Abstract
Despite most pregnant women safely tolerating heart diseases, they remain a leading cause of non-obstetric maternal mortality. Peripartum cardiomyopathy (PPCM) is a serious heart muscle disease caused or aggravated by pregnancy. It imposes significant risks for the development of pregnancy-associated heart failure with an elevated risk of fetomaternal morbidity and mortality. Its incidence has significance geographic variations with considerably higher rates in developing countries. With increased efforts to improve fetomaternal health in disadvantaged populations, there is need to improve the understanding of PPCM including its risk factors, diagnosis and clinical management. This review accumulates current evidence on research and clinical status as well as conducts a meta-analysis of diagnosis and clinical management to advance understanding.
Key-words
peripartum cardiomyopathy, pregnancy-associated heart failure, fetomaternal health
Introduction
Cardiac diseases are present in 1-4% of pregnancies and are a leading cause of non-obstetric maternal mortality [1]. Early diagnosis and appropriate management enable majorities of women to tolerate most cardiac diseases and safely come to term. However, some cardiac conditions such as peripartum cardiomyopathy (PPCM) are high-risk and an important cause of fetomaternal mortality [2,3]. Despite the high risk, a complete understanding of risk factors, etiopathogenesis and diagnosis of PPCM remains tenuous. This paper reviews published studies on PPCM with an emphasis on clinical definition, epidemiology, risk factors, clinical presentation, prognosis, etiopathogenesis, differential diagnosis and clinical management. The aim is to advance clinical knowledge and understanding of PPCM to improve diagnostic and therapeutic management.
Description
Historical Context
The seminal reference to PPCM in medical literature, described as idiopathic myocardial failure with onset of symptoms in early puerperium, was in 1849 by Ritchie in the article, “Clinical contributions to the pathology, diagnosis, and treatment of certain chronic diseases of the heart” [4]. Later in 1880, Virchow and Porak also described an association between heart failure and early puerperium in the absence of determinable cardiac disease [5]. However, formal recognition of PPCM as a distinct clinical entity was in 1937, when Gouley, et al. [6], and Hull and Hafkesbring [7] described an idiopathic origin of postpartal toxic heart disease in previously healthy women.
In 1971, Demakis and Rahimtoola [8] proposed the terminology peripartum cardiomyopathy in place of postpartum cardiomyopathy, which excluded patients with onset of symptoms antepartum. The duo also proposed three diagnostic criteria for PPCM: (i) has onset of acute heart failure in the last month of pregnancy or within five months postpartum; (ii) has an idiopathic origin; and (iii) develops in the absence of any determinable heart disease [8,9]. Later in 1999, Hibbard, Lindheimer and Lang [10] proposed an additional diagnostic criterion based on echocardiographically defined left ventricular (LV) systolic dysfunction as LV ejection fraction (LVEF) of < 45% and LV enlargement. More recently, Elkayam, et al. [11] described 23 cases of idiopathic dilated cardiomyopathy (DCM) with onset of symptoms earlier than the last gestational month but indistinguishable from classic PPCM (occurring in the last month of pregnancy or early puerperium).
PPCM has had several clinical definitions. Although all the definition agrees that PPCM occur a few weeks prior to and after pregnancy, they differ in the description of the actual condition. It is a rare form of cardiomyopathy presenting with LV systolic dysfunction and idiopathic heart failure (HF) [12]; acute heart failure in the absence of heart diseases prior to the last month of pregnancy [13]; and idiopathic cardiomyopathy manifesting with HF secondary to LV systolic dysfunction [14] (Table 1).
Table 1. Clinical Definition of PPCM based on Regional and National Health Bodies.
Source (Health Organization) |
Clinical Definition |
The American Heart Association (AHA) on contemporary definitions and classification of the cardiomyopathies [12] |
A rare dilated form of cardiomyopathy associated with LV systolic dysfunction and idiopathic heart failure presenting in the third trimester of pregnancy or within five months postpartum and whose diagnosis requires a high index of suspicion. It frequently affects obese and multiparous women with preeclampsia aged > 30 years |
The National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) Workshop [13] |
Defines PPCM based on the four criteria proposed by Demakis, et al. [9] and Hibbard, et al. [10]. PPCM is acute heart failure developing in the last gestational month or within five months postpartum in the absence of demonstrable heart disease prior to the last month of pregnancy characterized by echocardiographically defined LV systolic dysfunction |
The Heart Failure Association of the European Society of Cardiology (ESC) Working Group on peripartum cardiomyopathy [14] |
Idiopathic cardiomyopathy manifesting with heart failure secondary to LV systolic dysfunction towards the end of pregnancy or within five months after delivery and diagnosis is often always by exclusion. Left ventricle (LV) may or may not be dilated but often always LV ejection fraction is reduced <45% |
Despite the definitional variations across regional and national health organizations, there is a unanimous consensus that PPCM is a clinical entity (heat muscle failure) distinct from pre-existing forms of cardiomyopathies, facilitated or aggravated by pregnancy-induced stress and diagnosed primarily through the exclusion of other cardiac diseases [12-14]. Taking together the definitions, PPCM may be described as an idiopathic myocardial condition with onset of acute heart failure in the third trimester of pregnancy or five months postpartum with echocardiography demonstrated LV systolic dysfunction (LVEF < 45% and enlarged LV).
The exact incidence of PPCM remains poorly defined chiefly due to the lack of population-based estimates and universal diagnostic criteria. The existing small-scale population-based studies may also suffer from dependence on the experience of the institution, referral bias or individual practice patterns that could potentially underreport incidence rate but otherwise provide important estimates of incidence of PPCM [3,13]. The reported incidence rates have large temporal and geographic variations ranging between 1 in 1,485 [15] and 1 in 15,000 [16]. The rates are higher in developing countries such as Nigeria 1 in 100 [17] and Haiti 1 in 300 [18] compared to more developed countries such as South Africa at 1 in 1,000 [19] and the U.S. at 1 in 3-4,000) [20]. In support, several studies on incidence of PPCM since 1985 with a sample of more than 25 patients, using either or both clinical tests and echocardiography-defined LV systolic dysfunction as diagnostic criteria indicate incidence of PPCM ranges between 300 and 4,025 per 10,000 persons [18,21-27] (Table 2).
Table 2. Summary of Studies on Incidence of PPCM.
First Author [Ref #] |
Study Period |
Country |
Type |
Design |
Incidence |
Mean Age |
Mielniczuk, et al. [22] |
1990-2002 |
U.S. |
PB |
R |
1:3,189 |
30 |
Brar, et al. [23] |
1996-2005 |
U.S. |
PB |
R |
1:4,025 |
33 |
Fett, et al. [18] |
2000-2005 |
Haiti |
CS |
P |
1:300 |
32 |
Chapa, et al. [24] |
1988-2001 |
U.S. |
CS |
P |
1:1,149 |
27 |
Desai, et al. [25] |
1986-1989 |
S. Africa |
CS |
P |
1:1,000 |
29 |
Witlin, et al. [26] |
1986-1994 |
U.S. |
CS |
R |
1:2,406 |
-- |
Gunderson, et al. [27] |
1995-2004 |
U.S |
CS |
R |
1:2,066 |
-- |
PB: Population-based study; CS: Case Series single center study; R: Retrospective study; P: Prospective study
Risk Factors
Several risk factors have been associated with the development of PPCM but many have not been evaluated in epidemiology studies and their contribution to the development of PPCM remains inconclusive [21,28]. Risk factors can be classified as probable, proposed or emerging (Figure 1).
Figure 1. Risk Factors for the Development of PPCM [28].
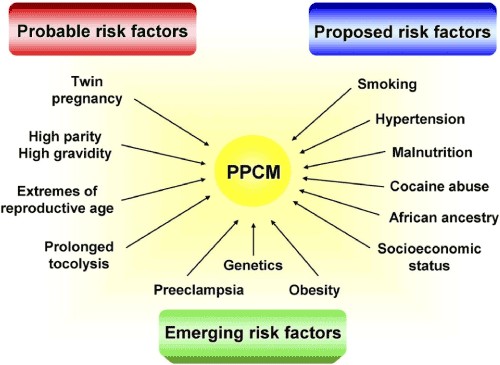
Risk factors for the development of PPCM have been classified as probable (twin pregnancy, high parity/gravidity, extreme reproductive age and prolonged tocolysis); proposed (smoking, hypertension, malnutrition, cocaine use, African ancestry and socio-economic status); and emerging (genetics, pre-eclampsia and obesity).
Probable/Independent
Probable risk factors for the development of PPCM include extreme maternal age greater than 30 years, multiparous women, multiple gestations, presence of pre-eclampsia or prolonged tocolytic therapy [11,13,29]. In a systematic review and meta-analysis of 22 studies (N=979), Bello, et al. [30] find the prevalence of both pre-eclampsia and multiparity in PPCM women is significantly higher than that found in the general population strongly suggesting shared pathogenesis. The study also finds hypertension and multiple gestations are strong risk factors for developing PPCM. In a case-series epidemiology study in Northern California delivery hospitals, Gunderson, et al. [27] supports that maternal age greater than 25 years, multiparity, multiple gestation and hypertensive disorders are independent risk factors for the development of PPCM. However, in a recent U.S study, Elkayam, et al. [11] showed statistically insignificant association between multiparity and the development of PPCM where 40% of PPCM incidence occurred in the first pregnancy and greater than 50% in the second pregnancy.
Proposed risk factors for PPCM include smoking, hypertension, malnutrition, ethnicity (mainly African descent), cocaine abuse and socio-economic status. They are proposed because they require additional studies to determine whether they have an independent effect or derive their effect from interaction between race, hypertension and low social-economic status. Genetics and obesity are emerging risk factors whose contribution to the development of PPCM is still under investigation [28]. In a study of 115 German cohort of PPCM women, Haghikia, et al. [31] found a positive family history of cardiomyopathy in 16.5% but did not suggest it has an independent effect on the development of PPCM. Additional systematic studies on genetic basis of PPCM would provide important insight into the role of genetics in the development of PPCM. In another case-controlled study in Georgia, U.S., Gentry, et al. [32] found African-American women are 15.7 more likely to develop PPCM compared to non-African American, which could not be explained by other factors. However, Elkayam, et al. [11] showed a weak association between race (African American) and the development of PPCM. Additional large-scale studies or meta-analysis studies are warranted to determine whether the presence of genetic or environmental factors in African women could underlie or explain the higher risk. Clearly identifying risk factors for the development of PPCM has important clinical implications in developing recommendations for screening high-risk population [33].
Clinical Presentation
Onset of symptoms
The onset of clinical symptoms in PPCM patients varies significantly but a majority (78%) develop symptoms in the first four months postpartum, 9% in the last gestational month and 13% either one month prior to delivery or more than four months after delivery [15]. In a few countries such as South Africa, some patients have the onset of PPCM symptoms later than five months after delivery but have not been included in any study because they do not fall within the accepted clinical definition of PPCM [14].
Signs and Symptoms
The initial signs and symptoms of PPCM mimic those of normal physiology in pregnancy such as pedal edema, dyspnea on exertion, orthopnea, paroxysmal nocturnal dyspnea, and persistent cough [14] (Table 3).
Table 3. Summary of Clinical Signs and Symptoms Associated with PPCM [28].
Clinical Symptoms |
Clinical Signs |
|
|
|
|
|
|
|
- Third heart sound/Gallop rhythm
|
- Paroxysmal nocturnal dyspnea
|
- New murmur (mitral/tricuspid regurgitation)
|
|
- Lateral/downward displacement of point of maximal impulse
|
|
- Jugular venous distention
|
- Decreased exercise tolerance
|
|
|
|
|
|
|
|
These symptoms are also indistinguishable to those of heart failure in the setting of idiopathic dilated cardiomyopathy [21]. The similarity of symptoms could delay or even miss the diagnosis of PPCM potentially leading to the development of preventable major adverse cardiac events requiring cardiac transplantation or in rare cases culminating in cardiac death. PPCM patients also frequently present with cough, chest pain and abdominal pain, which complicates initial clinical assessment [28].
Recovery (or normalization) of LV function is defined as LVEF ≥ 50% or an improvement > 20% [3]. Unlike other forms of non-ischemic cardiomyopathies, a majority of PPCM patients have a spontaneous recovery of LV function [34]. Several studies have reported 23% to 54% of PPCM patients recover between three and six months postpartum and over 70% by 12 months postpartum [11,18,34-36]. About 10% take up to 48 months to recover while fewer (with treatment) have a good LV function in the range 30-49% and 2% may require heart transplantation [37]. Even after full recovery, PPCM patients have a high risk of recurrence in subsequent pregnancies with a marked decline of LVEF [38]. In a U.S. study of 44 PPCM patients with subsequent pregnancies, LVEF dysfunction was more severe in those with partial recovery (44%) compared to those with complete recovery (21%) [39]. More patients with a baseline LVEF < 55% have PPCM recurrence in subsequent pregnancies than those with LVEF ≥ 55% (17%) [40]. Whereas exercise stress echocardiography can uncover subtle residual LV dysfunction that subsequent pregnancy can exacerbate [40], current guidelines recommend against future pregnancies [14].
Echocardiographically defined LV functional properties are the most frequently researched predictors of recovery in PPCM patients. Baseline LV ejection fraction (LVEF) > 30% has been shown to predict favorable prognosis [11]. In a study of South African women diagnosed with PPCM, multiple regression analysis reveals after adjustment of New York Heart Association (NYHA) functional class, ethnicity (African American), LVEF and LV end-diastolic dimension (LVEDD), smaller LV end-systolic dimension (LVESD) (Odds Ratio: OR 1.08, 95%: CI 1.01-1.17) and older age (OR 0.92, 95%: CI 0.86-0.98) are independent predictors of favorable prognosis [41]. Patients with baseline LVEF ≥ 30% are five times more likely to completely recover LV function compared to those with LVEF < 30% [42]. Other studies reveal LVEDD [26,43], higher LVEF [35,44], higher fractional shortening [42] and the absence of thrombus [26] may predict better LV recovery. On the other hand, high troponin levels and LVEDD > 5.6 cm, late diagnosis > one week, high NYHA functional class, African American, multiparity and co-existing illnesses predict poor LV recovery [11,32,42,45]. Patient with low-level recovery often require heart transplantation [40].
Race, especially African women, have been suggested to be an independent predictor of mortality. Blauwet, et al. [41] find 13% mortality rate among African women diagnosed with PPCM. In two other studies [35,36], univariate analysis reveals the main predictors of PPCM-related mortality were the extent of LV enlargement and higher NYHA functional class at baseline. Other emerging predictors of mortality were younger age at diagnosis and lower body mass index (BMI). However, the low number of deaths was insufficient to conduct multiple regression and thus studies recruiting a large sample are warranted to validate whether race, LV enlargement, NYHA functional class, young age and lower BMI are predictors of mortality in PPCM patients [41].
The exact etiopathogenesis of PPCM remains incompletely understood but several hypotheses have been advanced to explain etiologic or pathologic processes underlying PPCM. These include stress-activated cytokines, unbalanced oxidative stress, myocardial inflammation, pathological maternal autoimmune response, genetics factors and pathological response to pregnancy-associated hemodynamic stress.
Sliwa, et al. [46] discovered high concentration of serum markers of inflammation including cytokines such as tumor necrosis factor alpha (TNFα), interferon gamma, interleukin-6 (IL-6), C-reactive protein (CRP) and apoptosis antigen 1 to suggest inflammatory processes underlie pathophysiologic development of PPCM [47]. However, it is not known whether the high concentration of cytokines is reactive or causative [28].
Pregnancy normally induces pathophysiologic changes characterized with increases in maternal antioxidant defense mechanism [47]. However, PPCM patients have higher levels of oxidative stress evident in elevated (unbalanced) concentration of oxidized low-density lipoprotein [48]. Unbalanced oxidative stress mediates the cleavage of prolactin hormone into 16kDa increasing its antiangiogenic and pro-apoptotic properties that destroys cardiovascular tissues [48]. This finding forms the basis of the suggestion that blocking the splitting of prolactin hormone may confer a therapeutic value for PPCM [21].
Reports of a majority of PPCM patients having viral or antigen-induced myocardial inflammation contributed to the hypothesis that it may contribute to pathophysiologic mechanisms of PPCM. However, the link between PPCM and myocardial inflammation has remained inconclusive [3]. The incidence of myocarditis in PPCM patients ranges between 29% and 100% [49]. The explanation for the wide variation remains unknown but Rizeq, et al. [50] theorizes that the cause could be endomyocardial biopsy only detects myocarditis when performed at or closer to the onset of PPCM, performed at the wrong site or misdiagnosis of another form of heart failure.
Maternal immune system usually destroys fetal microchimeric cells escaping into the maternal circulation system [51]. However, due to a weakened maternal immune system during pregnancy, some of the fetal microchimeric cells may evade destruction and accumulate in the maternal heart. During puerperium, the maternal immune system normalizes and triggers a pathological immune response to fetal microchimeric cells deposited on the heart [52]. Ansari, et al. [51] report high concentration of antibodies directed at cardiac myosin heavy chains in the serum of PPCM patients but absent in idiopathic DCM patients or in healthy individuals [51]. However, a causal relationship between the high concentration of antibodies and PPCM has not been established [3].
Few data, largely case studies, have been published to evaluate genetic basis of PPCM. High incidences in certain geographical regions supports the hypothesis that genetic predisposition may have an important role in the pathophysiology of PPCM [29]. Further, accumulating research evidence indicate a subset of PPCM patients have mothers or sisters also with PPCM [53-57] or have genetic mutations – MYBPC3, MYH6, MYH7, SCN5A, TNNC1 and TNNT2 – associated with dilated cardiomyopathy, suggesting overlapping clinical spectrum of the two cardiomyopathy phenotypes [57-59]. However, additional randomized controlled trials are warranted to establish the genetic basis of PPCM, which will pave way for genetic screening of PPCM patients.
Pathological Response to Hemodynamic Stress
Hemodynamic changes during pregnancy leads to an exaggerated decrease in LV systolic function. Plasma volumes go up by 50% and the mass of red cells increases by 20%, decreased blood pressure and increased heart rate, decreased pulmonary/systemic resistances and increased cardiac output [60]. These hemodynamic changes peak during the second and third trimester of pregnancy and fully resolves around three months postpartum [28]. Echocardiography findings in normal pregnancy reveal LVED volume increases by 10% resulting into transient LV wall thickening and reversible LV systolic dysfunction from the second and third trimester and early puerperium [28].
Diagnosis
Diagnosis Pathway
The American Heart Association (AHA) statement on current diagnostic and treatment strategies for specific dilated cardiomyopathies recommend confirmatory diagnosis of PPCM should involve several methods including full patient history, physical examination and diagnostic testing to exclude previous history of cardiac disease and other cardiomyopathies. The initial clue for PPCM diagnosis is patient history confirming the onset of HF began in the last months of pregnancy or months after pregnancy, and cardiac evaluation should confirm LV dysfunction. The usual laboratory assessment for cardiomyopathies such as chest radiograph, ECG and assessment for cardiac biomarkers [12].
Diagnosis pathway for PPCM begins with patient history to identify symptoms of heart failure in the third trimester or the first few weeks postpartum. Initial clinical examination usually excludes hypertension then considers echocardiographic tests for LV dysfunction defined by LVEF ≤ 45% [12. Additional tests including chest x-ray, electrocardiography and lab tests could assist to confirm PPCM diagnosis. Other tests such as cardiac MRI are useful when echo findings are non-diagnostic while biopsy are useful for patients with ventricular tachycardia or high-grade heart failure, or patient’s refractory to optimal medical therapy [28] (Figure 2).
Figure 2.Differential Diagnostic Workup of PPCM [28].
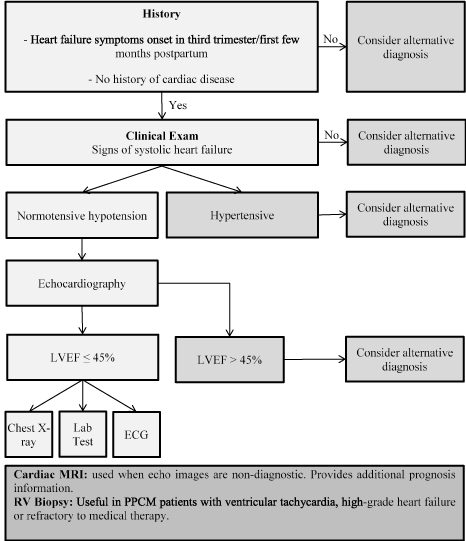
Diagnostic work-up of PPCM begins with examining patient history to find the onset of HF symptoms, then a confirmation of systolic HF using clinical examination and LV dysfunction using echocardiography imaging. Additional tests such as chest X-ray excludes other etiologies of HF symptoms including severe anemia, thyroid or liver disease; laboratory tests in select cases to exclude other etiologies; and ECG to exclude other etiologies such as CAD or thrombosis. Alternative diagnosis such as cardiac MRI are useful when echocardiography is non-conclusive and RV biopsy for patients with ventricular tachycardia or refractory to treatment.
If initial tests (clinical examination and echocardiography) do not provide a confirmatory diagnosis of abnormalities in cardiac functions, differential diagnosis should be considered to exclude other cardiac and non-cardiac conditions that share similar symptoms with PPCM. Frequently encountered heart etiologies for differential diagnosis include congenital heart disease or acquired disorders including LV dysfunction due to myocardial infarction, valvular heart disease or hypertension [14,61]. Table 4 summarizes frequently encountered cardiac and non-cardiac conditions mimicking PPCM, the relevant clinical tests and results to underpin the need for differential diagnosis.
Table 4. Differential Diagnosis of PPCM [14].
Condition |
Diagnostic Tests |
Findings |
Pre-existing idiopathic DCM unmasked by pregnancy. |
History, electrocardiography, BNP and echocardiography. |
PPCM presents postpartum while DCM in the second trimester. |
Pre-existing familial DCM unmasked by pregnancy |
Patient history, family screening, electrocardiography, echocardiography and genetic testing. |
PPCM presents postpartum while familial DCM in the second trimester. |
Cardiomyopathy due to HIV/AIDS |
HIV test |
HIV cardiomyopathy presents with non-dilated ventricles. |
Pre-existing valvular heart disease unmasked by pregnancy |
Patient history, physical examination, ECG and Echo. |
PPCM presents postpartum while valvular disease in the second trimester. |
Hypertensive heart disease |
-- |
Exclude hypertension presenting before delivery. |
Pre-existing idiopathic congenital heart disease |
Patient history, ECG and Echo. |
PPCM presents postpartum while congenital heart disease in the second trimester. |
Pregnancy-associated myocardial infarction |
History, ECG, BNP, coronary angiography and Echo |
Patient history |
Pulmonary embolus |
History, ECG, echo, perfusion scan, CT and pulmonary angiography. |
Patient history |
Differential Diagnosis
Biomarkers for Diagnosis
PPCM symptoms may overlap with those of heart failure and of normal pregnancy to delay a definitive differential diagnosis. These symptoms include exertional shortness of breath, nocturnal dyspnea, palpitations, fatigue, cough and pedal edema. In addition, specialists other than cardiologists usually see PPCM patients, which further complicates diagnosis [61,62]. Despite these complications, biomarkers provide important information that raise the suspicion of PPCM and inform referrals to expert physicians for additional diagnostic evaluation. Table 5 provides a summary of the major biomarkers screened of PPCM patients and their relevance in the diagnosis of PPCM.
Table 5. Biomarkers Raising Suspicion of PPCM [62].
Biomarker |
Relevance for PPCM |
NT-proBNP |
Commercial marker for screening of PPCM but non-specific – also elevated in preeclampsia, pulmonary embolism and heart failure. |
16-kDa Prolactin |
Pathophysiologic factor for PPCM but diagnostic accuracy requires confirmation |
Interferon Alpha |
Elevated plasma levels in PPCM patients but diagnostic accuracy requires further evaluation |
Asymmetric Dimethylarginine |
Marker for endothelial dysfunction and cardiac risk. |
Cathepsin D |
Elevated activity in plasma of PPCM patients |
Soluble fms-like tyrosine kinase-1 |
Elevated activity in plasma of PPCM patients |
MicroRNA-146a |
Pathophysiologic factor for PPCM but diagnostic accuracy requires further evaluation |
Electrocardiogram
Electrocardiogram (ECG) should be performed when symptoms of heart failure are present or suspected to identify and rule out pulmonary embolism or acute ischemic event [3]. Although pothognomic ECG patterns have not been identified for PPCM patients, wide ranges of ECG abnormalities are common in PPCM patients [63]. The most frequently encountered ECG abnormalities are LV hypertrophy and ST-T wave abnormalities [8]. A small subset of PPCM patients may also have intraventricular conductance abnormalities such as left bundle branch block (LBBB). Other cardiac complications such as atrial fibrillation, atrial flutter, prolonged PR, and QRS intervals may be detected in some PPCM patients [3].
The use of cardiac protein assays in the diagnosis of PPCM requires further clinical evaluation. However, it shows promising results for determining diagnosis and prognosis of PCM [3]. B-type Natriuretic peptide (BNP) tests for systolic dysfunction. In PPCM patients, systolic dysfunction causes an elevated LV-end diastolic pressure leading to increased plasma levels of BNP or N-terminal pro-BNP (NT-proBNP) [64]. In analysis of 38 PPCM patients, Sliwa, et al. [14] find all had elevated NT-proBNP (mean = 1727.2fmol/mL) compared to 21 healthy women (mean = 339.5 fmol/mL).
Whereas diagnosis of PPCM is by exclusion, echocardiography is the current gold standard for diagnostic confirmation and prognostication of PPCM. The modality assesses and monitors regional and global ventricular function based on onset of HF, no pre-existing heart condition, idiopathic and echocardiography findings (LVEDD > 2.7 cm/m3, LVEF < 45% and M-mode fractional shortening < 30% (Table 6).
Table 6. Diagnostic Criteria for PPCM [10].
Criterion |
Clinical/Echocardiographic Description |
1 |
Onset of heart failure in the last gestational month or 5 months postpartum |
2 |
No demonstrable pre-existing heart disorder |
3 |
Idiopathic (no determinable cause) |
4 |
Echocardiography findings: (a and b or c, or all a-c) |
- LVEDD > 2.7 cm/m3
|
- M-mode fractional shortening < 30%
|
- LVEF < 45%
|
LV: Left Ventricular; LVEF: Left Ventricular Ejection Fraction: LVEDD: Left Ventricular End Diastolic Dimension
Echocardiography tests should be performed in all suspected cases of PPCM. It obviates the need for invasive procedures such as cardiac catheterization and endomyocardial biopsy [3] but in some rare cases, invasive procedures may be required to obtain additional information about ischemic causes of heart failure [62,65]. The Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy recommends repeat echocardiography at 6 weeks and 6 months to obtain prognostic information.
Chest X-Ray
Chest x-ray visualizes cardiomegaly in the setting of LV enlargement and/or pericardial effusion. Chest x-ray may be useful to assess and exclude other etiologies for heart failure symptoms such as pneumonia and pneumothorax [28].
Cardiac MRI
Cardiac Magnetic Resonance Imaging (MRI) is usually indicated for women having symptoms of heart failure to determine diagnosis, and in the case of PPCM, to provide prognostic information [14]. Cardiac MRI provides more detailed information than echocardiography about myocardial morphology and RV function [66]. Cardiac MRI T2-weighted spin echo sequence helps in the precise diagnosis of myocardial inflammation, cell necrosis, the presence of LV thrombus and provides accurate quantification of ventricular volumes [62]. Cardiac MRI also assists in the identification of sites for endomyocardial biopsy. However, the European Society of Radiology contraindicates contrast-enhanced cardiac MRI during prepartum because it crosses the placenta and may harm the developing fetus [3].
Meta-Analysis of Diagnostic/Prognostic Methods
Search Strategy
Studies investigating diagnosis and prognosis of PPCM were searched from three electronic databases (PubMed, Embase, and Cochrane) and Google Scholar. Broad search terms, “peripartum cardiomyopathy” OR “pregnancy associated cardiomyopathy” AND “diagnosis” OR “prognosis” were used to ensure all relevant studies were identified. Studies were included if they were (a) prospective or retrospective; (b) relied on echocardiography-defined LV dysfunction for diagnosis; (c) had a follow-up period of at least 6 months; and (d) had at least one outcome assessing predictors of prognosis (recovery of LV function), mortality, heart transplantation and predictors of mortality. Studies were excluded if (a) only abstract was available (difficult to extract data); (b) were conference papers (they are not final since they may be subjected to revision) and (c) were case series (studies one or a few cases of PPCM and thus insufficient for statistical analysis). Additional studies were included from a review of bibliographies of included studies. Each study included in the meta-analysis was read and data extracted on a Microsoft Excel Worksheet. The extracted data was name of first author, study period, number of patients, diagnosis criteria used, LV recovery (%), predictors of LV recovery, mortality rate, heart transplantation rate and predictors of mortality (Table 7). [67-74].
Table 7. Major Studies on Diagnosis and Prognosis of PPCM at 6 Months Follow-up.
1st Author [Ref. #] |
Study Period |
No. of Patients |
Diagnosis Criteria |
LV Recovery (LVEF > 50%) (%) |
Predictors of LV Recovery |
Mortality (%) |
Heart Transplantation (%) |
Predictors of Mortality |
Fett, et al. [18] |
2000-2005 |
92 |
LVEF < 45% |
32 |
LVEF ≥ 30%;
FS ≥ 20% |
15.3 |
-- |
-- |
Brar, et al. [23] |
1996-2005 |
60 |
LVEF < 50% |
-- |
-- |
3.3 |
-- |
-- |
Chapa, et al. [24] |
1988-2001 |
32 |
FS < 30%; LVEDD ≥ 4.8 cm |
41 |
LVEDD ≥ 6cm; FS > 20% |
9.6 |
6.5 |
-- |
Desai, et al. [25] |
1986-1999 |
99 |
LVEF < 45% |
|
|
14.0 |
|
-- |
Gentry, et al. [32] |
2003-2008 |
28 |
LVEF < 45% |
28 |
Non-African American |
-- |
-- |
-- |
Duran, et al. [35] |
1995-2007 |
33 |
LVEF < 45% |
24 |
LVEF > 27%; LVEDD ≥ 5.5 cm |
10.0 |
6 |
-- |
Sliwa, et al. [36] |
2003-2005 |
100 |
LVEF < 40% |
23 |
LVEF ≥ 30%, LVEDD |
15.0 |
-- |
Fas/Apo-1; NYHA FC |
Goland, et al. [42] |
1994-2004 |
187 |
LVEF < 45% |
61 |
LVEF ≥ 30% |
-- |
-- |
-- |
Amos, et al. [43] |
1990-2003 |
55 |
LVEF < 45% |
45 |
LVEDD > 5.6 cm; LVEF ≥ 30%; No LV thrombus |
0.0 |
10 |
-- |
Modi, et al. [44] |
1992-2003 |
44 |
LVEF < 45% |
35 |
LVEF ≥ 30% |
15.9 |
-- |
-- |
Sliwa, et al. [75] |
2005-2006 |
80 |
LVEF < 30% |
-- |
LVEF ≥ 30% |
10.0 |
-- |
-- |
Goland, et al. [76] |
2009-2011 |
182 |
LVEF < 45% |
49 |
LVEF ≥ 25% |
7.1 |
4.9 |
Age > 30yrs; LVEF ≤ 25%; Delayed diagnosis ≥ 1wk |
Felker, et al. [77] |
1982-1977 |
51 |
LVEF < 45% |
-- |
-- |
7.0 |
-- |
-- |
Mielniczuk, et al. [78] |
1990-2002 |
171 |
-- |
-- |
Younger age, non-African American |
1.4 |
-- |
-- |
Hasan, et al. [79] |
2003-2007 |
32 |
LVEF < 45% |
63 |
-- |
9.3 |
-- |
-- |
Safirstein, et al. [80] |
2005-2007 |
55 |
LVEF < 45% |
-- |
LVEF ≥ 35%; gHTN, breastfeeding; postpartum diagnosis |
-- |
|
-- |
Witlin, et al. [81] |
1986-1994 |
28 |
LVEF < 45% |
-- |
-- |
18.0 |
11 |
-- |
Total/Mean |
|
1329 |
|
40 |
|
9.7 |
7.7 |
|
LVEDD: Left Ventricular End Diastolic Dimension; FS: Fractional Shortening; gHTN: Gestational Hypertension
Study Characteristics
Seventeen (17) studies investigating diagnosis and prognosis of PPCM meeting the inclusion criteria were included in the present meta-analysis [18,23,24,25,32,35,36,42-44,75-81]. In total the recruited patient population in the 17 studies was (N=1,329). The patients were followed for a period of six months or above. One of the common diagnostic criterion in 16 studies was echocardiography-defined LVEF while one study [24] used echocardiography-defined fractional shortening (FS) and LV end diastolic dimension (LVEDD). The main prognostic information assessed were predictors of LV recovery in 10 studies [18,24,32,35,36,42-44,76,79], mortality in 13 studies [18,23-25,35,36,43,44,75-79,81], heart transplantation in five studies [24,35,43,76,81] and predictors of mortality in two studies [36,76].
Diagnosis of the 1,329 PPCM patients was through exclusion of other cardiac and non-cardiac condition but confirmation was through echocardiography-defined LV dysfunction, considered the gold standard of PPCM diagnosis. Whereas LVEF < 45% is considered one of the criterion for PPCM diagnosis, three studies used different LVEF criteria of < 30% [74], < 40% [36] and < 50% [23] suggesting the lack of a universal LVEF criteria. Only one study [24] used fractional shortening and LV end diastolic dimension for PPCM diagnosis. Echocardiography assessed LV dysfunction provided valuable prognostic information for PPCM status and clinical outcomes. Slightly less than half (40%) of the 1,320 patients recovered LV function (defined as LVEF > 50%) within the first six months postpartum. The major predictors of LV recovery were baseline LVEF ≥ 30% indicated by 52% of the studies, baseline LVEDD ≥ 5.5 cm indicated by 24% of the studies, fractional shortening ≥ 20% indicated by 12% of the studies, and non-African American ancestry indicated by 12% of the studies. Other predictors were no LV thrombus, younger age, gestational hypertension, postpartum diagnosis indicated by 6% (1) of the reviewed studies. Mortality rate was low (7.7%) predicted by Fas/Apo-1 plasma levels and NYHA functional class [36], and age > 30yrs, LVEF ≤ 25% and delayed diagnosis ≥ 1wk [76].
Discussion
According to AHA practice guidelines, the cornerstone of the diagnosis of PPCM is the onset of symptomatic HF a few months pre-partum or post-partum complemented with clinical evidence of LV systolic dysfunction [12]. Similarly, the present meta-analysis reveals that, following the initial clinical suspicion of PPCM based on the onset of HF symptoms, echocardiography is the most preferred imaging modality for identifying and characterizing LV dysfunction. However, the accuracy of echocardiography-based diagnosis is limited by the lack of a standardized definition of LV dysfunction. The greater majority of the studies (84%) defined LV dysfunction as LVEF < 45% while the remaining two studies used LVEF < 30% [75] and LVEF < 50% [23]. The variation in the definition of LVEF arises due the lack of published standardized LVEF parameters to define LV dysfunction in PPCM patients. The lack of standardization has implications on the accuracy of diagnosis and on the determination of the precise prevalence of PPCM in expectant or post-partum mothers [13]. In addition to LVEF, one study demonstrated that LV dysfunction in PPCM patients could be quantified using LV fractional shortening and/or LV end-diastolic dimension (LVEDD). However, additional studies are warranted to provide demonstrable evidence of the value of LV fractional shortening and LVEDD in quantifying LV dysfunction in PPCM women.
In addition to diagnosis, echocardiography imaging provides valuable prognostic information on PPCM. Generally, PPCM has a favorable prognosis with slightly over half of the patients having a spontaneous recovering of LV function six months post-partum [34-36]. Consistent with these previous studies, the present findings reveal 40% of the patients recovered their LV function six months after giving birth. The main predictors of LV recovery were LVEF ≥ 30%, LVEDD ≥ 5.5 cm, and LV fractional shortening ≥ 20% at presentation (baseline). In addition to LV recovery, a lower mortality rate (7.7%) indicated a favorable prognosis. However, delayed diagnosis, Fas/Apo-1 plasma levels, NYHA functional class and baseline LVEF ≤ 25% indicated a higher risk for mortality. Consistent with previous studies [11,43] LV functional parameters defined using echocardiography imaging modality is an accurate predictor of PPCM prognosis. Higher LVEF > 30%, LV fractional shortening, LVEDD and LVESD have a statistically significant correlation with subsequent improvement in LV function [42,43]. Although in general PPCM has a low mortality rate, patients African ancestry, very low LVEF < 30%, and higher NYHA functional class suggests a greater risk of death [35,36,41].
In summary, the diagnosis of PPCM is HF symptoms, which appear in the last month of pregnancy or within five months postpartum in the setting of clinically significant LV dysfunction. Cardiac imaging by echocardiography is able to characterize LV dysfunction but confirmatory diagnosis is usually obtained by additional tests including chest X-ray, ECG and laboratory examination to exclude other potential etiologies such as severe anemia, thyroid disease, liver disease, coronary artery disease (CAD) or thrombosis. Cardiac MRI is an alternative diagnostic method when echocardiography is inconclusive as well as provides additional prognostic information.
Clinical Management
PPCM occurs in the setting of pregnancy and clinical management requires a well-coordinated multi-disciplinary team involving cardiovascular drugs, obstetrics, immunology and pathology [62]. According to AHA, guideline-directed medical therapy known to have beneficial outcomes in treating LV dysfunction and heart failure should be considered. However, medication contra-indicated during pregnancy and/or breastfeeding should be considered [12]. In essence, clinical management of PPCM is similar to that of heart failure: relieves congestive symptoms, optimizes hemodynamics and improves acute to chronic prognosis [21].
Pharmacotherapy
Pharmacotherapy for PPCM follows the European Society of Cardiology (ESC) guidelines for management of heart failure [67]. However, restriction (contraindication) to the guidelines during pregnancy include angiotensin converting enzyme (ACE) inhibitors and angiotensin-II receptor blockers (ARB) because of increased risk of serious renal and fetal toxicity [68,69]. Table 8 outlines drugs for management of PPCM, and their maternal and fetal precautions during antepartum and postpartum [3,14,28,70].
Table 8. Drugs for PPCM Management and Their Maternal/Fetal Precaution.
Drug |
Maternal Precautions |
Fetal Precautions |
Loop Diuretic (Furosemide) |
Electrolyte abnormalities, hypotension & azotemia. |
Insufficient uterine/placental perfusion/inhibit lactation. |
Beta-Blockers (Carvedilol/Bisoprolol/ Metoprolol) |
Bradycardia |
Long-term use could cause bradycardia/hypoglycemia/low birth weight |
Peripheral vasodilator (Hydralazine/nitrate) |
Hypotension |
None |
Digoxin |
Arrhythmias |
Serum levels should be monitored to avoid toxicity |
Inotropes (Dobutamine/
Dopamine/Milrinon) |
Arrhythmias |
None |
ACE-Inhibitor/ARBs |
Renal toxicity |
Fetal and renal toxicity anteparturm |
Anticoagulants (Unfractionated heparin/low molecular weight heparin) |
Considered for patients LVEF > 35% |
Consider fetotoxicity/teratogenic of Warfarin during pregnancy. |
Currently, pharmacotherapy lacks consensus on the ideal period for managing heart failure and its prophylactic value in subsequent pregnancies. The optimal clinical approach has been to give medication over time while gradually tampering the dose between 6 to 12 months as clinical and echocardiographic findings show favorable prognosis until complete recovery [3]. Other drugs on experiment but with promising results include immunosuppression (intravenous immunoglobulin), pentoxifylline (inhibit production of TNF-α) and bromocriptine (prolactin blocker) [74].
Mechanical Circulatory Support
Mechanical circulatory support devices have been used as a bridge to recovery or to heart transplantation [71]. Intra-aortic balloon pumps or extracorporeal membrane oxygenation have been used in PPCM patients with cardiogenic shock to restore normal ventricular circulation [3,14]. PPCM patients with deteriorating clinical conditions non-responsive to optimal pharmacotherapy may benefit from left ventricular (LV) assist devices (LVAD) [14]. Since PPCM resolves within six months after delivery, in the case of fulminant PPCM, LVAD could serve as a bridge to recovery [3]. However, when LVEF remains significantly depressed or continues to decline, LVAD may also serve as a bridge to heart transplantation [71]. Recent reports indicate prolonged mechanical circulatory support has decreased PPCM patients seeking heart transplantation from 33% to between 4 and 7% [11].
Implantable Cardioverter-De?brillators
PPCM patients with persistent LV dysfunction six months since onset of the condition may benefit from implantable cardioverter-de?brillator (ICD), or together with cardiac resynchronization therapy (CRT) if the patient presents with NYHA functional class III symptoms and QRS duration of 120 ms [14]. Since PPCM resolves within 2-6 months, wearable/subcutaneous ICD is advisable [73,74].
Heart Transplantation
Heart transplantation is indicated for PPCM patients with symptoms refractory to both optimal pharmacotherapy and mechanical circulatory support. Clinical outcomes of heart transplantation in PPCM patients is comparable to patients transplanted for other etiologies [28]. Although heart transplantation has been successful in many PPCM patients, there is a risk of organ rejection, infection, allograft vasculopathy and even mortality [74].
Meta-analysis of PPCM Clinical Management
Search Strategy
Clinical management for PPCM lacks definitive guidelines. Current guidelines adopt treatment developed for LV dysfunction and heart failure, which does not consider changes brought about by pregnancy or breastfeeding. To improve understanding on clinical management approaches, this meta-analysis combines research findings on treatment methods for PPCM. Electronic search for studies on clinical management of PPCM was conducted in PubMed, Embase and Cochrane online databases, and Google Scholar. The broad-based search terms used were “medication therapy” OR “drug therapy” AND “mechanical support” OR “heart transplantation” OR “cardiac transplantation” AND “peripartum cardiomyopathy”.
The inclusion criteria for studies were. (a) A prospective or retrospective study. (b) Included at least one clinical management approach for PPCM (medication, mechanical support or heart transplantation therapy). (c) Had a follow-up period of six months or more since commencement of therapy (because in majority of patients PPCM usually resolves by the sixth month postpartum). (d) Included one quantified clinical outcome (LV recovery and/or mortality). The exclusion criteria was the study were: (a) the study is a case series; (b) conference paper; or (c) had only abstract available (Table 9).
Table 9. Summary of Studies on Clinical Management of PPCM.
1st Author [Ref. #] |
Study Period |
No. of Patients |
Mean Age |
Clinical Management Method |
Follow-up (Months) |
LV Recovery (%) |
Mortality |
Haghikia, et al. [31] |
2004-2012 |
115 |
34 |
Beta blocker, ACE-inhibitor/ARB, bromocriptine |
6 |
47 |
2 |
Amos, et al. [43] |
1990-2003 |
55 |
|
ACE-Inhibitors/ Beta Blockers/ARB |
43 |
62 |
0 |
Forster, et al. [64] |
2008 |
43 |
30 |
ACE-inhibitors, beta-blockers and diuretics |
6 |
58 |
7 |
Gevaert, et al. [71] |
2000-2010 |
6 |
35 |
Mechanical ventilatory support (IABP, ECMO, LVAD) |
24 |
33 |
17 |
Sliwa, et al. [82] |
1996-1997 |
29 |
29 |
ACE-Inhibitors/ ARB Beta Blockers |
6 |
34 |
32 |
Midei, et al. [83] |
1983-1988 |
18 |
28 |
Diuretic &
Digoxin, immunosuppressive therapy |
6 |
-- |
6 |
Bozkurt, et al. [84] |
1991-1998 |
6 |
26 |
ACE-Inhibitors, digoxin, loop diuretics Immunosuppressive therapy (immune globulin) |
12 |
50 |
0 |
Pillarisetti, et al. [85] |
1999-2012 |
100 |
30 |
Beta blockers, ACE-inhibitors/ARB and ICD (13)/ CRT(2) |
33 |
23 |
11 |
McNamara, et al. [86] |
2009-2012 |
100 |
30 |
Beta blockers, ACE-inhibitors/ARB and ICD/CRT |
12 |
72 |
6 |
Sliwa, et al. [87] |
2002 |
30 |
33 |
ACE-inhibitors, Beta-blockers and pentoxifylline. |
6 |
27 |
3 |
Elkayam & Goland [88] |
2010 |
10 |
24 |
ACE-Inhibitor, Beta-blockers and Bromocriptine |
6 |
58 |
10 |
Kishimoto, et al. [89] |
2003 |
9 |
26 |
Intravenous immunoglobulin |
6 |
16 |
0 |
Biteker, et al. [90] |
2011 |
12 |
27 |
ACE-Inhibitor, Beta Blocker & Inodilator agent (Levosimendan) |
21 |
42 |
33 |
Duncker, et al. [91] |
2012-2013 |
12 |
34 |
Wearable Cardioverter Defibrillator (WCD) |
12 |
25 |
0 |
Saltzberg, et al. [92] |
2003-2009 |
107 |
30 |
Wearable Cardioverter Defibrillator (WCD) |
-- |
-- |
0 |
Keogh, et al. [93] |
1983-1991 |
10 |
-- |
Heart Transplantation (HT) |
24 |
NA |
12 |
Rickenbacher, et al. [94] |
1994 |
8 |
-- |
Heart Transplantation (HT |
6 |
NA |
25 |
Total /Mean |
|
670 |
25.47 |
|
15 |
30.92 |
9.65 |
IABP: Intra-Aortic Balloon Pump: LVAD: Left Ventricular Assist Devices; ECMO: Extra Corporeal Membrane Oxygenation; WCD: Wearable Cardioverter Defibrillator; HT: Heart Transplantation
Study Characteristics
Screening of all potential studies against the inclusion criteria yielded 17 studies that were included in this meta-analysis [31,43,64,71,82-94]. The 17 studies spanned a period of three decades, published between 1983 and 2013. They provided accumulated evidence on the development of clinical management approaches for PPCM over time. Medication (drug) therapy was the most studied clinical management methods by 71% (n=12) of the included studies [31,43,64,82-90]. This was followed by wearable cardioverter defibrillator (WCD) by 12% (n=2) of the included studies [91,92], heart transplantation by 12% (n=2) [93,94] and finally mechanical/ventilatory support (which included intra-aortic balloon pump, left ventricular assist devices (LVAD) and extra corporeal membrane oxygenation) by 6% (n=1) [71]. The 17 studies recruited a combined population of 670 PPCM patients with a mean age 25 years. The mean follow up period was 15 months in which clinical management approaches used indicated promising results with about third (30%) having a complete LV recovery (LVEF > 50%) and fewer deaths (9%).
Study Outcomes
Medication remains the first-line clinical therapy used in the management of PPCM patients diagnosed with mildly severed LV dysfunction (LVEF > 30%) as evident in the majority of studies (71%) examining its use and clinical outcomes. Although medication therapy for PPCM is similar to that of other non-ischemic cardiomyopathies, the studies emphasize on the need to choose medication that are safe in pregnancy and lactation to avoid adverse effects to mother and fetus. Mechanical support for ventilation is an important therapy for patients presenting with symptoms of acute heart failure who require supplemental oxygen. Frequently used mechanical support devices include intra-aortic balloon pump, left ventricular assist devices and extra corporeal membrane oxygenation. Mechanical ventilation has promising recovery rates (33%) within 24 months but suffer from unacceptably high mortality rates (17%) [71]. For PPCM patients refractory to optimal medication therapy and mechanical support with severe LV dysfunction (LVEF < 30%) ICD [91,92] and heart transplantation may be considered [93,94].
Discussion
The current findings suggest pregnancy and breastfeeding complicate clinical management of PPCM. Current therapeutic approaches adopt guidelines that have proved efficacious in the treatment of LV dysfunction and symptomatic heart failure. Medication remains the frontline therapy for alleviating HF symptoms and treating LV dysfunction in PPCM patients. In the present findings, 71% of the authors report medication was indicated in PPCM patients with mildly severe LV function (LVEF > 30%). Despite its efficacy, the AHA [12] and the ESC [67], caution its use due to potential adverse effects associated with pregnancy (to the mother) or lactation (to the fetus). Its use should be on an individual basis. This is especially true because several current heart failure medications have been contra-indicated in pregnant and lactating women, which may reduce the efficacy of medical treatment. In addition, medical therapy for PPCM lacks standardized period for management of heart failure and there is little knowledge on the prophylactic effect of medication on subsequent pregnancies [74].
For PPCM patients diagnosed with significantly depressed LV function (LVEF < 25%) or acute heart failure symptoms and may require supplementary oxygen, mechanical support for circulation is the initially indicated non-pharmacological therapy. Mechanical support is also considered for patients who are non-responsive to optimal medical therapy. It has promising rates of recovery (33%). Mechanical support such as intra-aortic balloon pumps or extracorporeal membrane oxygenation together with cardiogenic shock are effective in restoring normal LV function as well as a bridge to heart transplantation [14,71]. LV assist devices are indicated as a bridge to recover for acute and severe onset of PPCM. Mechanical circulatory support also reduces the need for heart transplantation in almost a third of PPCM patients but may suffer from high mortality rates. When patients with severely depressed LV function (LVEF > 25%-30%) and are non-responsive to both optimal medical and mechanical circulatory support, heart transplantation is the last medical therapy [93,94]. However, when indicating heart transplantations, complications such as organ rejection, infection and allograft vasculopathy should be considered.
In summary, clinical management of PPCM depends on treatment guidelines developed for LV dysfunction and symptomatic heart failure. Medical therapy is the initial treatment option but potential adverse effect to mother and fetus should be considered. When optimal medical therapy is non-effective, mechanical circulatory support using intra-aortic balloon pumps, extracorporeal membrane oxygenation or LV assist devices should be considered. Heart transplantation is indicated when symptoms are refectory to optimal medical therapy and mechanical circulatory support.
Conclusion
Peripartum cardiomyopathy (PPCM) is a rare form of heart failure associated with pregnancy. It occurs in the setting of LV systolic dysfunction with onset in the third trimester or within six months postpartum in the absence of any demonstrable underlying cardiac condition. It is prevalent in about 1 in 1,500 to 15,000 with higher rates in developing countries. Independent risk factors are maternal age > 30 years, multiparous women, multiple gestation, pre-eclampsia and prolonged tocolytic therapy. Smoking, hypertension, malnutrition, African ancestry cocaine use and socio-economic status are emerging as probable risk factors. Prognosis is favorable with more than half of patients spontaneously recovering their LV function within six months post-partum. Baseline LVEF > 30%, higher LVEDD and fractional shortening predict favorable prognosis. Despite spontaneous recovery, PPCM remains a leading cause of non-obstetric fetomaternal morbidity and mortality. Its etiopathogenesis remains poorly understood but it is hypothesized that stress-activated cytokines, unbalanced oxidative stress, pathological maternal autoimmune response or pathological response to hemodynamic stress may contribute to the development of PPCM. The mainstay of PPCM diagnosis is the onset of heart failure symptoms in the last month of pregnancy or within five months post-partum. Definitive diagnosis is however by exclusion of other probable cardiac and non-cardiac etiologies presenting with similar symptoms using chest X-ray, electrocardiography, laboratory examination and cardiac MRI. Although PPCM lacks definitive treatment, it adopts conventional therapy used for LV dysfunction and heart failure while considering contraindications associated with pregnancy and breastfeeding. Pharmacotherapy remains the main clinical management approach but in the case of persistence LV systolic dysfunction, mechanical circulatory support or ICD may be considered. If conditions remain refractory to both medical therapy and mechanical circulatory support, heart transplantation may be considered but it is rare because of the short duration of the condition (2-6 months) and potential complications.
References
- Elkayam U, Goland S, Pieper PG, Silverside CK (2016) High-Risk Cardiac Disease in Pregnancy: Part I. J Am Coll Cardiol 68: 396-410. [Crossref]
- Burt CC, Durbridge J (2009) Management of cardiac disease in pregnancy. Contin Educat Anaesth Crit Care & Pain 9: 44-47.
- Bhattacharyya A, Basra SS, Sen P, Kar B (2012) Peripartum cardiomyopathy: A review. Texas Heart Inst J 39: 8.
- Ritchie C (1849) Clinical contributions to the pathology, diagnosis, and treatment of certain chronic diseases of the heart. Edin Med Surg J 2: 333.
- Laverde-Sábogál CE, Garnica-Rosas LM, Correa-González N (2016) Peripartum cardiomyopathy–Rare, unknown and life threatening. Colomb J Anesthesiol 44: 63-68.
- Gouley BA, Mcmillan TM, Bellet S (1937) Idiopathic myocardial degeneration associated with pregnancy and especially the puerperium. Am J Med Sci 194: 185-199.
- Hull E, Hafkesbring E (1937) Toxic postpartal heart disease. New Orleans M and S J 89: 550-557.
- Demakis JG, Rahimtoola SH (1971) Peripartum cardiomyopathy. Circulation 44: 964-968.
- Demakis JG, Rahimtoola SH, Sutton GC, Meadows WR, Szanto PB, et al. (1971) Natural course of peripartum cardiomyopathy. Circulation 44: 1053-1061. [Crossref]
- Hibbard JU, Lindheimer M, Lang RM (1999) A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstet Gynecol 94: 311-316. [Crossref]
- Elkayam U, Akhter MW, Singh H, Khan S, Bitar F, et al. (2005) Pregnancy-associated cardiomyopathy. Circulation 111: 2050-2055.
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, et al. (2006) Contemporary definitions and classification of the cardiomyopathies. Circulation 113: 1807-1816. [Crossref]
- Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, et al. (2000) Peripartum cardiomyopathy: National Heart, Lung, And Blood Institute and Office of Rare Diseases (national institutes of health) workshop recommendations and review. JAMA 283: 1183-1188. [Crossref]
- Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, et al. (2010) Current state of knowledge on etiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 12: 767-778. [Crossref]
- Lampert MB, Lang RM (1995) Peripartum cardiomyopathy. Am Hear J 130: 860-870.
- Cunningham FG, Pritchard JA, Hankins GD, Anderson PL, Lucas MJ, et al. (1986) Peripartum heart failure: Idiopathic cardiomyopathy or compounding cardiovascular events? Obstet Gynecol 67: 157-168. [Crossref]
- Sanderson JE, Adesanya CO, Anjorin FI, Parry EH (1979) Postpartum cardiac failure – heart failure due to volume overload. Am Heart J 97: 613-621. [Crossref]
- Fett JD, Christie LG, Carraway RD, Murphy JG (2005) Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc 80: 1602-1606. [Crossref]
- Sliwa K, Damasceno A, Mayosi BM (2005) Epidemiology and etiology of cardiomyopathy in Africa. Circulation 112: 3577-3583.
- Ventura SJ, Peters KD, Martin JA, Maurer JD (1997) Births and deaths: United States, 1996. Mon Vital Stat Rep 46: 1-40. [Crossref]
- Anderson JL, Horne BD (2010) Birthing the genetics of peripartum cardiomyopathy. Circulation 121: 2157-2159. [Crossref]
- Mielniczuk LM, Williams K, Davis DR, Tang AS, Lemery R, et al. (2006) Frequency of peripartum cardiomyopathy. Am J Cardiol 97: 1765-1768. [Crossref]
- Brar SS, Khan SS, Sandhu GK, Jorgensen MB, Parikh N, et al. (2007) Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol 100: 302-304. [Crossref]
- Chapa JB, Heiberger HB, Weinert L, Decara J, Lang RM, et al. (2005) Prognostic value of echocardiography in peripartum cardiomyopathy. Obstet Gynecol 105: 1303-1308. [Crossref]
- Desai D, Moodley J, Naidoo D (1995) Peripartum cardiomyopathy: Experiences at King Edward VIII Hospital, Durban, South Africa and a review of the literature. Trop Doct 25: 118-123. [Crossref]
- Witlin AG, Mabie WC, Sibai BM (1997) Peripartum cardiomyopathy: an ominous diagnosis. Am J Obstet Gynecol 176: 182-188. [Crossref]
- Gunderson EP, Croen LA, Chiang V, Yoshida CK, Walton D, et al. (2011) Epidemiology of peripartum cardiomyopathy: incidence, predictors, and outcomes. Obstet Gynecol 118: 583-591. [Crossref]
- Blauwet LA, Cooper LT (2011) Diagnosis and management of peripartum cardiomyopathy. Heart 97: 1970-1981. [Crossref]
- Sliwa K, Fett J, Elkayam U (2006) Peripartum cardiomyopathy. The Lancet 368: 687-693.
- Bello N, Rendon ISH, Arany Z (2013) The relationship between pre-eclampsia and peripartum cardiomyopathy: A systematic review and meta-analysis. J Am Coll Cardiol 62: 1715-1723. [Crossref]
- Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, et al. (2013) Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol 108: 366. [Crossref]
- Gentry MB, Dias JK, Luis A, Patel R, Thornton J, et al. (2010) African-American women have a higher risk for developing peripartum cardiomyopathy. J Am Coll Cardiol 55: 654-659. [Crossref]
- Abboud J, Murad Y, Chen-Scarabelli C, Saravolatz L, Scarabelli TM (2007) Peripartum cardiomyopathy: A comprehensive review. Int J Cardiol 118: 295-303. [Crossref]
- Cooper LT, Mather PJ, Alexis JD, Pauly DF, Torre-Amione G, et al. (2012) Myocardial recovery in peripartum cardiomyopathy: prospective comparison with recent onset cardiomyopathy in men and nonperipartum women. J Card Fail 18: 28-33. [Crossref]
- Duran N, Günes H, Duran I, Biteker M, Ozkan M (2008) Predictors of prognosis in patients with peripartum cardiomyopathy. Int J Gynaecol Obstet 101: 137-140. [Crossref]
- Sliwa K, Förster O, Libhaber E, Fett JD, Sundstrom JB, et al. (2005) Peripartum cardiomyopathy: Inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J 27: 441-446. [Crossref]
- Fett JD, Sannon H, Thélisma E, Sprunger T, Suresh V (2009) Recovery from severe heart failure following peripartum cardiomyopathy. Int J Gynaecol Obstet 104: 125-127. [Crossref]
- Mandal D, Mandal S, Mukherjee D, Biswas SC, Maiti TK, et al. (2011) Pregnancy and subsequent pregnancy outcomes in peripartum cardiomyopathy. J Obstet Gynaecol Res 37: 222-227. [Crossref]
- Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, et al. (2001) Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med 344: 1567-1571. [Crossref]
- Fett JD, Fristoe KL, Welsh SN (2010) Risk of heart failure relapse in subsequent pregnancy among peripartum cardiomyopathy mothers. Int J Gynaecol Obstet 109: 34-36. [Crossref]
- Blauwet LA, Libhaber E, Forster O, Tibazarwa K, Mebazaa A, et al. (2013). Predictors of outcome in 176 South African patients with peripartum cardiomyopathy. Heart 99: 308-313. [Crossref]
- Goland S, Bitar F, Modi K, Safirstein J, Ro A, et al. (2011) Evaluation of the clinical relevance of baseline left ventricular ejection fraction as a predictor of recovery or persistence of severe dysfunction in women in the United States with peripartum cardiomyopathy. J Card Fail 17: 426-430. [Crossref]
- Amos AM, Jaber WA, Russell SD (2006) Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J 152: 509-513. [Crossref]
- Modi KA, Illum S, Jariatul K, Caldito G, Reddy PC (2009) Poor outcome of indigent patients with peripartum cardiomyopathy in the United States. Am J Obstet Gynecol 201: 171-e1-5. [Crossref]
- Homans DC (1985) Peripartum cardiomyopathy. N Eng J Med 312: 1432-1437.
- Sliwa K, Förster O, Libhaber E, Fett JD, Sundstrom JB, et al. (2005) Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J 27: 441-446. [Crossref]
- Burton GJ, Jauniaux E (2011) Oxidative stress. Best Pract Res Clin Obstet Gynaecol 25: 287-299. [Crossref]
- Hilfiker-Kleiner D, Meyer GP, Schieffer E, Goldmann B, Podewski E, et al. (2007) Recovery from postpartum cardiomyopathy in 2 patients by blocking prolactin release with bromocriptine. J Am Coll Cardiol 50: 2354-2355. [Crossref]
- Bhakta P, Biswas BK, Banerjee B (2007) Peripartum cardiomyopathy: Review of the literature. Yonsei Med J 48: 731-747. [Crossref]
- Rizeq MN, Rickenbacher PR, Fowler MB, Billingham ME (1994) Incidence of myocarditis in peripartum cardiomyopathy. Am J Cardiol 74: 474-477. [Crossref]
- Ansari AA, Fett JD, Carraway RE, Mayne AE, Onlamoon N, et al. (2002) Autoimmune mechanisms as the basis for human peripartum cardiomyopathy. Clin Rev Allergy Immunol 23: 301-324. [Crossref]
- Lapaire O, Hösli I, Zanetti-Daellenbach R, Huang D, Jaeggi C, et al. (2007). Impact of fetal–maternal microchimerism on women's health—a review. J Matern Fetal Neonatal Med 20: 1-5. [Crossref]
- Pearl W (1995) Familial occurrence of peripartum cardiomyopathy. Am Heart J 129: 421-422. [Crossref]
- Pierce JA, Price BO, Joyce JW (1963) Familial occurrence of postpartal heart failure. Arch Int Med 111: 651-655.
- Meyer GP, Labidi S, Podewski E, Sliwa K, Drexler H, et al. (2010) Bromocriptine treatment associated with recovery from peripartum cardiomyopathy in siblings: two case reports. J Med Case Rep 4: 80. [Crossref]
- Fett JD, Sundstrom BJ, Etta King M, Ansari AA (2002) Mother–daughter peripartum cardiomyopathy. Int J Cardiol 86: 331-332. [Crossref]
- Van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, van der Werf R, Jongbloed JD, et al. (2010) Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation 121: 2169-2175.
- Morales A, Painter T, Li R, Siegfried JD, Li D, et al. (2010) Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy. Circulation 121: 2176-2182.
- van Spaendonck-Zwarts KY, Posafalvi A, van den Berg MP, Hilfiker-Kleiner D, Bollen IA, et al. (2014) Titin gene mutations are common in families with both peripartum cardiomyopathy and dilated cardiomyopathy. Eur Heart J 35: 2165-2173. [Crossref]
- Lamparter S, Pankuweit S, Maisch B (2007) Clinical and immunologic characteristics in peripartum cardiomyopathy. Int J Cardiol 118: 14-20. [Crossref]
- Pyatt JR, Dubey G (2011) Peripartum cardiomyopathy: current understanding, comprehensive management review and new developments. Postgrad Med J 87: 34-39. [Crossref]
- Hilfiker-Kleiner D, Haghikia A, Nonhoff J, Bauersachs J (2015) Peripartum cardiomyopathy: current management and future perspectives. Eur Heart J 36: 1090-1097. [Crossref]
- Tibazarwa K, Mayosi B, Sliwa K, Carrington M, Stewart S, et al. (2012) The 12-lead ECG in peripartum cardiomyopathy: Cardiovascular topics. Cardiovasc J Afr 23: 322-329. [Crossref]
- Forster O, Hilfiker-Kleiner D, Ansari AA, Sundstrom JB, Libhaber E, et al. (2008) Reversal of IFN-γ, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur J Heart Fail 10: 861-868. [Crossref]
- Hilfiker-Kleiner D, Sliwa K (2014) Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol 11: 364-370. [Crossref]
- Mouquet F, Lions C, de Groote P, Bouabdallaoui N, Willoteaux S, et al. (2008) Characterization of peripartum cardiomyopathy by cardiac magnetic resonance imaging. Eur Radiol 18: 2765-2769. [Crossref]
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, et al. (2008) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur J Heart Fail 10: 933-989. [Crossref]
- Schaefer C (2003) Angiotensin II-receptor-antagonists: further evidence of fetotoxicity but not teratogenicity. Birth Defects Res A Clin Mol Teratol 67: 591-594. [Crossref]
- Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, et al. (2006) Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 354: 2443-2451.
- Briggs GG, Freeman RK, Yaffe SJ (2012). Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. Philadelphia: Lippincott Williams & Wilkins.
- Gevaert S, Van Belleghem Y, Bouchez S, Herck I, De Somer F, et al. (2011) Acute and critically ill peripartum cardiomyopathy and 'bridge to' therapeutic options: A single center experience with intra-aortic balloon pump, extra corporeal membrane oxygenation and continuous-flow left ventricular assist devices. Crit Care 15: R93. [Crossref]
- Reek S, Geller JC, Meltendorf U, Wollbrueck A, Szymkiewicz SJ, et al. (2003) Clinical efficacy of a wearable defibrillator in acutely terminating episodes of ventricular fibrillation using biphasic shocks. Pacing Clin Electrophysiol 26: 2016-2022. [Crossref]
- Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, et al. (2010) An entirely subcutaneous implantable cardioverter–defibrillator. N Engl J Med 363: 36-44.
- Elkayam U (2011) Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol 58: 659-670. [Crossref]
- Sliwa K, Forster O, Tibazarwa K, Libhaber E, Becker A, et al. (2011) Long-term outcome of peripartum cardiomyopathy in a population with high seropositivity for human immunodeficiency virus. Int J Cardiol 147: 202-208. [Crossref]
- Goland S, Modi K, Bitar F, Janmohamed M, Mirocha JM, et al. (2009) Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail 15: 645-650. [Crossref]
- Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, et al. (2000) Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 342: 1077-1084. [Crossref]
- Mielniczuk LM, Williams K, Davis DR, Tang AS, Lemery R, et al. (2006) Frequency of peripartum cardiomyopathy. Am J Cardiol 97: 1765-1768. [Crossref]
- Hasan JA, Qureshi A, Ramejo, BB, Kamran A (2010) Peripartum cardiomyopathy characteristics and outcome in a tertiary care hospital. J Pak Med Assoc 60: 377-380. [Crossref]
- Safirstein JG, Ro AS, Grandhi S, Wang L, Fett JD, et al. (2012) Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the internet. Int J Cardiol 154: 27-31. [Crossref]
- Witlin AG, Mabie WC, Sibai BM (1997) Peripartum cardiomyopathy: an ominous diagnosis. Am J Obstet Gynecol 176: 182-188. [Crossref]
- Sliwa K, Skudicky D, Bergemann A, Candy G, Puren A, et al. (2000). Peripartum cardiomyopathy: Analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/APO-1. J Am Coll Cardiol 35: 701-705. [Crossref]
- Midei MG, DeMent SH, Feldman AM, Hutchins GM, Baughman KL (1990) Peripartum myocarditis and cardiomyopathy. Circulation 81: 922-928.
- Bozkurt B, Villaneuva FS, Holubkov R, Tokarczyk T, Alvarez RJ Jr, et al. (1999) Intravenous immune globulin in the therapy of peripartum cardiomyopathy. J Am Coll Cardiol 34: 177-180. [Crossref]
- Pillarisetti J, Kondur A, Alani A, Reddy M, Reddy M, et al. (2014) Peripartum cardiomyopathy: predictors of recovery and current state of implantable cardioverter-defibrillator use. J Am Coll Cardiol 63: 2831-2839. [Crossref]
- McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, et al. (2015) Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol 66: 905-914. [Crossref]
- Sliwa K, Skudicky D, Candy G, Bergemann A, Hopley M, et al. (2002) The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur J Heart Fail 4: 305-309. [Crossref]
- Elkayam U, Goland S (2010) Bromocriptine for the treatment of peripartum cardiomyopathy. Circulation 1463-1464.
- Kishimoto C, Shioji K, Kinoshita M, Iwase T, Tamaki S, et al. (2003) Treatment of acute inflammatory cardiomyopathy with intravenous immunoglobulin ameliorates left ventricular function associated with suppression of inflammatory cytokines and decreased oxidative stress. Int J Cardiol 91: 173-178. [Crossref]
- Biteker M, Duran NE, Kaya H, Gündüz S, Tanboga HÎ, et al. (2011) Effect of levosimendan and predictors of recovery in patients with peripartum cardiomyopathy, a randomized clinical trial. Clin Res Cardiol 100: 571-577. [Crossref]
- Duncker D, Haghikia A, König T, Hohmann S, Gutleben KJ, et al. (2014) Risk for ventricular fibrillation in peripartum cardiomyopathy with severely reduced left ventricular function—value of the wearable cardioverter/defibrillator. Eur J Heart Fail 16: 1331-1336. [Crossref]
- Saltzberg MT, Szymkiewicz, S, Bianco NR (2012) Characteristics and outcomes of peripartum versus nonperipartum cardiomyopathy in women using a wearable cardiac defibrillator. J Card Fail 18: 21-27. [Crossref]
- Keogh A, Macdonald P, Spratt P, Marshman D, Larbalestier R, et al. (1994) Outcome in peripartum cardiomyopathy after heart transplantation. J Heart Lung Transplant 13: 202-207. [Crossref]
- Rickenbacher PR, Rizeq MN, Hunt SA, Billingham ME, Fowler MB (1994) Long-term outcome after heart transplantation for peripartum cardiomyopathy. Am Heart J 127: 1318-1323. [Crossref]
- Elkayam U, Goland S, Pieper PG, Silverside CK (2016) High-Risk Cardiac Disease in Pregnancy: Part I. J Am Coll Cardiol 68: 396-410. [Crossref]
- Burt CC, Durbridge J (2009) Management of cardiac disease in pregnancy. Contin Educat Anaesth Crit Care & Pain 9: 44-47.
- Bhattacharyya A, Basra SS, Sen P, Kar B (2012) Peripartum cardiomyopathy: A review. Texas Heart Inst J 39: 8.
- Ritchie C (1849) Clinical contributions to the pathology, diagnosis, and treatment of certain chronic diseases of the heart. Edin Med Surg J 2: 333.
- Laverde-Sábogál CE, Garnica-Rosas LM, Correa-González N (2016) Peripartum cardiomyopathy–Rare, unknown and life threatening. Colomb J Anesthesiol 44: 63-68.
- Gouley BA, Mcmillan TM, Bellet S (1937) Idiopathic myocardial degeneration associated with pregnancy and especially the puerperium. Am J Med Sci 194: 185-199.
- Hull E, Hafkesbring E (1937) Toxic postpartal heart disease. New Orleans M and S J 89: 550-557.
- Demakis JG, Rahimtoola SH (1971) Peripartum cardiomyopathy. Circulation 44: 964-968.
- Demakis JG, Rahimtoola SH, Sutton GC, Meadows WR, Szanto PB, et al. (1971) Natural course of peripartum cardiomyopathy. Circulation 44: 1053-1061. [Crossref]
- Hibbard JU, Lindheimer M, Lang RM (1999) A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstet Gynecol 94: 311-316. [Crossref]
- Elkayam U, Akhter MW, Singh H, Khan S, Bitar F, et al. (2005) Pregnancy-associated cardiomyopathy. Circulation 111: 2050-2055.
- Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, et al. (2006) Contemporary definitions and classification of the cardiomyopathies. Circulation 113: 1807-1816. [Crossref]
- Pearson GD, Veille JC, Rahimtoola S, Hsia J, Oakley CM, et al. (2000) Peripartum cardiomyopathy: National Heart, Lung, And Blood Institute and Office of Rare Diseases (national institutes of health) workshop recommendations and review. JAMA 283: 1183-1188. [Crossref]
- Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, et al. (2010) Current state of knowledge on etiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 12: 767-778. [Crossref]
- Lampert MB, Lang RM (1995) Peripartum cardiomyopathy. Am Hear J 130: 860-870.
- Cunningham FG, Pritchard JA, Hankins GD, Anderson PL, Lucas MJ, et al. (1986) Peripartum heart failure: Idiopathic cardiomyopathy or compounding cardiovascular events? Obstet Gynecol 67: 157-168. [Crossref]
- Sanderson JE, Adesanya CO, Anjorin FI, Parry EH (1979) Postpartum cardiac failure – heart failure due to volume overload. Am Heart J 97: 613-621. [Crossref]
- Fett JD, Christie LG, Carraway RD, Murphy JG (2005) Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc 80: 1602-1606. [Crossref]
- Sliwa K, Damasceno A, Mayosi BM (2005) Epidemiology and etiology of cardiomyopathy in Africa. Circulation 112: 3577-3583.
- Ventura SJ, Peters KD, Martin JA, Maurer JD (1997) Births and deaths: United States, 1996. Mon Vital Stat Rep 46: 1-40. [Crossref]
- Anderson JL, Horne BD (2010) Birthing the genetics of peripartum cardiomyopathy. Circulation 121: 2157-2159. [Crossref]
- Mielniczuk LM, Williams K, Davis DR, Tang AS, Lemery R, et al. (2006) Frequency of peripartum cardiomyopathy. Am J Cardiol 97: 1765-1768. [Crossref]
- Brar SS, Khan SS, Sandhu GK, Jorgensen MB, Parikh N, et al. (2007) Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol 100: 302-304. [Crossref]
- Chapa JB, Heiberger HB, Weinert L, Decara J, Lang RM, et al. (2005) Prognostic value of echocardiography in peripartum cardiomyopathy. Obstet Gynecol 105: 1303-1308. [Crossref]
- Desai D, Moodley J, Naidoo D (1995) Peripartum cardiomyopathy: Experiences at King Edward VIII Hospital, Durban, South Africa and a review of the literature. Trop Doct 25: 118-123. [Crossref]
- Witlin AG, Mabie WC, Sibai BM (1997) Peripartum cardiomyopathy: an ominous diagnosis. Am J Obstet Gynecol 176: 182-188. [Crossref]
- Gunderson EP, Croen LA, Chiang V, Yoshida CK, Walton D, et al. (2011) Epidemiology of peripartum cardiomyopathy: incidence, predictors, and outcomes. Obstet Gynecol 118: 583-591. [Crossref]
- Blauwet LA, Cooper LT (2011) Diagnosis and management of peripartum cardiomyopathy. Heart 97: 1970-1981. [Crossref]
- Sliwa K, Fett J, Elkayam U (2006) Peripartum cardiomyopathy. The Lancet 368: 687-693.
- Bello N, Rendon ISH, Arany Z (2013) The relationship between pre-eclampsia and peripartum cardiomyopathy: A systematic review and meta-analysis. J Am Coll Cardiol 62: 1715-1723. [Crossref]
- Haghikia A, Podewski E, Libhaber E, Labidi S, Fischer D, et al. (2013) Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol 108: 366. [Crossref]
- Gentry MB, Dias JK, Luis A, Patel R, Thornton J, et al. (2010) African-American women have a higher risk for developing peripartum cardiomyopathy. J Am Coll Cardiol 55: 654-659. [Crossref]
- Abboud J, Murad Y, Chen-Scarabelli C, Saravolatz L, Scarabelli TM (2007) Peripartum cardiomyopathy: A comprehensive review. Int J Cardiol 118: 295-303. [Crossref]
- Cooper LT, Mather PJ, Alexis JD, Pauly DF, Torre-Amione G, et al. (2012) Myocardial recovery in peripartum cardiomyopathy: prospective comparison with recent onset cardiomyopathy in men and nonperipartum women. J Card Fail 18: 28-33. [Crossref]
- Duran N, Günes H, Duran I, Biteker M, Ozkan M (2008) Predictors of prognosis in patients with peripartum cardiomyopathy. Int J Gynaecol Obstet 101: 137-140. [Crossref]
- Sliwa K, Förster O, Libhaber E, Fett JD, Sundstrom JB, et al. (2005) Peripartum cardiomyopathy: Inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J 27: 441-446. [Crossref]
- Fett JD, Sannon H, Thélisma E, Sprunger T, Suresh V (2009) Recovery from severe heart failure following peripartum cardiomyopathy. Int J Gynaecol Obstet 104: 125-127. [Crossref]
- Mandal D, Mandal S, Mukherjee D, Biswas SC, Maiti TK, et al. (2011) Pregnancy and subsequent pregnancy outcomes in peripartum cardiomyopathy. J Obstet Gynaecol Res 37: 222-227. [Crossref]
- Elkayam U, Tummala PP, Rao K, Akhter MW, Karaalp IS, et al. (2001) Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med 344: 1567-1571. [Crossref]
- Fett JD, Fristoe KL, Welsh SN (2010) Risk of heart failure relapse in subsequent pregnancy among peripartum cardiomyopathy mothers. Int J Gynaecol Obstet 109: 34-36. [Crossref]
- Blauwet LA, Libhaber E, Forster O, Tibazarwa K, Mebazaa A, et al. (2013). Predictors of outcome in 176 South African patients with peripartum cardiomyopathy. Heart 99: 308-313. [Crossref]
- Goland S, Bitar F, Modi K, Safirstein J, Ro A, et al. (2011) Evaluation of the clinical relevance of baseline left ventricular ejection fraction as a predictor of recovery or persistence of severe dysfunction in women in the United States with peripartum cardiomyopathy. J Card Fail 17: 426-430. [Crossref]
- Amos AM, Jaber WA, Russell SD (2006) Improved outcomes in peripartum cardiomyopathy with contemporary. Am Heart J 152: 509-513. [Crossref]
- Modi KA, Illum S, Jariatul K, Caldito G, Reddy PC (2009) Poor outcome of indigent patients with peripartum cardiomyopathy in the United States. Am J Obstet Gynecol 201: 171-e1-5. [Crossref]
- Homans DC (1985) Peripartum cardiomyopathy. N Eng J Med 312: 1432-1437.
- Sliwa K, Förster O, Libhaber E, Fett JD, Sundstrom JB, et al. (2005) Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J 27: 441-446. [Crossref]
- Burton GJ, Jauniaux E (2011) Oxidative stress. Best Pract Res Clin Obstet Gynaecol 25: 287-299. [Crossref]
- Hilfiker-Kleiner D, Meyer GP, Schieffer E, Goldmann B, Podewski E, et al. (2007) Recovery from postpartum cardiomyopathy in 2 patients by blocking prolactin release with bromocriptine. J Am Coll Cardiol 50: 2354-2355. [Crossref]
- Bhakta P, Biswas BK, Banerjee B (2007) Peripartum cardiomyopathy: Review of the literature. Yonsei Med J 48: 731-747. [Crossref]
- Rizeq MN, Rickenbacher PR, Fowler MB, Billingham ME (1994) Incidence of myocarditis in peripartum cardiomyopathy. Am J Cardiol 74: 474-477. [Crossref]
- Ansari AA, Fett JD, Carraway RE, Mayne AE, Onlamoon N, et al. (2002) Autoimmune mechanisms as the basis for human peripartum cardiomyopathy. Clin Rev Allergy Immunol 23: 301-324. [Crossref]
- Lapaire O, Hösli I, Zanetti-Daellenbach R, Huang D, Jaeggi C, et al. (2007). Impact of fetal–maternal microchimerism on women's health—a review. J Matern Fetal Neonatal Med 20: 1-5. [Crossref]
- Pearl W (1995) Familial occurrence of peripartum cardiomyopathy. Am Heart J 129: 421-422. [Crossref]
- Pierce JA, Price BO, Joyce JW (1963) Familial occurrence of postpartal heart failure. Arch Int Med 111: 651-655.
- Meyer GP, Labidi S, Podewski E, Sliwa K, Drexler H, et al. (2010) Bromocriptine treatment associated with recovery from peripartum cardiomyopathy in siblings: two case reports. J Med Case Rep 4: 80. [Crossref]
- Fett JD, Sundstrom BJ, Etta King M, Ansari AA (2002) Mother–daughter peripartum cardiomyopathy. Int J Cardiol 86: 331-332. [Crossref]
- Van Spaendonck-Zwarts KY, van Tintelen JP, van Veldhuisen DJ, van der Werf R, Jongbloed JD, et al. (2010) Peripartum cardiomyopathy as a part of familial dilated cardiomyopathy. Circulation 121: 2169-2175.
- Morales A, Painter T, Li R, Siegfried JD, Li D, et al. (2010) Rare variant mutations in pregnancy-associated or peripartum cardiomyopathy. Circulation 121: 2176-2182.
- van Spaendonck-Zwarts KY, Posafalvi A, van den Berg MP, Hilfiker-Kleiner D, Bollen IA, et al. (2014) Titin gene mutations are common in families with both peripartum cardiomyopathy and dilated cardiomyopathy. Eur Heart J 35: 2165-2173. [Crossref]
- Lamparter S, Pankuweit S, Maisch B (2007) Clinical and immunologic characteristics in peripartum cardiomyopathy. Int J Cardiol 118: 14-20. [Crossref]
- Pyatt JR, Dubey G (2011) Peripartum cardiomyopathy: current understanding, comprehensive management review and new developments. Postgrad Med J 87: 34-39. [Crossref]
- Hilfiker-Kleiner D, Haghikia A, Nonhoff J, Bauersachs J (2015) Peripartum cardiomyopathy: current management and future perspectives. Eur Heart J 36: 1090-1097. [Crossref]
- Tibazarwa K, Mayosi B, Sliwa K, Carrington M, Stewart S, et al. (2012) The 12-lead ECG in peripartum cardiomyopathy: Cardiovascular topics. Cardiovasc J Afr 23: 322-329. [Crossref]
- Forster O, Hilfiker-Kleiner D, Ansari AA, Sundstrom JB, Libhaber E, et al. (2008) Reversal of IFN-γ, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur J Heart Fail 10: 861-868. [Crossref]
- Hilfiker-Kleiner D, Sliwa K (2014) Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol 11: 364-370. [Crossref]
- Mouquet F, Lions C, de Groote P, Bouabdallaoui N, Willoteaux S, et al. (2008) Characterization of peripartum cardiomyopathy by cardiac magnetic resonance imaging. Eur Radiol 18: 2765-2769. [Crossref]
- Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, et al. (2008) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008. Eur J Heart Fail 10: 933-989. [Crossref]
- Schaefer C (2003) Angiotensin II-receptor-antagonists: further evidence of fetotoxicity but not teratogenicity. Birth Defects Res A Clin Mol Teratol 67: 591-594. [Crossref]
- Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, et al. (2006) Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 354: 2443-2451.
- Briggs GG, Freeman RK, Yaffe SJ (2012). Drugs in pregnancy and lactation: a reference guide to fetal and neonatal risk. Philadelphia: Lippincott Williams & Wilkins.
- Gevaert S, Van Belleghem Y, Bouchez S, Herck I, De Somer F, et al. (2011) Acute and critically ill peripartum cardiomyopathy and 'bridge to' therapeutic options: A single center experience with intra-aortic balloon pump, extra corporeal membrane oxygenation and continuous-flow left ventricular assist devices. Crit Care 15: R93. [Crossref]
- Reek S, Geller JC, Meltendorf U, Wollbrueck A, Szymkiewicz SJ, et al. (2003) Clinical efficacy of a wearable defibrillator in acutely terminating episodes of ventricular fibrillation using biphasic shocks. Pacing Clin Electrophysiol 26: 2016-2022. [Crossref]
- Bardy GH, Smith WM, Hood MA, Crozier IG, Melton IC, et al. (2010) An entirely subcutaneous implantable cardioverter–defibrillator. N Engl J Med 363: 36-44.
- Elkayam U (2011) Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol 58: 659-670. [Crossref]
- Sliwa K, Forster O, Tibazarwa K, Libhaber E, Becker A, et al. (2011) Long-term outcome of peripartum cardiomyopathy in a population with high seropositivity for human immunodeficiency virus. Int J Cardiol 147: 202-208. [Crossref]
- Goland S, Modi K, Bitar F, Janmohamed M, Mirocha JM, et al. (2009) Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail 15: 645-650. [Crossref]
- Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, et al. (2000) Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med 342: 1077-1084. [Crossref]
- Mielniczuk LM, Williams K, Davis DR, Tang AS, Lemery R, et al. (2006) Frequency of peripartum cardiomyopathy. Am J Cardiol 97: 1765-1768. [Crossref]
- Hasan JA, Qureshi A, Ramejo, BB, Kamran A (2010) Peripartum cardiomyopathy characteristics and outcome in a tertiary care hospital. J Pak Med Assoc 60: 377-380. [Crossref]
- Safirstein JG, Ro AS, Grandhi S, Wang L, Fett JD, et al. (2012) Predictors of left ventricular recovery in a cohort of peripartum cardiomyopathy patients recruited via the internet. Int J Cardiol 154: 27-31. [Crossref]
- Witlin AG, Mabie WC, Sibai BM (1997) Peripartum cardiomyopathy: an ominous diagnosis. Am J Obstet Gynecol 176: 182-188. [Crossref]
- Sliwa K, Skudicky D, Bergemann A, Candy G, Puren A, et al. (2000). Peripartum cardiomyopathy: Analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/APO-1. J Am Coll Cardiol 35: 701-705. [Crossref]
- Midei MG, DeMent SH, Feldman AM, Hutchins GM, Baughman KL (1990) Peripartum myocarditis and cardiomyopathy. Circulation 81: 922-928.
- Bozkurt B, Villaneuva FS, Holubkov R, Tokarczyk T, Alvarez RJ Jr, et al. (1999) Intravenous immune globulin in the therapy of peripartum cardiomyopathy. J Am Coll Cardiol 34: 177-180. [Crossref]
- Pillarisetti J, Kondur A, Alani A, Reddy M, Reddy M, et al. (2014) Peripartum cardiomyopathy: predictors of recovery and current state of implantable cardioverter-defibrillator use. J Am Coll Cardiol 63: 2831-2839. [Crossref]
- McNamara DM, Elkayam U, Alharethi R, Damp J, Hsich E, et al. (2015) Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol 66: 905-914. [Crossref]
- Sliwa K, Skudicky D, Candy G, Bergemann A, Hopley M, et al. (2002) The addition of pentoxifylline to conventional therapy improves outcome in patients with peripartum cardiomyopathy. Eur J Heart Fail 4: 305-309. [Crossref]
- Elkayam U, Goland S (2010) Bromocriptine for the treatment of peripartum cardiomyopathy. Circulation 1463-1464.
- Kishimoto C, Shioji K, Kinoshita M, Iwase T, Tamaki S, et al. (2003) Treatment of acute inflammatory cardiomyopathy with intravenous immunoglobulin ameliorates left ventricular function associated with suppression of inflammatory cytokines and decreased oxidative stress. Int J Cardiol 91: 173-178. [Crossref]
- Biteker M, Duran NE, Kaya H, Gündüz S, Tanboga HÎ, et al. (2011) Effect of levosimendan and predictors of recovery in patients with peripartum cardiomyopathy, a randomized clinical trial. Clin Res Cardiol 100: 571-577. [Crossref]
- Duncker D, Haghikia A, König T, Hohmann S, Gutleben KJ, et al. (2014) Risk for ventricular fibrillation in peripartum cardiomyopathy with severely reduced left ventricular function—value of the wearable cardioverter/defibrillator. Eur J Heart Fail 16: 1331-1336. [Crossref]
- Saltzberg MT, Szymkiewicz, S, Bianco NR (2012) Characteristics and outcomes of peripartum versus nonperipartum cardiomyopathy in women using a wearable cardiac defibrillator. J Card Fail 18: 21-27. [Crossref]
- Keogh A, Macdonald P, Spratt P, Marshman D, Larbalestier R, et al. (1994) Outcome in peripartum cardiomyopathy after heart transplantation. J Heart Lung Transplant 13: 202-207. [Crossref]
- Rickenbacher PR, Rizeq MN, Hunt SA, Billingham ME, Fowler MB (1994) Long-term outcome after heart transplantation for peripartum cardiomyopathy. Am Heart J 127: 1318-1323. [Crossref]


