Case report
A 62-year-old man on immunosuppressive therapy because of heart transplant 15 years before, presented with progressive paraparesis, sensory loss with T10 level, lower limbs areflexia and sphincteric abnormalities.
A spinal MRI (T1 and T2 weighted) showed spinal cord swelling without contrast-enhancement. Because of the doubtful MRI results and the lack of a precise target site for biopsy a 18F-FDG PET/MRI was proposed to the patient in order to better clarify the nature of the lesion.
A limited number of papers [1-2] describing small case series of spinal cord tumors (using 18F-FDG or 11C-Methionine PET/CT alone or combined to MRI) reports a variable degree of uptake of lesions partially corresponding to the area of constrast enhancement. Plasma glucose was 108 mg/dL before PET acquisition (performed about 60 minutes after injection of 170 MBq of 18F-FDG). PET images were reconstructed using Dixon sequence for attenuation correction.
PET-MRI (T2, T1, with and without fat saturation) showed diffuse irregular spinal uptake of 18F-FDG (Figure1) without areas of clear, high focal uptake. A pan-medullary tumor was then suspected because no increased uptake of 18F-FDG is physiologically evident in the normal spinal cord and because no other traumatic, inflammatory or infective clinical data explained PET data. Caudally to D10-D11 level the uptake of 18F-FDG was however slightly lower and therefore spinal cord biopsy was performed at D9 level (Figure2).
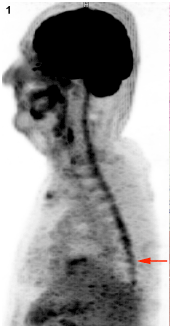
Figure 1. Diffuse spinal 18F-FDG uptake (MIP, sagittal). Arrow: site of surgery.
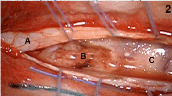
Figure 2. Intra-operative view after T9-T10 laminectomy: congested diffusely infiltrated spinal cord (A: dura; B: surgical cavity; C: spinal cord).
Histology showed low-grade astrocytoma (grade II, WHO classification) (Figure 3-4). Primary pan-medullary tumors are rare, and the diagnosis is challenging. In our case combined 18F-FDG PET-MRI was crucial to confirm the clinical suspect and to plan the site of spinal cord biopsy.
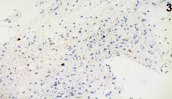
Figure 3. Immunohistochemistry (X40): MIB1 cells reactivity in scattered, atypical nuclei.
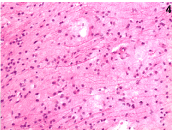
Figure 4. Histopathology (H&E X40): neoplastic astrocytes with large and hyperchromatic nuclei.
Compliance with ethical standards
Funding
This study was NOT funded by grants.
Conflict of interest
All the authors declare that they have no conflict of interest.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Individual contributions of the authors
Chiara Briani is the neurologist in charge of the patient.
Alessandro Della Puppa is the neurosurgeon who performed the spinal cord biopsy.
Diego Cecchin is the nuclear medicine physician who performed and interpreted the PET-MRI.
Mariagiulia Anglani is the radiologist physician who jointly interpreted the MRI portion of the PET/MRI.
Marina Paola Gardiman is the neuropathologist who made the histological diagnosis.
Chiara Briani, Alessandro Della Puppa, and Diego Cecchin wrote the draft of the manuscript.
All the authors helped in the analysis and interpretation of data.
All the Authors have read and approved the final version of the manuscript.
All the Authors report no disclosures.
References
- Wilmshurst JM, Barrington SF, Pritchard D, Cox T, Bullock P, et al. (2000) Positron emission tomography in imaging spinal cord tumors. J Child Neurol. 15:465-72.
- Tomura N, Ito Y, Matsuoka H, Saginoya T, Numazawa SI, et al. (2013) PET Findings of Intramedullary Tumors of the Spinal Cord Using [18F] FDG and [11C] Methionine. AJNR Am J Neuroradiol 34: 1278-1283. [Crossref]




