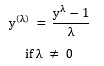Background: Chronic Obstructive Pulmonary Disease (COPD) can be divided into emphysema dominant, airway dominant, or mixed phenotypes using CT.
Objective: We aimed to classify subjects with COPD in Tokyo based on CT findings and to investigate how these findings relate to clinical manifestation.
Methods: The percentage of lung low attenuation area (%LAA) on chest CT was used to evaluate emphysema while airway wall thickness (Pi10) was used to evaluate airway disease. We introduced PVA on chest CT as an index of pulmonary vessel area. Reference ranges of %LAA and Pi10 were established based on chest CT normal subjects. Subjects with COPD were divided into four phenotypes using upper limits of reference ranges. Correlation between clinical findings and quantitative CT parameters were estimated. The proportions of each phenotype were evaluated based on Chronic Obstructive Lung Disease (GOLD) assessment.
Results: Reference ranges were established based on 14 chest CT normal subjects. Twenty-five subjects with COPD were classified as emphysema dominant phenotype (52%), mixed phenotype (24%), airway dominant phenotype (12%), and CT normal phenotype (12%). %LAA correlated with FEV1/FVC more than with Pi10 and %PVA. CAT and mMRC scores significantly correlated with FEV1/FVC. No differences in the proportions of each phenotype were observed upon GOLD assessment.
Conclusion: The emphysema dominant COPD phenotype was the most prevalent in Tokyo.
COPD, emphysema, small airway desease, %FEV1,
Abbreviations
COPD: Chronic Obstructive Pulmonary Disease; CT: Computed Tomography; GOLD: Global Initiative for Chronic Obstructive Lung Disease; LAA: Low Attenuation Area; FEV1: Forced Expiratory Volume in one second; FVC: Forced Vital Capacity; CAT: COPD Assessment Test; mMRC: modified Medical Research Council; HU: Hounsfield Unit; Pi10: square root of wall area of a 10-mm lumen perimeter; %FEV1: percent predicted Forced Expiratory Volume in one second; PVA: Pulmonary Vessel Area; QOL: Quality Of Life
Chronic Obstructive Pulmonary Disease (COPD) is a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases [1]. In addition to the considerable morbidity and mortality associated with COPD, this disease incurs significant healthcare and societal costs [2]. The chronic airflow limitation, characteristic of COPD, is caused by a mixture of parenchymal destruction (emphysema) and small airways disease (e.g., obstructive bronchiolitis). Emphysema is the pathological lesion that correlates most closely with loss of lung elastic recoil,while the airway component is characterized by thickening and narrowing of the membranous bronchioles [3]. Quantitative computed tomography (CT) has been used to quantify the extent of emphysema and airway wall thickness [3-5].
Classifying patients with COPD into the emphysema dominant, airway dominant, or mixed phenotypes may aid in the study of its pathogenesis, pharmacological interventions and, ultimately, in the choice of patient-specific therapy [3]. We hypothesize that the phenotype of COPD varies depending on the geographical region. However, such a study has not been done yet.
The aim of this study is to use CT quantitative measurements to classify subjects with COPD in Tokyo into four phenotypes: emphysema dominant, airway dominant, mixed phenotype, or normal phenotype, and then examine how they are related to the severity of airflow limitation and ABCD assessment by Global Initiative for Chronic Obstructive Lung Disease (GOLD) [1].
Study subjects
The subjects were recruited while visiting the Tokyo Medical and Dental University Medical Hospital for treating COPD (subjects with COPD) or other diseases (chest CT normal subjects), who underwent chest CT examination. Subjects with severe liver, kidney, heart, or blood disease, or with other serious complications, and subjects whose attending physician regarded as inappropriate for the study were excluded. Subjects without 1.0 mm thickness CT images were excluded. Also, subjects with COPD with abnormal findings other than low attenuation area (LAA) or airway wall thickening on the CT slice to be analyzed were excluded. Subjects showing normal findings on the CT slice to be analyzed were selected as chest CT normal subjects.
COPD was defined according to the GOLD criteria [1]: a postbronchodilator FEV1/FVC (forced expiratory volume in one second /forced vital capacity) ratio below 0.70. Informed written consents were obtained from all subjects.
Pulmonary function tests
Spirometry measurements were carried out using the Autospirometer System21 [MINATO MEDICAL SCIENCE CO., LTD. Osaka, Japan] according to the Japanese Respiratory Society guideline [6].
Symptom assessment
Health status measures were obtained from the COPD assessment test (CAT) [7] and the modified Medical Research Council (mMRC) questionnaire [7].
The CAT assesses several aspects affecting COPD patients, ranging from symptoms, health status, and well-being [7]. Each item presents with a rating ranging from the best (score of 0) to the worst (score of 5) for that statement. The scores for each of the 8 items are then added up to give one final score (with a minimum of 0 and a maximum score of 40). Higher values of the total CAT score imply poor health status of the individual. We categorized 8 items into three categories; daily symptoms (cough, phlegm, and chest tightness), activity limitation (breathlessness and activities), and emotional health and feeling (confidence, sleep, and energy).
The mMRC dyspnea score is obtained from a questionnaire consisting of five statements, which provide a measure of perceived breathlessness [7]. High mMRC scores signify severe breathlessness.
CT scanning
All patients were scanned, by using the 64-slice CT scanner, Toshiba Aquilion TSX-101A (Toshiba Corp, Tokyo, Japan), while holding their breath at deep inspiration. Scan data were obtained in spiral mode setting 120 kVp 500 mA. The CT images were reconstructed with 1.0 mm thickness and 512 × 512 matrix and then exported as Digital Imaging and Communications in Medicine (DICOM) [Medical Imaging & Technology Alliance (MITA), VA, USA] files.
Quantification of emphysema
All CT images were analyzed using the Image J 1.50i software (Wayne Rasband National Institutes of Health, USA; https://imagej.nih.gov/ij/). Three slices were selected from the upper lung field (1 cm above the aortic arch) (Fig. 1a), the middle lung field (1 cm below the trachea bifurcation), and the lower lung field (1 cm below the lower right pulmonary vein).
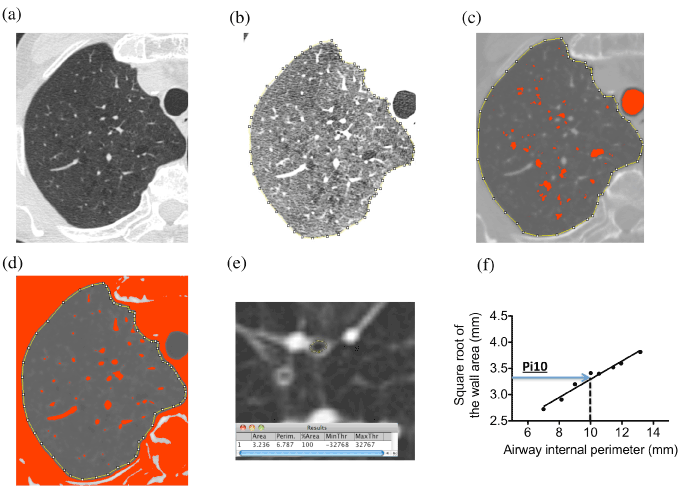
Figure 1. Determination of total lung area, LAA, PVA, and Pi10 on CT.
(a) Upper right lung area of subjects with COPD on CT image.
(b) Manually surrounded lung total area of (a).
(c) Lung area of (a) with the threshold values < -950 HU (LAA).
(d) Lung area of (a) with the threshold values > -700 HU (PVA).
(e) Measurement of bronchi.
(f) Linear regression after plotting the square root of the airway wall area against the internal perimeter.
To obtain areas of lung fields, we manually surrounded the left and right lung fields (total of 6 lung fields) using the Image J software (Fig. 1b). The extent of emphysema was assessed by measuring the percentage of lung area with low attenuation area (%LAA) < -950 Hounsfield units (HU) [8]. The areas of LAA in the same lung fields were calculated by using the image J software (Fig. 1c). %LAA was calculated by dividing the total area of LAA by the total area of lung fields.
Quantification of large pulmonary vessels
The %PVA (pulmonary vessels area) was calculated in the same lung fields as the %LAA. We defined PVA as > -700 HU. PVA was mainly composed of pulmonary vessels (Fig. 1d). The percentage PVA (%PVA) was calculated by dividing the total area of PVA by the total area of lung fields.
Quantification of airway wall thickness
We selected 8 airways in the subsegmental and subsubsegmental bronchi in the right superior lobe apical segment, left superior lobe apical-posterior segment, and right and left inferior lobe posterior-basal segment for each patient based on previous studies [9,10]. To overcome possible errors from sampling bias and from airway size variation in different individuals, a standardized measure for airway wall thickness was derived for each subject by plotting the square root of the airway wall area against the internal perimeter of each airway cut in cross-section [4,8-13] (Fig. 1e, 1f). The resulting regression line was used to predict the square root of the wall area of an arbitrary airway with an internal perimeter of 10 mm and to calculate its average wall thickness (Pi10) (Fig. 1f) using TREND function of Microsoft Excel for Mac 2011 version 14.4.8.
COPD phenotypes
As previously reported, subjects with COPD can be divided into four groups based on their CT measurements [3,12]. We set upper limits of normal (ULN) for the CT measurements of %LAA and Pi10 from chest CT normal subjects.
%LAA from chest CT normal subjects did not show normal distribution. To obtain a reference range of %LAA, results from normal subjects were transformed to a more normal distribution using Box-Cox transformation [14]. The one-parameter Box-Cox transformation was defined as:[14]
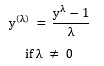
After confirming a normal distribution of the Box-Cox transformed values by D’Agostino and Pearson omnibus normality test, the Box-Cox transformed reference range was set as the average transformed value ± 2 standard deviation (SD).
Pi10 from chest CT normal subjects showed normal distribution, therefore, a reference range was set as an average value ± 2SD.
The four groups were: emphysema dominant phenotype, %LAA > ULN and Pi10 ≤ ULN; airway dominant phenotype, %LAA ≤ ULN and Pi10 > ULN; mixed phenotype, %LAA > ULN and Pi10 > ULN; and CT normal phenotypes, %LAA ≤ ULN and Pi10 ≤ ULN; respectively.
Correlation between FEV1/FVC (%) and quantitative CT parameters in subjects with COPD
In order to evaluate the relationship between pulmonary function and changes in pulmonary structure, the correlation between FEV1/FVC and quantitative CT parameters (%LAA, Pi10, and %PVA) were examined by the Spearman’s rank correlation coefficient.
Correlation of CAT and mMRC with FEV1/FVC, %LAA, and Pi10 in subjects with COPD
The relationships between health status and pulmonary function or changes in pulmonary structure were examined by the Spearman’s rank correlation coefficient.
GOLD assessment
Subjects were stratified by using the GOLD 2019 comprehensive assessment [1]. Subjects were stratified on the severity of airflow limitation; grade 1 (FEV1 ≥ 80% predicted), grade 2 (50% ≤ FEV1 < 80% predicted), grade 3 (30% ≤ FEV1 < 50% predicted), grade 4 (FEV1 < 30% predicted). Subjects were also stratified based on symptom burden and risk of exacerbation. Symptom burden with a score (mMRC grade 0–1 and CAT < 10) results in two low-symptom categories (group A and group C), and with a score (mMRC grade ≥2 or CAT ≥ 10) results in two high-symptom categories (group B and group D). Exacerbation risk was assessed only by COPD exacerbation history in the previous year (0 vs ≥1 hospitalization); this allowed stratification of patients into low-risk categories (group A and group B) and high-risk (group C and group D) categories. In the two categories of GOLD grade and group, we examined the proportion of emphysema dominant, airway dominant, mix, and normal phenotypes of COPD.
Statistical analyses
Statistical analyses were carried out using the GraphPad Prism version 5.0 (GraphPad Software, San Diego, USA). D’Agostino and Pearson omnibus normality test was used to examine the normality of CT parameters for chest CT normal subjects. The Mann-Whitney's U test or the Chi-square test was used to compare groups. The Spearman’s rank correlation coefficient was used to evaluate relationships between variables.
The values of l in the Box-Cox transformations were calculated using the “power Transform function” in the “car” package in R ver. 3.4.1 (The R Foundation for Statistical Computing R; https://www.r-project.org/foundation/) with EZR ver. 1.36 (Saitama Medical Center, Jichi Medical University) [15], a graphical user interface for R used to import data. P < 0.05 were regarded as statistically significant.
This study was approved by the ethics committee of the Tokyo Medical and Dental University (approval number M295-554).
Subjects characteristics
Among the 44 subjects recruited, 3 subjects were excluded because CT images of 1.0 mm thickness were not obtained while 2 subjects were excluded because fibrosis was observed on CT image.
The characteristics of the 25 subjects with COPD and 14 chest CT normal subjects are shown in Table 1. Subjects with COPD were significantly older and had more pack-years of smoking history when compared with chest CT normal subjects. The spirometry results from subjects with COPD showed obstructed lung disorder. The pack-years and mMRC score were significantly higher in subjects with COPD. The CAT score was higher in subjects with COPD, however, their difference was not significant. The airway wall thickness, assessed by Pi10, was thicker and the extent of emphysema, assessed by %LAA, were more severe in subjects with COPD when compared with those of subjects with normal chest CT (p < 0.05).
Table 1. Subject characteristics
|
chest CT normal subjects
(n = 14) |
COPD
(n = 25) |
p value |
Age |
64.6 ± 10.9 |
72.2 ± 6.54 |
0.016 |
BMI |
21.8 ± 2.66 |
22.1 ± 3.28 |
0.97 |
Pack-Years |
13.2 ± 29.3 |
65.5 ±33.1 |
<0.0001 |
CAT (total) |
9.36 ± 10.0 |
8.84 ± 5.90 |
0.62 |
CAT (daily symptom) |
3.93 ± 4.14 |
3.56 ± 2.53 |
0.71 |
CAT (activity limitation) |
2.93 ± 2.76 |
3.04 ± 1.97 |
0.54 |
CAT (emotional health and feeling ) |
2.50 ± 3.84 |
2.24 ± 2.50 |
0.67 |
mMRC |
0.36 ± 0.84 |
1.04 ± 1.02 |
0.01 |
FEV1/FVC (%) |
73.4 ± 2.65 |
51.4 ± 11.9 |
<0.0001 |
%FEV1 |
96.7 ± 7.09 |
67.3 ± 24.7 |
0.0020 |
%LAA |
0.44 ± 0.62 |
10.1 ± 10.1 |
<0.0001 |
Pi10 (mm) |
3.26± 0.11 |
3.4 ± 0.22 |
0.029 |
%PVA |
6.06 ± 1.52 |
5.88 ± 1.4 |
0.69 |
Data are mean ± SD
Comparisons between two groups were carried out by Mann-Whitney's U test.
COPD, chronic obstructive pulmonary disease; BMI, body mass index; CAT, COPD Assessment Test; mMRC, modified Medical Research Council; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; %LAA, percentage of low attenuation area; Pi10, square root of wall area of a 10-mm lumen perimeter; %PVA, percentage of pulmonary large vessels area
Comparisons data among CT-based phenotypes
Based on the ULN for %LAA and Pi10 the following classification of the 25 subjects was done: emphysema dominant phenotype (n = 13, 52%), airway dominant phenotype (n = 3, 12%), mixed phenotype (n = 6, 24%), and normal phenotype (n = 3, 12%). Figure 2 shows the distribution of subjects with COPD in relation to %LAA and Pi10.
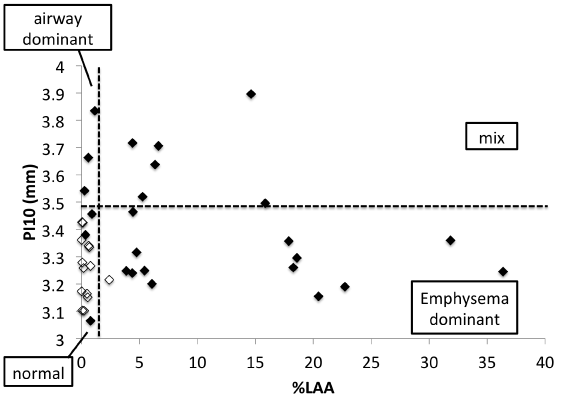
Figure 2. Relationship between Pi10 and the %LAA.
Filled symbols represent subjects with COPD (n = 25), open symbols represent chest CT normal subjects (n = 14).
Vertical line shows mean + 2SD of %LAA of the chest CT normal subjects (upper limits of normal (ULN)). Horizontal line shows the mean + 2SD of Pi10 of the chest CT normal subjects (ULN). Using these cut-off values, subjects with COPD can be divided into 4 phenotypes; emphysema dominant (n = 13), %LAA > ULN and Pi10 ≤ ULN; airway dominant (n = 3) %LAA ≤ ULN and Pi10 > ULN; mixed %LAA > ULN and Pi10 > ULN (n = 6); and normal phenotypes (n = 3), %LAA ≤ ULN and Pi10 ≤ ULN, respectively.
Table 2 shows the correlation of subject characteristics and CT parameters in subjects with COPD. With regards to FEV1/FVC, a stronger correlation was observed with %LAA than with Pi10 (Fig. 3a, 3b). The %PVA had a negative correlation with %LAA but it had a positive correlation with Pi10 and FEV1/FVC (Fig. 3c).
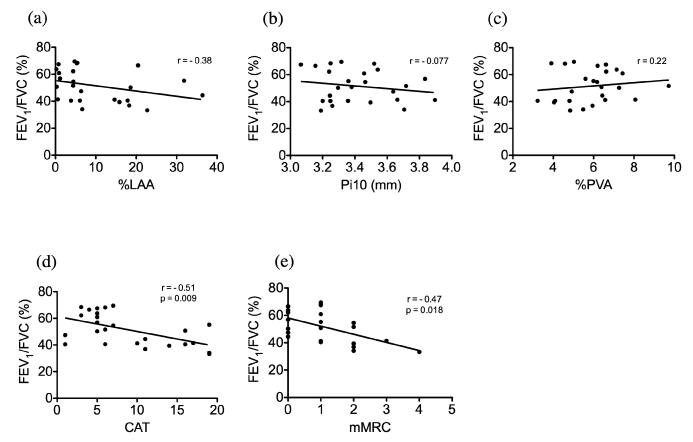
Figure 3. Correlation between FEV1/FVC and quantitative CT parameters in subjects with COPD.
(a) Relationship between FEV1/FVC and %LAA.
(b) Relationship between FEV1/FVC and Pi10.
(c) Relationship between FEV1/FVC and %PVA.
(d) Relationship between FEV1/FVC and CAT.
(e) Relationship between FEV1/FVC and mMRC.
Table 2. Spearman’s rank correlation coefficient for %LAA, Pi10 and %PVA in subjects with COPD
|
%LAA |
Pi10 |
%PVA |
r |
p |
r |
p |
r |
p |
Age |
-0.32 |
0.11 |
-0.05 |
0.78 |
0.066 |
0.75 |
BMI |
-0.12 |
0.57 |
0.19 |
0.57 |
0.11 |
0.61 |
Pack-years |
-0.20 |
0.37 |
0.16 |
0.43 |
0.37 |
0.083 |
FEV1/FVC |
-0.38 |
0.06 |
-0.077 |
0.72 |
0.22 |
0.28 |
%FEV1 |
-0.24 |
0.25 |
-0.16 |
0.50 |
0.033 |
0.88 |
%PVA |
-0.34 |
0.09 |
0.18 |
0.39 |
|
|
Table 3 shows the correlation of CAT and mMRC with FEV1/FVC, %LAA, and Pi10 in subjects with COPD. The total CAT and mMRC scores had significant negative correlations with FEV1/FVC (Fig. 3d, 3e). After CAT stratification, FEV1/FVC correlated significantly with the daily symptoms and activity limitations but not with the emotional health and feelings of subjects (Table 3). The %LAA and Pi10 did not correlate significantly with CAT or mMRC (Table 3).
Table 3. Spearman’s rank correlation coefficient between CAT, mMRC and FEV1/FVC , %LAA, Pi10 in subjects with COPD
|
FEV1/FVC |
%LAA |
Pi10 |
r |
p |
r |
p |
r |
p |
CAT (total) |
-0.51 |
0.009 |
0.19 |
0.36 |
0.18 |
0.38 |
CAT (daily symptom) |
-0.43 |
0.030 |
0.18 |
0.40 |
0.18 |
0.39 |
CAT (activity limitation) |
-0.56 |
0.003 |
0.17 |
0.40 |
0.085 |
0.69 |
CAT (emotional health and feeling ) |
-0.35 |
0.087 |
0.064 |
0.76 |
0.044 |
0.84 |
mMRC |
-0.47 |
0.018 |
0.010 |
0.97 |
0.15 |
0.46 |
BMI: body mass index; CAT: COPD Assessment Test; mMRC: modified Medical Research Council; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; %LAA: percentage of low attenuation area; Pi10: square root of wall area of a 10-mm lumen perimeter; %FEV1; %PVA: percentage of pulmonary vessels area
The proportion each phenotype of COPD in the two categories of GOLD grade (1, 2, 3, 4) and group (A, B, C, D) are shown in Fig. 4. There were no significant differences in the percentage of the four phenotypes among the GOLD grades and groups.
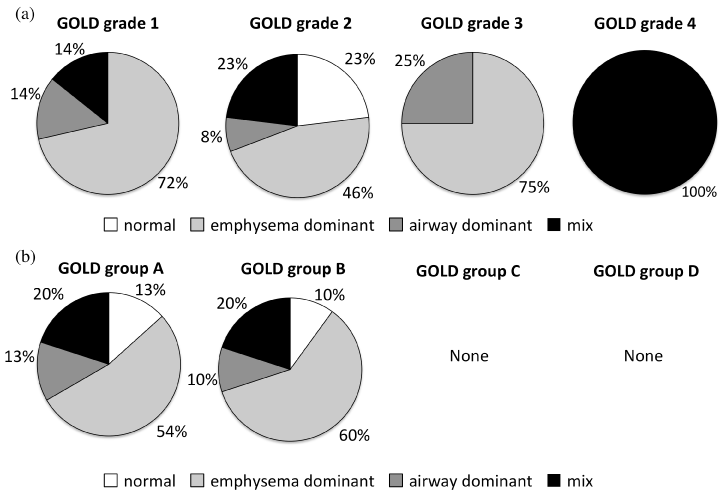
Figure 4. Phenotypic proportion by GOLD 2019 assessment.
(a) Stratification by severity of airflow limitation.
(b) Stratification by symptom burden and risk of exacerbation.
Based on the ULNs for %LAA and Pi10, subjects with COPD were classified into four distinct phenotypes; over half was classified as the emphysema dominant phenotype. We had hypothesized that there were many emphysema dominant patients in Tokyo, and this study confirmed our assumption. Previous studies investigated the ratio of each phenotype in some regions [3,12,16]. They also showed that the emphysema dominant phenotype accounts for the largest proportion of COPD type in Kyoto and Shiga, Japan, and in Ho Chi Minh, Vietnam.
Our study showed that FEV1/FVC and %FEV1 correlated with the %LAA more than with Pi10. Our result was consistent with previous studies [10,12,17].
Since the GOLD grade classification of airflow limitation is based on %FEV1, we hypothesized that the proportion of emphysema type increased as airflow limitation grade progressed. Although we could not find significant differences among grades, we could not draw conclusions because of the low numbers in each grade.
Patients with frequent exacerbations belong to group C or D in the GOLD classification. We had hypothesized that the proportion of airway dominant or mixed COPD phenotype is larger in patients with frequent exacerbations because inflammation in the bronchi is supported to be involved in COPD exacerbation. However, no COPD patients with frequent exacerbation were recruited in this study and, therefore, we could not draw any conclusion.
The CAT total score correlated significantly with FEV1/FVC. In addition to an overall score, we further stratified the CAT questionnaire to identify specific areas of greater severity as the consequence of impaired respiratory physiological functions and pulmonary morphological changes. As the result, the respiratory physiological functions (FEV1/FVC) was strongly correlated with daily symptoms, activity limitation, and emotional health and feeling.
We think that the decrease in quality of life (QOL) of patients with COPD in Tokyo is more related to FEV1/FVC than to emotional health and feeling. Our results would provide the basis for further research about the regional differences on COPD.
In COPD, blood vessels are lost with pulmonary parenchyma. We introduced PVA as the novel index of pulmonary vessel area. PVA is supposed to mainly reflect the area of large pulmonary vessels. Our findings that %PVA was decreased as COPD progressed demonstrate that %PVA can be a good marker of the proportion of pulmonary blood area. Future studies can, therefore, utilize PVA for pulmonary circulation research such as pulmonary blood pressure or cardiac function in COPD research.
Regional differences in races are seen among the Japanese people, especially in Okinawa and Hokkaido, which are thought to be genetically different from the mainland [18]. In emphysema, pulmonary alveoli, blood vessels, and pulmonary parenchyma are thought to be mainly destroyed by matrix metalloproteinases [19,20]. Fibrosis caused by Transforming Growth Factor-ß is thought to be involved in airway diseases in COPD [21]. The regional differences in COPD phenotypes can be explained by the differences of inhaled substances and the genetic differences in the reactions to stimulants. Based on our results in Tokyo, future research focusing on the COPD phenotype in each region will clarify the pathogenesis of emphysema and airway disease in relation to genetic backgrounds and differences of inhaled substances.
In conclusion, among the four COPD phenotypes, the emphysema dominant phenotype was the most prevalent in Tokyo. There were no significant differences in the percentage of the four phenotypes among the GOLD grades and groups.
Authorship statement
Authors’ contributions Takao Miyoshi and Yuki Sumi designed the study and contributed to collection, analysis, and interpretation of data, and wrote the manuscript. All other authors have contributed to collect and interpret the data. All authors approved the final version of the manuscript, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure statement
The authors declare no conflicts of interest associated with this manuscript.
We would like to thank Editage (www.editage.jp) for English language editing.
- 2019 Global strategy for prevention, diagnosis and management of COPD [Accessed December 14, 2018]. Available from: https://goldcopd.org/gold-reports/
- Mannino DM, Kiriz VA (2006) Changing the burden of COPD mortality. Int J Chron Obstruct Pulmon Dis 1: 219-233. [Crossref]
- Ogawa E, Nakano Y, Ohara T, Muro S, Hirai T, et al. (2009) Body mass index in male patients with COPD: correlation with low attenuation areas on CT. Thorax 64: 20-25. [Crossref]
- Mohamed Hoesein FA, de Jong PA, Lammers JW, Mali WP, Schmidt M, et al. (2015) Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur Respir J 45:644-651. [Crossref]
- Coxson HO, Mayo J, Lam S, Santyr G, Parraga G, et al. (2009) New and current clinical imaging techniques to study chronic obstructive pulmonary disease. Am J Respir Crit Care Med 180: 588-597. [Crossref]
- Japan Respiratory Society Pulmonary Physiology Special Committee. Respiratory function tests guideline – Spirometry, Flow-volume curve, Diffusion capacity of the lung -. Medical Review Co., Ltd, Tokyo, 2011.
- Sciriha A, Lungaro-Mifsud S, Scerri J, Magro R, Camilleri L, et al. (2017) Health status of COPD patients undergoing pulmonary rehabilitation: A comparative responsiveness of the CAT and SGRQ. Chron Respir Dis 14: 352–359. [Crossref]
- Camiciottoli G, Bigazzi F, Paoletti M, Cestelli L, Lavorini F, et al. (2013) Pulmonary function and sputum characteristics predict computed tomography phenotype and severity of COPD. Eur Respir J 42: 626-635. [Crossref]
- Martinez CH, Chen YH, Westgate PM, Liu LX, Murray S, et al. (2012) Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax 67: 399-406. [Crossref]
- Nambu A, Zach J, Schroeder J, Jin G, Kim SS, et al. (2016) Quantitative computed tomography measurements to evaluate airway disease in chronic obstructive pulmonary disease: Relationship to physiological measurements, clinical index and visual assessment of airway disease. Eur J Radiol 85: 2144-2151. [Crossref]
- Johannessen A, Skorge TD, Bottai M, Grydeland TB, Nilsen RM, et al. (2013) Mortality by level of emphysema and airway wall thickness. Am J Respir Crit Care Med 187: 602-608. [Crossref]
- Higami Y, Ogawa E, Ryujin Y, Goto K, Seto R, et al. (2016) Increased Epicardial Adipose Tissue Is Associated with the Airway Dominant Phenotype of Chronic Obstructive Pulmonary Disease. PLoS One 11: e0148,9794. [Crossref]
- Grydeland TB, Dirksen A, Coxson HO, Eagan TM, Thorsen E, et al. (2010) Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med 181: 353-359. [Crossref]
- Box GEP, Cox DR (1964) An analysis of transformations. Journal of the Royal Statistical Society, Series B. 26: 211-52. Available at https://www.ime.usp.br/~abe/lista/pdfQWaCMboK68.pdf
- Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48: 452-458. [Crossref]
- Van Tho N, Ogawa E, Trang le TH, Ryujin Y, Kanda R, et al. (2015) A mixed phenotype of airway wall thickening and emphysema is associated with dyspnea and hospitalization for chronic obstructive pulmonary disease. Ann Am Thorac Soc 12: 988-996. [Crossref]
- Tho NV, Ryujin Y, Ogawa E, Trang le TH, Kanda R, et al. (2015) Relative contributions of emphysema and airway remodelling to airflow limitation in COPD: Consistent results from two cohorts. Respirology 20: 594-601. [Crossref]
- Jinam T, Nishida N, Hirai M, Kawamura S, Oota H, et al. (2012) The history of human populations in the Japanese Archipelago inferred from genome-wide SNP data with a special reference to the Ainu and the Ryukyuan populations. J Hum Genet 57: 787-795. [Crossref]
- Dahl R, Titlestad I, Lindqvist A, Wielders P, Wray H, et al. (2012) Effects of an oral MMP-9 and -12 inhibitor, AZD1236, on biomarkers in moderate/severe COPD: a randomised controlled trial. Pulm Pharmacol Ther 25: 169-177.
- Navratilova Z, Zatloukal J, Kriegova E, Kolek V, Petrek M (2012) Simultaneous up-regulation of matrix metalloproteinases 1, 2, 3, 7, 8, 9 and tissue inhibitors of metalloproteinases 1, 4 in serum of patients with chronic obstructive pulmonary disease. Respirology 17: 1006-1012. [Crossref]
- Hoang LL, Nguyen YP, Aspeé R, Bolton SJ, Shen YH, et al. (2016) Temporal and Spatial Expression of Transforming Growth Factor-ß after Airway Remodeling to Tobacco Smoke in Rats. Am J Respir Cell Mol Biol 54: 872-881. [Crossref]