaluate the performance of nHAp in overcoming the acute renal failure induced by cisplatin in rats. Biochemical and histological kidney investigations were performed to evaluate the effectiveness of the prepared of nHAp.
Materials and methods
Chemicals
The chemicals used for n-HAp preparation were calcium nitrate tetrahydrate (Ca(NO3)2.4H2O, M. wt. 236.15 g/mole, Merk, Germany), diammonium hydrogen orthophosphate anhydrous ((NH4)2HPO4, 132.06 g/mole, S.D. Fine Chem. Ltd. Mumbai, India), polyvinyl alcohol (PVAl) (M. wt.≈160000 g/mole), and ammonium hydroxide (NH4OH, M. wt. 35.5 g/mole, May & Baker, England).
Cis-diaminedichloroplatinum (II) was obtained from Sigma-Aldrich (Germany) and provided as a solution (1 mg/ml). All chemicals were used in the experimental work without further purification.
Synthesis and characterization of nHAp
HAp nanoparticle was synthesized by prepared 5% of PVAL solution in 1-L flat bottom flask using deionized water with stirring at 80˚C for 30 min. Then calcium nitrate (Ca(NO3)2.4H2O) was added. Finally, diammonium hydrogen orthophosphate ((NH4)2HPO4) was added to the mixture with Ca/P atomic ratio 1.67 while stirring and heating at 80°C under PH control. The formed was dried at 100°C for 24 h and characterized using FTIR, XRD and TEM to prove the formation of hydroxyapatite structure. The synthesized nHAp was calcined using muffle furnace at 900°C for 7 h. The formed calcined powder was characterized using Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), X-ray diffraction (XRD), and transmission electron microscopy (TEM), to confirm the nano-size range of synthesized nHAp.
Animals
Forty male healthy albino rats (200 gm-220 gm) were used in the current study. They housed in a clean polypropylene cages and maintained at the room temperature 23°C-25°C with alternating 12 h light and dark cycle. The animals were fed on standard pellet diet and clean drinking water. The study was conducted in accordance with the guidelines set by the CIOHS & ICLAS International Guiding Principles for Biomedical Research involving animals (2012) which accordance with the Guide for the Care and Use of Laboratory Animals (Eighth Edition, 2011, published by The National Academies Press, 2101 Constitution Ave. NW, Washington, DC 20055, USA). This guide was approved by the Ethical Committee at National Center for Radiation Research, Egyptian Atomic Energy Authority, Cairo, Egypt (NCRR- EAEA).
Experimental design
The animals were randomly divided into four groups, each group from 10 animals. Rats in group I was served as negative control (C) and they were injected intravenous with a saline solution only. Animals in group II served as positive control (nHAp) and they were treated weekly with i.v. 100 mg/kg b.w. nHAp alone for three weeks. Animals in group III (CP) were received single dose of intraperitoneal (i.p.) CP at level of 5 mg/kg b.w. Animals in group IV (CP-nHAp) were received single dose of 5 mg/kg b.w CP (i.p.) and after 72 hours, they were received 100 mg/kg b.w. of nHAp (i.v.) weekly for three weeks. One week post the last dose of nHAp injection, the animals were anesthetized by diethyl ether and blood samples were drawn from the retro-orbital venous plexus in glass tubes, sera were separated by centrifugation at 3000 rpm for 10 minutes and kept frozen at -20˚C until the assessment of biochemical analyses. Then, all the animals were killed, and the abdomens were dissected to remove the kidneys immediately. Kidney tissue of each rat was divided into two parts, one part washed thoroughly with ice cold saline and kept at -80˚C for DNA electrophoresis and (Kim-1) estimation and the other part fixed in formalin solution (10%) for Histopathological examination.
DNA fragmentation assay
DNA was extracted from kidney tissue as previously described by Bortner, et al. [18]. A small piece of kidney (0.2 g) was put in a homogenizer with 0.8 ml of ice-cold PBS and homogenized for 1 min. to each 200 µL, 700 µl lysis buffer [50 mmoll−1 Tris+1 mmoll−1 Ethylene diamine tetra acetic acid (EDTA)+150 mmoll−1 sodium chloride (NaCl)+0.2% Triton X-100] and 50 µgml−1 of proteinase- K were added and incubated at 50˚C for 2 h. To 500 µl, 100 µl of ice-cold 5 moll−1 NaCl were added and vigorously vortexed, then 700 µl of ice-cold isopropanol were added and again vigorously vortexed. Precipitation was allowed to proceed overnight at −20˚C. After precipitation, DNA was recovered by pelleting for 10 min at 20,000 g at 4˚C. The supernatants were discarded, and the pellets were rinsed by adding 700 µl of ice-cold 70% ethanol and centrifuging at 20,000g for 10 min at 4˚C. The supernatants were again discarded, and the ethanol was removed, and the DNA pellet was dried and dissolved in 100 µl of Tris–EDTA buffer. The extent of DNA fragmentation was assessed by agarose gel electrophoresis containing 0.5 mgml−1ethidium bromide at 5 Vcm−1 for 1 h. DNA was visualized by placing the gel on a UV trans-illuminator and the amount of DNA fragmentation in individual rat livers was quantified using Image Analyzer software (Version 1.36.1).
Biochemical analysis
Measurements of serum Neutrophil Gelatinase- Associated Lipocalin (NGAL) and tumor necrosis factor (TNF-α) were performed according to the method of Ono, et al., and Stejskal, et al., [19,20], respectively. Serum monocytes chemoattractant protein-1 (MCP-1) and interlukin-10 (IL-10) activities were determined by the tests based on a solid phase enzyme linked immunosorbent assay (ELISA) from (Boster Biological Technology LTD, Malden, MA 02148 USA) according to Tietz [21], techniques. Urea and creatinine were evaluated according to the methods of (Sarcosine colormetric/Fluorometric Assay Kit. respectively. While, sodium (Na) and Potassium (K) were determined by AVL 9180 electrolyte analyzer method based on the ion-selective electrode (ISE) measurement as principle of Tietz [21]. The value of kidney Injury Molecules (Kim-1) was estimated in tissue by a commercially available ELISA kit (BD Pharmingen).
Histopathological examinations
Autopsy samples were taken from the kidneys of rats in different groups and fixed in 10% formalin for twenty-four hours, washed in tap water then serial dilutions of alcohol (methyl, ethyl and absolute ethyl) were used for dehydration. Specimens were cleared in xylene and embedded in paraffin at 56 degree in hot air oven for twenty-four hours. Paraffin bees wax tissue blocks were prepared for sectioning at 4 microns by sledges microtome. The obtained tissue sections were collected on glass slides, deparaffinized and stained by hematoxylin and eosin stains [22] for histopathological examination using the electric light microscope.
Statistical analysis
All experimental values were expressed as mean ± SE. Statistical analysis was performed t ' test. P values <0.05 were statistically significant.
Results
Characterization of the formed nano-HAp
The FTIR analysis of the synthesized nHAp after calcination at 900°C was shown in Figure 1. The characteristic bands of nHAp Ca10 (PO4)6(OH)2 which appeared at 629.4 cm-1 and 3566.72 cm-1 belonged to the vibration of hydroxyl OH. Those bands at 955.03 cm-1 and 1025.42 cm-1 were the characteristic bands for phosphate (PO43-) group stretching vibration, while the bands at 601.06 cm-1and 563.69 cm-1 were due to phosphate bending vibration [23].
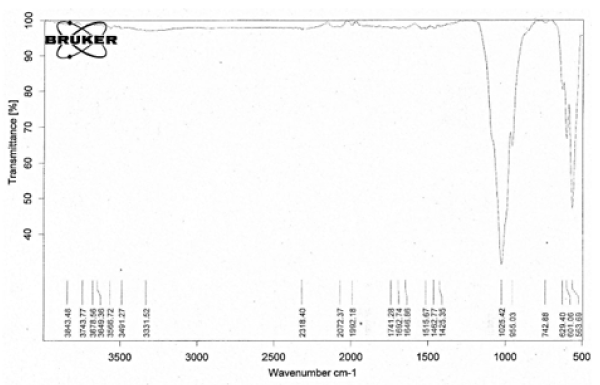
Figure 1. IR spectra of synthesized nano-HAp and calcined at 900°C
The main characteristic bands of nHAp crystal structure were appeared at d=2.81, 2.71 and 2.77 A from XRD analysis of the sample calcined at 900˚C for 7 h without any other calcium phosphate structure Figure. 2.
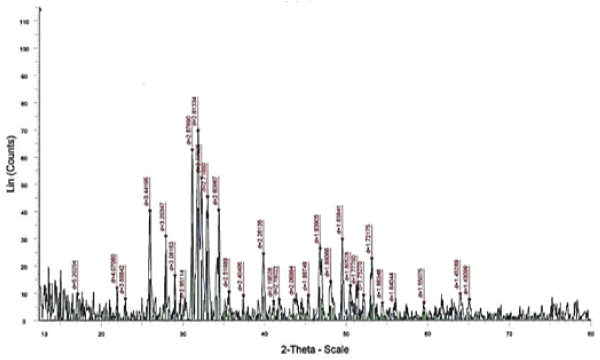
Figure 2. XRD patterns of the nHAp calcined at 900°C for 7 h
The transmission electron microscope of n-HAp illustrated ultra-small crystals distributed in matrix as shown in Figure 3, with average grain size of 40 nm. TEM studies showed a uniform distributed of n-HAp with self-assembled and aggregates of uniform size and morphology. Due to the very fine size of precipitated particles present in the aggregation, they could not be individually resolved, however, their crystalline nature and phase identification could be ascertained through selected area in the diffraction pattern, Figure. 3 that confirmed the formation of nano-hydroxyapatite phase.

Figure 3. TEM micrograph of calcined nHAp
DNA fragmentation results
Analyses of DNA by agarose gel electrophoresis showed that DNA was completely intact in kidney samples of both control and nHAp groups. Induction of kidney injury by CP resulted in elevation of DNA fragmentation, where, the intact DNA reduced to 60.48% and 70.30%, respectively, for the two replicate tested samples of CP kidney tissues. Treatment with nHAp for CP intoxicated rats was succeeded in repairing the fragmented DNA, since the intact DNA increased up to 87.3% and 77%, respectively, in the two tested kidney samples Table 1, Figure. 4.
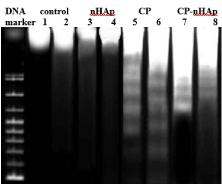
Figure 4. DNA electrophoresis of kidney tissue samples for all the studied groups. (n=2 samples per group)
|
Negative control
|
nHAp- group
|
CP- group
|
CP-nHAp group
|
|
Lane 1
|
Lane 2
|
Lane 3
|
Lane 4
|
Lane 5
|
Lane 6
|
Lane 7
|
Lane 8
|
|
BP
|
%
|
BP
|
%
|
BP
|
%
|
BP
|
%
|
BP
|
%
|
BP
|
%
|
BP
|
%
|
BP
|
%
|
|
3359
|
100
|
3272
|
100
|
3299
|
100
|
3321
|
100
|
3178
|
60.48
|
3023
|
70.3
|
3023
|
87.3
|
2945
|
77
|
| |
|
|
|
|
|
|
|
1202
|
2.85
|
869
|
4.54
|
811
|
3.3
|
869
|
11.73
|
| |
|
|
|
|
|
|
|
949
|
5.6
|
774
|
11.39
|
724
|
9.41
|
806
|
11.27
|
| |
|
|
|
|
|
|
|
834
|
9.59
|
590
|
6.75
|
|
|
|
|
| |
|
|
|
|
|
|
|
724
|
16.11
|
355
|
6.49
|
|
|
|
|
| |
|
|
|
|
|
|
|
562
|
5.38
|
|
|
|
|
|
|
Table 1. Analysis of DNA fragmentation in the different experimental groups
Biochemical results
As shown in Table 2, there was no significant difference between negative control and nHAp injected groups regarding to the values of NAGL and Kim-1. On the hand, administration of CP caused significant increase (p<0.05) in the levels of these two parameters as compared to both negative and positive control groups. These increases were reduced after the CP intoxicated rats had treated by nHAp and recorded significant differences relative to the three other preceding groups.
|
Groups
|
C
|
nHAp
|
CP
|
CP-nHAp
|
|
Parameters
|
|
NG-AL (ng/ml)
|
0.206 ± 0.0179
|
0.277∎ ± 0.043
|
2.063* ± 0.068
|
1.267*∎⍟ ± 0.071
|
|
Kim-1(pg/100mg)
|
52.08 ± 2.44
|
56.17∎ ± 2.30
|
159.00* ± 1.11
|
98.85*∎⍟ ± 3.18
|
Table 2. Levels of NG-AL and Kim -1 in different groups. Data are represented as mean of 10rats ± standard mean (SEM) error of
*: Significant difference in comparison with C group (P<0.05)
∎: Significant difference in comparison with CP group (P<0.05)
⍟: Significant difference in comparison with n-HAp group (P<0.05)
Table 3 manifested that injection of animals with nHAp (positive group) had no significant effects on serum contents of cytokines i.e. TNF-α, MCP-1 & IL-10. The administration of CP into rats (CP group) altered these cytokines concentrations to increase by more than 20 for TNF-α and 4 folds for MCP-1 while IL-10 activity was decreased by 2 folds compared with animals in both control groups. Treatment with nHAp composite, post CP intoxication, remodel the activities of the tested cytokines to a great extent but its contents still significantly differ from both control groups.
|
Groups
Parameters
|
C
|
nHAp
|
CP
|
CP-nHAp
|
|
TNF-α (pg/ml)
|
21.34 ± 1.01
|
23.32∎ ± 0.99
|
428.92* ± 11.39
|
242.30 *∎⍟ ± 13.98
|
|
MCP-1 (pg/ml)
|
110.5 ± 3.564
|
105.417∎ ± 1.575
|
465.13* ± 14.401
|
278.183*∎⍟ ± 5.698
|
|
IL-10 (pg/ml)
|
350.71 ± 5.45
|
375.78*∎ ± 2.32
|
168.85* ± 4.05
|
246.39*∎⍟ ± 19.18
|
Table 3. Serum cytokines activity in different groups. Data are represented as mean of 10 rats ± standard mean (SEM) error of
*: Significant difference in comparison with control group (P<0.05)
∎: Significant difference in comparison with CP group (P<0.05)
⍟: Significant difference in comparison with nHAp group (P<0.05)
There were no notable differences between negative control group and nHAp ones regarding the concentrations of creatinine, urea, Na and K. Administration of CP (CP group) to rats disposed significant elevations in serum creatinine, urea and K levels in addition to detectable decrease in Na concentration compared with the two control groups (Table 4). Injection of nHAp followed CP toxicity (CP-nHAp group) accompanied with recasting the contents of the kidney function biomarkers under consideration, but their activities, still significantly differ from C & nHAp groups except for Na content which adjusted nearly to the controls figure Table 4.
|
Groups
Parameters
|
C
|
nHAp
|
CP
|
CP-nHAp
|
|
Creatinine (mg/dl)
|
0.58 ± 0.01
|
0.58∎ ± 0.03
|
3.92* ± 0.21
|
1.76*∎⍟ ± 0.02
|
|
Urea (mg/dl)
|
16.10 ± 0.767
|
14.46∎ ± 0.10
|
45.94* ± 1.46
|
23.81*∎⍟ ± 0.99
|
|
Na (mEq/l)
|
146.64 ± 1.93
|
146.88∎ ± 1.09
|
135.01 ± 2.42
|
147.13∎ ± 2.4
|
|
K (mEq/l)
|
4.40 ± 0.14
|
4.54∎ ± 0.11
|
7.29* ± 0.10
|
6.37*∎⍟ ± 0.11
|
Table 4. Biomarkers of kidney functions in different groups. Data are represented as mean of 10 rats ± standard mean (SEM) error of
*: Significant difference in comparison with control group (P<0.05)
∎: Significant difference in comparison with CP group (P<0.05)
⍟: Significant difference in comparison with nHAp group (P<0.05)
Histopathological results
Section in kidney of negative control rats revealed normal histological structure of the glomeruli (g) surrounded by tubules (t) at the cortex (Figure. 5). Similarly, there was no histopathological alteration observed in kidney section for rats in positive control group administrated nHAp only (Figure. 6). Post CP intoxication, kidney sections showed degeneration and necrosis in the lining epithelium of the tubules in a diffuse manner (Figure. 7 A,B) associated with focal inflammatory cells infiltration in between the glomeruli and tubules at the cortex (Figure. 8). Treatment of CP intoxicated rats with nHAp surpassed to ingenious results, where, all the pathological phenomena were disappeared, and the kidney tissues regenerated their normal architecture structures as clarified in Figure 8.
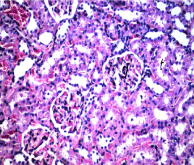
Figure 5. Section in kidney of negative control rat
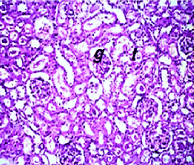
Figure 6. Section in kidney of positive control rat
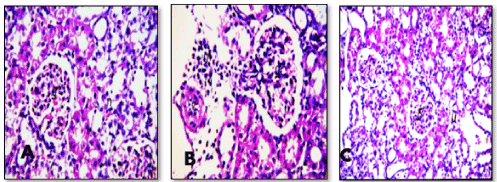
Figure 7(A, B, C). Sections in kidney of CP intoxicated rats
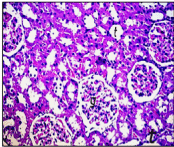
Figure 8. Section in kidney of CP intoxicated rats and treated with nHAp
Discussion
Researchers have shown a special interest for nHAp composite and its usage in the treatment of many organs dysfunction for its supervisor effect in inhibition the growth of some tumors through mitochondrion pathway of apoptosis [24-26]. But, there have been little researches on the effects of this nanoparticles on the kidney injury [27] induced by cisplatin chemotherapy. It well reported that the effect of cisplatin may be caused by direct action on tubular cells, forming cross-linkages of DNA, which causes mitochondrial dysfunction [28,29], generation of reactive oxygen species (ROS) [30], caspase activation [31], elevation of tumor necrosis factor (TNF-α) [32] and inflammatory cell migration in the kidney cells [33].
In tissue, this study illustrated that, CP caused fragmentation in DNA up to 30%-40% with different molecular weight bands when compared to control one which show 100% intact DNA. Meanwhile, the treatment of CP intoxicated rats with nHAp displayed a remarkable increase in the intact DNA and reduction in the fragmented DNA in compare with CP administered animals. On the other hand, the level of renal kim-1 markedly increased by CP intoxication, but post treatment with nHAp composite, the level of Kim-1 was significantly decreased. This damaged effect of cisplatin may be due to its special chemical composition that qualifies it for biological interaction inside the body. Whereas, cisplatin is an inorganic complex formed from an atom of platinum surrounded by chlorine and ammonia atoms in the cis position of a horizontal plane. Intracellularly, water displaces the chloride to form highly reactive charged platinum complexes. These complexes inhibit DNA through covalent binding leading to intra-strand, inter-strand, and protein cross-linking of DNA, leading to distortion of the duplex structure [34,35]. DNA damage signal is then relayed to activation of p-53, which then induces apoptotic cell death of renal tubular cells, in part via mitochondrial injury [36]. Under such conditions, the excess mitochondrial ROS may target and modify genomic DNA, which may eventually enhance the susceptibility to genomic stress by cisplatin-DNA adducts [37]. The reached data provided a clear and convenient evidence for the ability of nHAp in counteracted CP nephrotoxicity through its success in repairing the fragmented DNA by percentage above 17%. This result was consistent with the study of Abdel-Gawad and Awwad [8,11] who reported that nHAp has high capacity in chelating the heavy metals in vivo and in vitro and repairing the associated DNA fragmentation.
The increase of NGAL activity for rats in CP group was rational data because, NGAL is identified as one of the most highly induced genes and intensely expressed subsequent to cisplatin nephrotoxicity in animals [3]. NGAL was rapidly released in the kidney and detected as a punctuate distribution in the cytoplasm within tubule cells in case of CP nephrotoxicity [38]. The increase in NGAL production and release from tubular cells may have self-defensive intent based on the activation of specific iron-dependent pathways represented the mechanism through which NGAL promotes kidney growth and differentiation. Additionally, the level of NGAL is also important one for prediction the future appearance of acute kidney injury after treatments potentially detrimental to the kidney and even the acute worsening of unstable nephropathies.
The physiological role of TNF-α is based on its direct engaging with TNF-receptors, that may trigger tubular-cell death and tissue damage, and indirectly by mounting a robust inflammatory response. On the other hand, TNF-α coordinate the activation of a large network of pro-inflammatory cytokines such as IL-10 and monocyte chemotactic protein-1 (MCP-1), regulated the activation and stimulation of normal T cell expressed to secret cytokines [16]. The upregulation of MCP-1 by TNF-α may promote the migration of inflammatory cells into the renal parenchyma [39]. Thus, the infiltration of inflammatory cells may act as reservoirs of inflammatory cytokines and chemokines, which may enhance the cytotoxic effects of cisplatin [40,41]. The enhancements of inflammatory cytokines led to loss of kidney functions and fibrosis mediated by generation of ROS, nitrous oxide (NO), and pro-inflammatory cytokines [32]. For rats in CP group, the activities of TNF-α and MCP-1 were increased significantly as compared to either negative or positive control groups. In response to the treatment of nHAp, the activities of TNF-α and MCP-1 were readdressed and the level of IL-10 started to elevate. In order to explore plausible reasons for the lower TNF-α secretion at the crystalline nHAp, a thorough surface characterization was performed with the emphasis on surface parameters that may affect the adhesion and/or spread of the monocytes. Since the adhesion of the cells was mediated via the adsorbed protein layer. These results were in consistent with the previous studies by by Rydén, et al., and Albrecht, et al., [42,43], who illustrated that nHAp was able to elicit proliferation of the T- lymphocytes up to 21 days and has the potential activity to mount the body to be able to defense against the oxidative stress. HAp nanoparticle has been shown to decrease TNF-α from binding with the death receptor leading to attenuation of renal toxicity caused by cisplatin [33]. The production of TNF-α is highly dependent upon the reactive oxygen species generation by CP. The antioxidant efficiency of nHAp was previously reported due to its ability in scavenging of superoxide, which is the main component of oxidative stress [43] and/or to inhibition of oxidative and nitro-oxidative species formation [8,44]. On the other hand, nHAp stimulated the production of IL-10 which is a potent anti-inflammatory cytokine produced mainly by Th-2 cells, regulatory T cells, dendritic cells and macrophages. Dendritic cells are the master regulators of the immune response and the production of IL-10 is an important component of protective effects of dendritic cells in cisplatin-induced kidney injury [45].
On studying the renal function parameters, there was a kidney function impairment evidenced by markedly elevation in urea and creatinine levels as well as disturbance in hemostasis of related minerals such as Na and K. There was a possible involvement of ROS and reactive nitrogen species and reduction of the antioxidant defense system due to the CP nephrotoxicity. The mechanism by which platinum-induced nephrotoxicity was related to the accumulation of platinum in the proximal and distal epithelial cells where it could interact with sulfhydryl compounds resulted in an increased membrane fragility, inflammations, functional alteration of cell membrane transporters and depletion of intracellular glutathione [46,47]. Therefore, the most likely site for depressed renal sodium absorption is the proximal nephron where the bulk of filtered Na is reabsorbed. Decreased proximal reabsorption results in the delivery of greater amounts of sodium to the distal nephron; however, with platinum-mediated damage of the distal tubule, the expected compensatory distal increase in sodium transport is impaired or prevented, leading to net Na excretion [48].
On the other hand, the electrolytes disturbances may be due to an excess of water relative to solute and cisplatin has been associated with inappropriate antidiuretic hormone secretion. Serum Na concentration is tightly regulated principally by the antidiuretic actions of arginine vasopressin, which binds to the V2 receptor in the renal collecting ducts and stimulates translocation of the aquaporin-2 water channels, resulting in increased free-water reabsorption. The mineralocorticoid hormone, aldosterone, also regulates sodium and potassium levels via the amiloride‐sensitive sodium channel and Na–K adenosine triphosphatase (ATPase) pump.
Renal damage has several patterns of histological changes, including degeneration and tubular necrosis associated with focal inflammatory cells infiltration in between the glomeruli and tubules at the cortex. Such findings were correlated with previous observations by Palipoch & Punsawad [46] and Arunkumar, et al., [49]. As previously mentioned those kidney functions and cytokines activities accompanied by pathological changes have a close relationship with free radicals generated by cisplatin. The current results indicated that i.v. of nHAp resulted in complete recovery of all pathological phenomena appeared in kidney tissues and attenuation of its function biomarkers as well as cytokines activities. This is not the only confirmation for this study, but there are properties confirmed by other studies on the efficiency of nHAp, including a special crucial role to promote angiogenesis and vasculogenesis for endothelial cells mediated by nitric oxide synthase fibroblast growth factor-2 and sustained the survival and proliferation and preserves their morphology. Angiogenesis and vasculogenesis are contribute key functions for new tissue development, growth, and regeneration [50]. Such findings may be due to antioxidant property and anti- inflammatory properties of nHAp [8,43].
Conclusion
Based on the experimental results reached, it may be demonstrated that administration of nano- hydroxyapatite composite can regenerate the acute histopathological kidney injured in rats induced by one of chemotherapeutic agent, namely cisplatin within three weeks. The potential of the nHAp based on its capability to chelate the platinum part in the CP counteracting its oxidative stress action inside the living cells. The obtained data make an of doses used, as a safe and vast remedy for kidney dysfunctions post the chemotherapy including cisplatin.
References
- Wahl DA, Czernuszka JT (2006) Collagen-hydroxyapatite composites for hard tissue repair. Eur Cell Mater 11: 43-56.
- Aoki H, Aoki H, Kutsuno T, Li W, Niwa M (2000) An in vivo study on the reaction of hydroxyapatite-sol injected into blood. J Mater Sci Mater Med 11: 67-72.
- Huang J, Best SM, Bonfield W, Brooks RA, Rushton N, et al. (2004) In vitro assessment of the biological response to nano-sized hydroxyapatite. J Mater Sci Mater Med 15: 441-445.
- Bauer IW, Li SP, Han YC, Yuan L, Yin M (2008) Internalization of hydroxyapatite nanoparticles in liver cancer cells. J Mater Sci Mater Med 19: 1091-1095.
- Hu S, Li S, Yan Y, Wang Y, Cao XY (2005) Apoptosis of cancer cells induced by HAP nanoparticles. J Wuhan Univ Technol Mater Sci Ed 20: 13-15.
- Hu J, Liu ZS, Tang SL, He YM (2007) Effect of hydroxyapatite nanoparticles on the growth and p53/c-Myc protein expression of implanted hepatic VX2 tumor in rabbits by intravenous injection. Gastroenterol 13: 2798-2802.
- Pezzatini S, Solito R, Morbidelli L, Lamponi S, Boanini E, et al. (2006) The effect of hydroxyapatite nanocrystals on microvascular endothelial cell viability and functions. J Biomed Mater Res A 76: 656-663.
- Abdel- Gawad EI, Awwad SA (2011) In-vivo and in-vitro Prediction of the Efficiency of Nano-Synthesized Material in Removal of Lead Nitrate Toxicity. J Am Sci 7: 105-119.
- Xu Z, Sun J, Liu C, Wei J (2009) Effect of hydroxyapatite nanoparticles of different concentrations on rat osteoblast. Mater Sci Forum 610-613: 1364-1369.
- Hussain NS, Gomes PS, Fernandes MH, Lopes MA, Santos JD (2009) Assessment of the osteoblastic cell response to a zinc glass reinforced hydroxyapatite composite (Zn-GRHA). IJNBM 2: 100-109.
- Abdel-Gawad EI, Awwad S (2010) Biocompatibility of intravenous nanohydroxyapatite in male rats. Nat and Sci 8: 60-68.
- Ali BH, Ramkumar A, Madanagopal TT, Waly MI, Tageldin M, et al. (2014) Motor and behavioral changes in mice with cisplatin-induced acute renal failure. Physiol Res 63: 35-45.
- McWhinney SR, Goldberg RM, McLeod HL (2009) Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther 8: 10-16.
- Brock PR, Knight KR, Freyer DR, Campbell CM, Steyger PS (2012) Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. J Clin Oncol 30: 2408-2417.
- Fahmy EK, Edrees HM (2014) Protective Effect of Exendin-4 (Glp-1 Analogue) in acute kidney injury in experimental animals. Journal of Health Science 4: 64-71
- Zhang B, Ramesh G, Norbury CC, Reeves WB (2007) Cisplatin-induced nephrotoxicity is mediated by tumour necrosis factor-alpha produced by renal parenchymal cells. Kidney Int 72: 37-44.
- Lu LH, Oh DJ, Dursun B, He Z, Hoke TS, et al. (2008) Increased macrophage infiltration and fractalkine expression in cisplatin-induced acute renal failure in mice. J Pharmacol Exp Ther 324: 111-117.
- Bortner CD, Oldenburg NB, Cidlowski JA (1995) The role of DNA fragmentation in apoptosis. Trends Cell Biol 5: 21-26.
- Ono K, Matsumori A, Furukawa Y, Igata H, Shioi T, et al. (1999) Prevention of myocardial reperfusion injury in rats by an antibody against monocyte chemotactic and activating factor/monocyte chemoattractant protein-1. Lab Invest 79: 195-203.
- Stejskal D, Karpísek M, Humenanska V, Hanulova Z, Stejskal P, et al. (2008) Lipocalin-2: development, analytical characterization, and clinical testing of a new ELISA. Horm Metab Res 40: 381-385
- Tietz NW (1990) Clinical Guide to Laboratory Test. 2nd Ed, W.B. Saunders company, Philadelphia, 554-556.
- Banchroft JD, Stevens A, Turner DR (1996) Theory and practice of histological techniques, 4th ed. Churchill Livingstone, New York.
- Lee S, Kim G (2002) Characteristics and densification behaviour of anorthite powder synthesized by a solution process employing a polymer carrier. J of Ceramic Processing Research 3: 136-140.
- Qi ZT, Cao XY, Ran Y (2000) The effect of nano apatite on the expression of telomerase gene of human hepatocarcinoma. Wihan Ligong Daxue Xebao 26: 46-48.
- O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL (2004) Photothermal tumour ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett 209: 171-176.
- Mohammed M, Abdel-Gawad E, Awwad S, Kandil E, El-Agamy B (2016) Therapeutic role of a synthesized calcium phosphate nanocomposite material on hepatocarcinogenesis in rats. Biochem Cell Biol 94: 279-288.
- Zhi-Xin W, Yi H, Wei H, Kai-Chen W, Bao-Feng G, et al. (2011) Effects of hydroxyapatite nanoparticles on apoptosis and invasion of human renal cell carcinoma 786-0 cells. Chem Res Chinese Universities 27: 94-98.
- Katsarava DA, Makhalova Z, Liedert BJ (2007) Repair capacity for platinum-DNA adducts determines the severity of cisplatin-induced peripheral neuropathy. J Neurosci 27: 9451-9457.
- Dugbartey GJ, Peppone LJ, de Graaf IAM (2016) An integrative view of cisplatin-induced renal and cardiac toxicities: molecular mechanisms, current treatment challenges and potential protective measures. Toxicology 371: 58-66.
- Townsend D, Tew KD, Lin H, King JB, Hanigan MH (2009) Role of glutathione-S-transferase Pi in cisplatin-induced nephrotoxicity. Biomed & Pharmacother 63: 79-85.
- Jiang M, Wang CY, Huang S, Yang T, Dong Z (2009) Cisplatin-induced apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. Am J Physiol Renal Physiol 296: 983-993.
- Ramesh G, Kimball SR, Jefferson LS, Reeves WB (2007) Endotoxin and cisplatin synergistically stimulate TNF-alpha production by renal epithelial cells. Am J Physiol Renal Physiol 292: 812-819.
- Dong Z, Atherton SS (2007) Tumor necrosis factor-alpha in cisplatin nephrotoxicity: a homebred foe. Kidney Int 72: 5-7.
- Cohen SM, Lippard SJ (2001) Cisplatin: from DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol 67: 93-130.
- Siddik ZH (2003) Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22: 72657-7279.
- Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, et al. (2011) Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol 22: 902-913.
- Miller RP, Tadagavadi RK, Ramesh G, Reeves WB (2010) Mechanisms of Cisplatin nephrotoxicity. Toxins 2: 2490-2518.
- Devarajan P (2005) Novel biomarkers for the early prediction of acute kidney injury. Cancer Therapy 3: 477-488.
- Wei Q, Dong G, Franklin J, Dong Z (2007) The pathological role of Bax in cisplatin nephrotoxicity. Kidney Int 72: 53-62.
- Pabla N, Dong Z (2008) Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int 73: 994-1007.
- Ozkok A, Edelstein CL (2014) Pathophysiology of cisplatin-induced acute kidney injury. Biomed Res Int 2014: 17.
- Rydén L, Omar O, Johansson A, Jimbo R, Palmquist A, et al. (2017) Inflammatory cell response to ultra-thin amorphous and crystalline hydroxyapatite surfaces. J Mater Sci Mater 28: 9.
- Albrecht C, Scherbart AM, van Berlo D, Braunbarth CM, Schins RPF, et al. (2009) Evaluation of cytotoxic effects and oxidative stress with hydroxyapatite dispersions of different physicochemical properties in rat NR8383 cells and primary macrophages. Toxicol In Vitro 23: 520-530.
- Fouda MFA, Nemat A, Gawish A, Ayman R, Baiuomy AR (2009) Does the coating of titanium implants by hydroxyapatite affect the elaboration of free radicals. An Experimental Study. Aust J Basic and Appl Sci 3: 1122-1129.
- Iyer SS, Cheng G (2012) Role of Interleukin 10 Transcriptional Regulation in Inflammation and Autoimmune Disease. Crit Rev Immunol 32: 23-63.
- Palipoch S, Punsawad CJ (2013) Biochemical and histological study of rat liver and kidney injury induced by cisplatin. Toxicol Pathol 26: 293-299.
- Kamel KM, Fawzy HM, Metwally SA, Abd El-Latif HA, El-sayed ME (2015) Protective effects of onion oil and selenium against cisplatin-induced nephrotoxicity and oxidative stress in rats. Egypt J Hosp Med 58: 18-25.
- Oronsky B, Caroen SS, Oronsky AA, Dobalian VE, Oronsky N, et al. (2017) Electrolyte disorders with platinum-based chemotherapy: mechanisms, manifestation, and management. Cancer Chemother Pharmacol 80: 895–907.
- Arunkumar PA, Viswanatha VL, Radheshyam N, Mukund H, Belliyappa MS (2012) Science behind cisplatin-induced nephrotoxicity in humans: A clinical study. Asian Pac J Trop Biomed Aug 2: 640–644.
- Zhou C, Hong Y, Zhang X (2013) Applications of nano structured calcium phosphate in tissue engineering. Biomater Sci 1: 1012-1028.








