Abstract
Background: Perivascular niches are today regarded as crucial in the tumor progression and invasion of glioblastoma (GB). The main problem remains to establish when a perivascular distribution of tumor cells can be defined as niche.
Methods: By immunohistochemical analysis five vessel/tumor cell associations have been investigated in four different GB areas (mild and high infiltration, hyper-proliferation, regression) from 20 tumor tissue samples.
Results: Different types of relationship have been found between vessels and the various cell types surrounding them. Among these, microglia/macrophages have been accurately studied, because of their various aspects and immunophenotypes, as well as pericytes in their different evolutionary stages.
Conclusions: Niche function is closely related to the direct relationship between glioblastoma stem cells/progenitors and endothelial cells. Vessels not in direct contact with any cell types or with thick walls cannot behave as niches.
Key words
glioblastoma, microglia, pericytes, perivascular niches
Introduction
Tumor microenvironment is a dynamic concept that includes, beside tumor cells, stromal cells, soluble factors and signaling molecules promoting neoplastic transformation, tumor growth and invasion, protection from host immunity and resistance to therapies [1]. The cell components would be tumor cells, normal and reactive astrocytes, glioblastoma stem cells (GSCs)/progenitors, pericytes, microglia/macrophages, fibroblasts [2-4].
Since tumor progression largely depends on the exchange between endothelial cells and GSCs through Notch signaling, nitric oxide (NO) and vascular endothelial growth factor (VEGF) [2,3], perivascular niches (PVN) have been considered as the propulsion centers of the tumor. Their prototype in gliomas was the simple association of Nestin+ and CD133+ cells, as representatives of GSCs, with endothelial cells [5], in analogy with the sub-ventricular zone (SVZ) niche, where vasculature is strictly connected with normal neural stem cells [6,7].
PVN occur besides perinecrotic and invasive niches [8], but they should be further substantiated and specified because of the glioblastoma (GB) heterogeneity and the various forms of neo-vascularization [9]. An important role is played by glioma-associated microglia/macrophages (GAMs), chemokine signaling and pericytes [10,11].
The goal of this work is to analyze vessel/tumor relationships in the different areas of GB in order to verify which ones deserves the term “niche”.
Materials and methods
Surgical specimens of 20 adult GBs operated on at the Department of Neurosurgery of Città della Salute e della Scienza (Turin, Italy) were diagnosed according to the current World Health Organization (WHO) guidelines [12] and selected on the basis of the size of the surgical sample in order to have the maximum number of tumor areas. The study was in compliance with the ethical human subject principles of the World Medical Association Declaration of Helsinki Research. Written informed consent of patients was obtained after the Ethics Committee approval.
Surgical tumor samples were formalin fixed, paraffin embedded (FFPE) and cut in 5-micrometer-thick sections. Beside Haematoxylin and Eosin (H&E) staining, immunohistochemical analyses were performed using a Ventana Full BenchMark® XT automated immunostainer (Ventana Medical Systems Inc.) and the UltraView™ Universal DAB Detection Kit (Ventana Medical Systems Inc.) as detection system. Heat-induced epitope retrieval (HIER) was performed in Tris-EDTA, pH 8. Primary antibodies are listed in Table I. Nestin, SOX2 and SEL1L [13] were considered as representatives of GSCs/progenitors.
Table 1. List of primary antibodies used for immunohistochemistry.
|
Antibody
|
Source
|
Dilution
|
Code
|
Manufacturer
|
|
Nestin
|
Mouse
|
1: 200
|
MAB5326
|
Millipore
|
|
Vimentin
|
Mouse
|
Pre-diluted
|
790-2917
|
Ventana
|
|
GFAP
|
Mouse
|
1: 200
|
M0761
|
Dako
|
|
CD34
|
Mouse
|
Pre-diluted
|
790-2927
|
Ventana
|
|
Factor VIII (von Willebrand)
|
Rabbit
|
Pre-diluted
|
760-2642
|
Ventana
|
|
NG2
|
Rabbit
|
1: 50
|
NBP1-89682
|
Novus Biologicals biologicals
|
|
α-sm-actin
|
Mouse
|
Pre-diluted
|
760-2833
|
Ventana
|
|
PDGFRβ
|
Rabbit
|
1: 50
|
ab32570
|
Abcam
|
|
SOX2
|
Mouse
|
1: 100
|
MAB2018
|
R&D Systems
|
|
SEL1L
|
Mouse
|
1: 350
|
-
|
Kind gift
|
|
Iba1
|
Rabbit
|
1: 500
|
#019-19741
|
Wako Chemicals
|
|
CD68
|
Mouse
|
Pre-diluted
|
790-2931
|
Ventana
|
|
CD163
|
Mouse
|
Pre-diluted
|
760-4437
|
Ventana
|
|
CD45
|
Mouse
|
Pre-diluted
|
760-2505
|
Ventana
|
2021 Copyright OAT. All rights reserv
|
CD14
|
Rabbit
|
Pre-diluted
|
760-4523
|
Ventana
|
|
CD11b
|
Rabbit
|
1: 100
|
AB52478
|
Abcam
|
|
CXCL12/SDF-1
|
Rabbit
|
1: 100
|
Ab9797
|
Abcam
|
|
CXCR4
|
Mouse
|
1: 1000
|
MAB172
|
R&D Systems
|
|
CCL2
|
Mouse
|
1: 25
|
MAB2791
|
R&D Systems
|
|
CCR2
|
Mouse
|
1: 50
|
MAB150
|
R&D Systems
|
|
CX3CL1
|
Goat
|
1: 20
|
AF365
|
R&D Systems
|
|
CX3CR1
|
Mouse
|
1: 50
|
Ab184678
|
Abcam
|
|
CXCL2
|
Rabbit
|
1: 50
|
AHP773
|
BIO-RAD
|
|
CXCR2
|
Rabbit
|
1: 50
|
LS-A803
|
LSBio
|
Double immunostainings for GFAP/Nestin, CD34/GFAP, CD34/Nestin, Factor VIII/NG2 and CD34/Iba1 were performed both by immunohistochemistry, with the ultraViewTM Universal Alkaline Phosphatase Red Detection Kit (Ventana), and by immunofluorescence. Negative controls were obtained by omitting the primary antibodies. Observations were made on a Zeiss Axioskop fluorescence microscope (Carl Zeiss).
The GB areas considered were: mild infiltration, high infiltration, high proliferation with high small vessel density and circumscribed necroses, regressive and necrotic areas. In each of them, at least 5 vessels/tumor structures were studied.
Vessel density (VD) has been evaluated as the mean number of visible vessels by CD34 staining counted in five randomly selected microscopic high-power fields at x400 magnification (HPFs) per section. The number of vessel sprouts was calculated as well in the same way. The frequency of microglia/macrophages and of reactive astrocytes was quantified by counting the number of immunopositive cells for each antibody in five HPFs per section and by calculating the mean values.
Results
Mild infiltration areas: Infiltration of Nestin+ isolated tumor cells occurred (Figure 1A), not associated with a small vessel increase. GFAP+ reactive astrocytes showed long processes as end-feet on small vessels (10-15 cells per HPF). VD, revealed by CD34 staining (Figure 1B), was 8.3, as in normal peritumor tissue and no sprout was observed. Approaching high infiltration areas VD slightly increased and sprouts appeared. Iba1+ (Figure 1C) and CD163− (Figure 1D) reactive ramified microglia (RRM) was observed with a frequency of 20 cells per HPF and with no crowding on vessels.
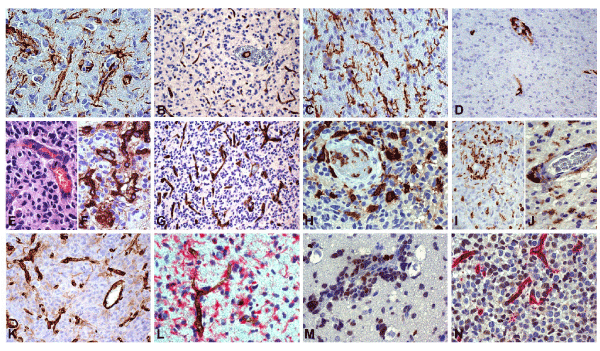
Figure 1. Glioblastoma. Immunohistochemical analysis. (A) Mild infiltration area. GSCs/progenitors on small vessels. Nestin, DAB, x400. (B) Vessel density (VD) does not increase in mild infiltration. CD34, DAB, x200. (C) Mild infiltration area. Increase of RRM not associated with vessels. Iba1, DAB, x400. (D) Mild infiltration area. Perivascular macrophages. CD163, DAB, x200. (E) High infiltration area. Initial endothelial proliferation. H&E, x400. (F) High infiltration area. New-formed vessels and sprouts. Factor VIII, DAB, x400. (G) VD increases in high infiltration. CD34, DAB, x200. (H) High infiltration area. GAM increase as macrophages or intermediate forms, both on vessels and in the parenchyma. Iba1, DAB, x400. (I) High proliferation area. Macrophages, both perivascular and scattered in the parenchyma. CD163, DAB, x200. (J) High proliferation area. Macrophages, both perivascular and scattered in the parenchyma. CD45, DAB, x400. (K) High proliferation area. New-formed vessels and sprouts. CD34, DAB, x200. (L) Nestin-positive GSCs/progenitors on a small vessel. Nestin/CD34, DAB/RED, x400. (M) High infiltration area. Perivascular cuffs of GSCs/progenitors. SOX2, DAB, x400. (N) High infiltration area. Positive GSCs/progenitors around new-formed small vessels. SEL1L/CD34, DAB/RED, x400.
High infiltration areas: An increase of tumor cells took place either as isolated cells or as cuffs of tumor cells around small sized vessels in the known aspect of co-option. VD increased to 19.6 and many vessels (arterioles and venules) appeared as “mother vessels” [14] with leakiness and extravasation, hypertrophy and hyperplasia of endothelial cells (Figure 1E), as observed by Factor VIII (Figure 1F) and CD34 (Figure 1G) immunostaining. Microglia increased, but changing its aspect from RRM into that of intermediate forms (IF) or of frank macrophages approaching the high proliferation area [15]. Iba1+ (Figure 1H), CD163+ (Figure 1I), CD68+, CD45+ (Figure 1J) and CD14+ macrophages appeared around the vessels. Sprouts of different length emerged perpendicularly from preexisting or new vessels with a mean value of 1.6. Reactive astrocytes increased in number up to 20-25 cells per HPF, sending processes on vessels, but keeping their cell body at a certain distance from them.
Hyper-proliferation areas: Their aspect appeared as the reinforcement of the previous ones. VD increased to 40 and sprouts were more frequent and longer than in the previous area (Figure 1K) with a mean value of 4. The new and old vessels were immersed in areas crowded with highly proliferative Nestin+ (Figure 1L), SOX2+ (Figure 1M) and SEL1L+ (Figure 1N) tumor cells, in direct contact with endothelial cells. The number of pericytes increased, as detected by NG2 (Figure 2A), α-sm-actin (Figure 2B) and PDGFRβ (Figure 2C) positivity, and Iba1+, CD163+ and CD45+ macrophages adhered to sprouts and vessels. Reactive astrocytes by GFAP (Figure 2D) were still visible at a distance from the vessels, and their end-feet on them were sometimes still recognizable.
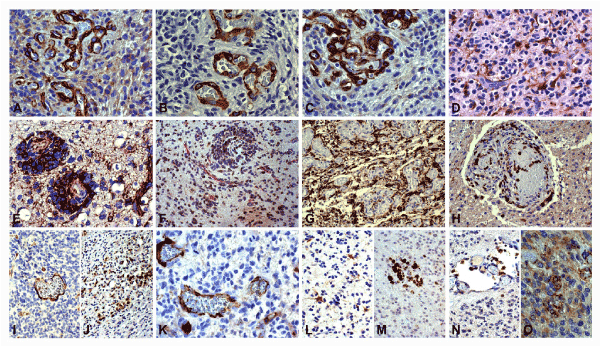
Figure 2. Glioblastoma. Immunohistochemical analysis. (A) High infiltration area. Positive pericytes in new-formed vessels. NG2, DAB, x400. (B) High infiltration area. Positive pericytes in new-formed vessels. α-sm-actin, DAB, x400. (C) High infiltration area. Positive pericytes and endothelial cells in new-formed vessels. PDGFR, DAB, x400. (D) Infiltration area. Reactive astrocytes: cell bodies distant from vessels and end-feet adherent to them. GFAP, DAB, x400. (E) Co-option area. Positive cells in the inner part of perivascular cuffs. Nestin, DAB, x400. (F) Co-option area. Positive cells in the inner part of perivascular cuffs. SEL1L/CD34, DAB/RED, x200. (G) High proliferation area. Abundant macrophages around glomeruli. CD163, DAB, x200. (H) Glomerulus. Positive cells around channels and in the blood. CD11b, DAB, x200. (I) Positive endothelia. CXCL12, DAB, x200. (J) Positive cells in the tumor proliferation. CXCR4, DAB, x200. (K) Positive endothelia. CX3CR1, DAB, x400. (L) Positive reactive astrocytes. CCL2, DAB, x200. (M) Positive GAMs in the tumor. CCR2, DAB, x200. (N) Positive peri- and intravascular GAMs. CXCL2, DAB, x200. (O) Positive endothelia and tumor cells. CXCR2, DAB, x400.
In co-option areas, usually merging with the previous ones, cuffs of Nestin+ (Figure 2E), SOX2+ and SEL1L+ (Figure 2F) tumor cells surrounded small vessels recognizable by Factor VIII+ and CD34+ endothelial cells intermingled with NG2+, α-sm-actin+ and PDGFRβ+ pericytes. Reactive astrocytes no longer appeared in the cuffs. Iba1+, CD163+ and CD45+ microglia/macrophages adhered to the vessels.
The sprouts often gave origin to channels of proliferated endothelial cells which terminated with multi-channel formations or glomeruli that could be found still in the same areas or in adjoining areas depending on the length of the sprouts. Glomeruli did not appear to have any relation with the surrounding tissue, towards which there was a sharp interface, with the exception of macrophages, flattened on it. Within glomeruli many lumina were delimited by Vimentin+, Nestin+, CD34+ and Factor VIII+ endothelial cells and NG2+, α-sm-actin+, and PDGFRβ+ pericytes. Ibal+, CD163+ (Figure 2G), CD45+ and CD14+ cells surrounded glomeruli, strongly adherent to them, but often they were located inside the glomeruli as CD11b+ cells (Figure 2H). Rarely, lumina surrounded by pericytes, but not by endothelial cells, were observed.
Regressive areas: They were hemorrhagic, edematous or necrotic with mainly large or even preexisting vessels with thickened walls or thrombosed or of venous type, with a hypertrofic or thinned endothelium. All the vessels could be provided with pericytes and surrounding macrophages, but never showed GSCs/progenitors around them.
Sprouts, microvascular proliferations and glomeruli could be found mainly in avascular areas containing circumscribed necroses, but also distant from them.
Of all the vascular structures studied, the only ones with a direct contact of endothelial cells with GSCs/progenitors were found in infiltration and hyper-proliferation areas. Associated pericytes were a constant finding, followed by macrophages and, much more rarely, by reactive astrocytes as remnants of end-feet.
Chemokines and receptors: CXCL12/SDF-1 was found to be expressed in endothelial cells (Figure 2I) and its receptor CXCR4 in tumor cells (Figure 2J); CX3CL1 expression was not observed in our samples whereas CX3CR1 was found on endothelial cells and GAMs (Figure 2K); CCL2 was observed in reactive astrocytes (Figure 2L) and CCR2 in CD163+ GAMs (Figure 2M); CXCL2 was expressed in peri- and intravascular GAMs (Figure 2N) and CXCR2 in endothelial and tumor cells (Figure 2O).
Discussion
The direct contact of endothelial cells with GSCs/progenitors seems to be mandatory for the recognition of a niche in accordance with its definition [5]. GSCs/progenitors activate endothelial cells for angiogenesis by VEGF [16] and endothelial cells keep GSC/progenitor stemness and migration capacity by Notch signaling and NO [2,5,8,17] that condition tumor progression [18]. The perivascular position of GSCs/progenitors is the consequence of the occurrence of angiogenesis in high infiltration and hyper-proliferation areas, composed mainly by GSCs/progenitors [19-21] and regulated by tumor microenvironment. From the reciprocal signaling exchange between endothelial cells and GSCs/progenitors tumor progression and angiogenesis are triggered [22] with the latter originating from the switch from an avascular to a vascular state with matrix degradation and basal membrane dissolution. At the same time, blood-brain barrier (BBB) is disrupted [14], entailing macrophage release from blood that becomes a mark of GB. Bone marrow-derived infiltrating macrophages are recruited to the tumor early during GB development [23], i.e. starting when infiltration is still mild.
Our observations demonstrate that the cells exerting an influence on the niche function are, in order, pericytes and macrophages, followed at a distance by reactive astrocytes.
It has been recently demonstrated in mice xenografts of GL261 glioma cells that GAMs originate, at least initially, mainly from resident microglia [24] and only later blood-borne GAMs appear [11]. Most observations [25-29] are today in favor of an immunosuppressive, pro-tumor function of GAMs (30, 31), despite some doubts exist [32]. GAMs increase with malignancy [15,33-35], but only if RRM of low-grade gliomas (LGG) or of infiltrating areas of GB are disregarded [36]. In solid GB, GAMs are mainly represented by CD163+, CD45+, CD68+ cells, i.e. originating from BBB disruption that is a mark of malignancy [37]. They are mostly absent in LGG or in mild infiltration areas of GB, but they become frequent after BBB disruption and the consequent starting of angiogenesis. Chemokines and their receptors largely take part in this process [27], among which CXCL12 (SDF-1)/CXCR4, CX3CL1/CX3CR1 and CCL2/CCR2 are mainly involved, also in the niche function [8,38,39]. Of particular significance seems to be the expression of CXCL2 and VEGF in GAMs and of CXCR2 on endothelial cells that would indicate the angiogenic function of GAMs [11].
The second most important cell type in PVN is given by pericytes, demonstrated by NG2, PDGFRβ and α-sm-actin. In response to angiogenic signals, pericytes initially dissociate and lose the capacity of suppressing endothelial proliferation. On the other hand, pericytes derive from precursors, such as mesenchymal stem cells (MSCs) or hematopoietic precursors, induced to differentiate by the tumor microenvironment [40] and by transforming growth factor-β (TGF-β) [10]. Their recruitment depends on matrix metalloprotease (MMP-9) produced by CD45+ cells via SDF-1/CXCR4 signaling [41,42] and VEGF released from the extracellular matrix. It has been demonstrated that in mice with NG2 deficiency, as well as with collagen VI deficiency [43], the tumor growth is reduced, and that GSCs give rise to pericytes to support tumor growth and vessel function through remodeling PVN [10].
Reactive astrocytes are known not only to participate in BBB function with their end-feet, but also to favor tumor cell invasiveness [44-46]. However, in infiltrating tumor their cell bodies remain distant from vessels, whereas their end-feet on them mostly disappear with advancing cell invasion. Their role in niche function could be limited to the initial stages when they can induce GAMs through CCL2 production.
Conclusions
The niche function, as expression of the microenvironment, could be maintained by the interaction between endothelial cells and GSCs/progenitors with the participation of the signaling starting in GAMs and pericytes and, in a minor extent, in reactive astrocytes.
Acknowledgements
This work was supported by Cassa di Risparmio di Vercelli Foundation, Vercelli, Italy and by the Grant n. 2016.AAI2705.U3302 from Compagnia di San Paolo Foundation, Turin, Italy.
We thank Dr. Ida Biunno, Institute for Genetic and Biomedical Research, National Research Council, Milan, Italy, for kindly providing the SEL1L antibody.
Conflict of interest
Authors declare no conflict of interest.
References
- Swartz MA, Iida N, Roberts EW (2012) Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res 72: 2473-2480.
- Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H (2012) The brain tumor microenvironment. Glia 60: 502-514. [Crossref]
- Filatova A, Acker T, Garvalov BK (2013) The cancer stem cell niche(s): the crosstalk between glioma stem cells and their microenvironment. Biochim Biophys Acta 1830: 2496-2508.
- Lorger M (2012) Tumor microenvironment in the brain. Cancers (Basel) 4: 218-243. [Crossref]
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, et al. (2007) A perivascular niche for brain tumor stem cells. Cancer Cell 11: 69-82. [Crossref]
- Palmer TD, Willhoite AR, Gage FH (2000) Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425: 479-494. [Crossref]
- Shen Q, Goderie SK, Jin L (2004) Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science 304: 1338-1340.
- Hambardzumyan D, Bergers G (2015) Glioblastoma: Defining Tumor Niches. Trends Cancer 1: 252-265. [Crossref]
- Hardee ME, Zagzag D (2012) Mechanisms of glioma-associated neovascularization. Am J Pathol 181: 1126-1141. [Crossref]
- Cheng L, Huang Z, Zhou W (2013) Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 153: 139-152.
- Brandenburg S, Müller A, Turkowski K (2016) Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathol 131: 365-378.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (2016) WHO classification of tumours of the Central Nervous System. Revised 4th ed. Lyon, France: IARC pp: 1-408.
- Mellai M, Cattaneo M, Storaci AM (2015) SEL1L SNP rs12435998, a predictor of glioblastoma survival and response to radio-chemotherapy. Oncotarget 6: 12452-12467.
- Dvorak HF (2015) Tumors: wounds that do not heal-redux. Cancer Immunol Res 3: 1-11. [Crossref]
- Annovazzi L, Mellai M, Bovio E, Mazzetti S, Pollo B, et al. (2017) Microglia Immunophenotyping In Gliomas. Oncol Lett. In press.
- Bao S, Wu Q, Sathornsumetee S (2006) Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res 66: 7843-7848.
- Jeon HM, Kim SH, Jin X (2014) Crosstalk between glioma-initiating cells and endothelial cells drives tumor progression. Cancer Res 74: 4482-4492.
- Gilbertson RJ, Rich JN (2007) Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer 7: 733-736. [Crossref]
- Schiffer D, Mellai M, Annovazzi L, Caldera V, Piazzi A, et al. (2014) Stem cell niches in glioblastoma: a neuropathological view. Biomed Res Int 2014: 725921. [Crossref]
- Schiffer D, Mellai M, Annovazzi L, Casalone C, Cassoni P (2015) Tumor microenvironment-Perivascular and perinecrotic niches. In: Lichtor T, ed. Tumors of the Central Nervous System. (1st Edn). Rijeka, Croatia: InTech 49-82.
- Schiffer D, Annovazzi L, Mazzucco M, Mellai M (2015) The Microenvironment in Gliomas: Phenotypic Expressions. Cancers (Basel) 7: 2352-2359. [Crossref]
- Ho IAW, Shim WSN (2017) Contribution of the Microenvironmental Niche to Glioblastoma Heterogeneity. Biomed Res Int 2017: 9634172. [Crossref]
- Chen Z, Feng X, Herting CJ (2017) Cellular and Molecular Identity of Tumor-Associated Macrophages in Glioblastoma. Cancer Res 77: 2266-2278.
- Müller A, Brandenburg S, Turkowski K, Müller S, Vajkoczy P (2015) Resident microglia, and not peripheral macrophages, are the main source of brain tumor mononuclear cells. Int J Cancer 137: 278-288.
- Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, et al. (2011) Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One 6: e23902.
- Li W, Graeber MB (2012) The molecular profile of microglia under the influence of glioma. Neuro Oncol 14: 958-978. [Crossref]
- Glass R, Synowitz M (2014) CNS macrophages and peripheral myeloid cells in brain tumours. Acta Neuropathol 128: 347-362. [Crossref]
- Szulzewsky F, Pelz A, Feng X (2015) Glioma-associated microglia/macrophages display an expression profile different from M1 and M2 polarization and highly express Gpnmb and Spp1. PLoS One 10: e0116644.
- Zhai H, Heppner FL, Tsirka SE (2011) Microglia/macrophages promote glioma progression. Glia 59: 472-485. [Crossref]
- Kennedy BC, Showers CR, Anderson DE, Anderson L, Canoll P, et al. (2013) Tumor-associated macrophages in glioma: friend or foe? J Oncol 2013: 486912. [Crossref]
- Kaminska B (2014) Microglia in Gliomas: Friend or Foe? In: Sedo A, Mentlein R [Eds] Glioma Cell Biology. Berlin, Springer-Verlag, Germany pp: 241-270.
- Schiffer D, Mellai M, Bovio E, Annovazzi L (2017) The neuropathological basis to the functional role of microglia/macrophages in gliomas. Neurol Sci 38: 1571-1577. [Crossref]
- Komohara Y, Ohnishi K, Kuratsu J, Takeya M (2008) Possible involvement of the M2 anti-inflammatory macrophage phenotype in growth of human gliomas. J Pathol 216: 15-24.
- Prosniak M, Harshyne LA, Andrews DW, Kenyon LC, Bedelbaeva K, et al. (2013) Glioma grade is associated with the accumulation and activity of cells bearing M2 monocyte markers. Clin Cancer Res 19: 3776-3786. [Crossref]
- Ding P, Wang W, Wang J, Yang Z, Xue L (2014) Expression of tumor-associated macrophage in progression of human glioma. Cell Biochem Biophys 70: 1625-1631. [Crossref]
- Simmons GW, Pong WW, Emnett RJ (2011) Neurofibromatosis-1 Heterozygosity Increases Microglia in a Spatially- and Temporally-Restricted Pattern Relevant to Mouse Optic Glioma Formation and Growth. J Neuropathol Exp Neurol 70: 51-62.
- Hambardzumyan D, Gutmann DH, Kettenmann H (2016) The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci 19: 20-27. [Crossref]
- Terasaki M, Sugita Y, Arakawa F, Okada Y, Ohshima K, et al. (2011) CXCL12/CXCR4 signaling in malignant brain tumors: a potential pharmacological therapeutic target. Brain Tumor Pathol 28: 89-97.
- Hira VV, Ploegmakers KJ, Grevers F (2015) CD133+ and Nestin+ Glioma Stem-Like Cells Reside Around CD31+ Arterioles in Niches that Express SDF-1a, CXCR4, Osteopontin and Cathepsin K. J Histochem Cytochem 63: 481-493.
- Birnbaum T, Hildebrandt J, Nuebling G, Sostak P, Straube A (2011) Glioblastoma-dependent differentiation and angiogenic potential of human mesenchymal stem cells in vitro. J Neurooncol 105: 57-65.
- Cheng M, Qin G (2012) Progenitor cell mobilization and recruitment: SDF-1, CXCR4, α4-integrin, and c-kit. Prog Mol Biol Transl Sci 111: 243-264. [Crossref]
- Du R, Lu KV, Petritsch C (2008) HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13: 206-220.
- You WK, Bonaldo P, Stallcup WB (2012) Collagen VI ablation retards brain tumor progression due to deficits in assembly of the vascular basal lamina. Am J Pathol 180: 1145-1158.
- Marchetti D, Li J, Shen R (2000) Astrocytes contribute to the brain-metastatic specificity of melanoma cells by producing heparanase. Cancer Res 60: 4767-4770.
- Le DM, Besson A, Fogg DK (2003) Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator-plasmin cascade. J Neurosci 23: 4034-4043.
- Kostianovsky AM, Maier LM, Anderson RC, Bruce JN, Anderson DE (2008) Astrocytic regulation of human monocytic/microglial activation. J Immunol 181: 5425-5432.


