Cellulose obtained from the bark, wood or leaves of plants is a natural binder which possesses many unique properties. Many synthetic binders are biodegradable and can be tailor-made easily. However, cellulose is completely biodegradable polymers and has potential applications in biomedical and environmental fields. Therefore, it received great attention and was extensively investigated. In the current work porous hydroxyapatite scaffolds are prepared by polymeric replication method using cellulose (a natural binder). The influence of binder concentration on the structure and mechanical properties of scaffold is studied. HAP scaffolds are characterized for phase purity, structural analysis and mechanical properties. It is inferred that it is possible to produce bio compatible hydroxyapatite scaffolds with highly inter connected macro and micro pores with an ultimate compressive strength of 0.65 MPa
hydroxyapatite scaffolds, 3D porous structure, natural gum, cellulose, compressive strength
Tissue engineering is a field where cells bone/ scaffolds and signals/factors are mutually joined with the endeavour to re-establish, preserve and improve tissue and organ utility. Calcium phosphates are amongst the most widely used resources for bone tissue regeneration. They can be man-made as gels, pastes and solid blocks or even as porous matrices, with orthopaedics and dentistry being their main areas of relevance. Hydroxyapatite (HAP) are the most frequently used calcium phosphates, owed to their Stoichiometric ratio (Ca/P) ratios close to that of natural bone and also for their stability when in contact with physiological environment. HAP is a major constituent of bone resource and is resorbed after a long time in the body, due to its biocompatibility [1]. The porous network or interconnected pores in HAP structure permit the tissue to penetrate, which further enhances the implant tissue attachment [2].
Several methods have been investigated to achieve the required porous scaffolds. Sopyan, et al. has studied with pore-creating volatile particles, ceramic foaming methods and polymeric sponge process [3]. The polymeric sponge technique, which offers great flexibility, is particularly of interest due to its greater advantages such as opportunity to control the pore size, forseveral required complex shapes and straightforward process (Tian and Tian 2001). on the other hand, the properties of the Hydroxyapatite scaffold prepared through the polymeric sponge technique highly depending on the slurry properties together with homogeneity, rheology and dispersion. Monmaturapoj has reported the dispersant on the rheological behaviour of concentrated hydroxyapatite suspensions [4]. In this study, porous hydroxyapatite scaffolds were obtained using the polymer replication method, using cellulose as a binding agent and the morphology and its physio-chemical properties were studied. The structure of cellulose is shown in Figure 1, it is an polysaccharide and an organic compound with the formula (C6H10O5)n, and also an important structural component of the primary cell all of green plants. It is one of the most abundant organic polymer on Earth, which constitutes to about 90% in cotton fibre, 40–50% in wood and approximately 57% in dried hemp. Hence, these scaffolds made from cellulose can be used as matrices for bone tissue engineering or as specific release vehicles. Also, they may be functionalized with molecules such as collagen, Chitosan, etc, in order to enhance their biological responses [5].
Sample Preparation: The Hydroxyapatite powder with an average crystallite size of approximately40nm was prepared according to our previous work by sol-gel [6,7]. The template used to prepare scaffolds were polyurethane sponges with an average pore size of 400 microns. The Cellulose used as a binder was made into a homogenous mixture by mixing for 2h with water at 50ºC. To fabricate scaffolds, a ceramic slurry containing 6 g of Hydroxyapatite powder, binder, tensioactive agent (A40V Dispexfrom BASF) and water [8] were blended again for 2 h to disperse thoroughly. The template was removed by heating in a muffle furnace at 600 ͦ C for 2 hrs, followed by the densification of scaffold by sintering at 1150 ͦ C for 4 h. Now, these prepared scaffolds were cut into cubes for further characterization.
Physio-Chemical Characterization
The microstructure of the hydroxyapatite scaffolds were characterized using optical stereo zoom microscope. The phase purity of the Hydroxyapatite scaffolds was determined by X-ray diffraction using a X’pertPro, Philips, TheNetherlands with CuKα radiation over the 2θ range of 10°-80° with a step size of 0.05°. The functional group analysis of HAP scaffolds was carried out in the spectral range from 4000to 650cm−1 using a single beam Fouriertransform infrared spectrometer (Agilent, Cary 630). The compressive strength of scaffolds were tested with an INSTRON materials testing machine (model 3365, Instron Corp., UK) using a cross-head speed of 1mm/min with a maximum load of 30N load cell. The porosity and density measurements of the scaffolds were calculated by simple displacement techniques [8].
It is found that the scaffolds replicated the pores in the sponges with a pore size of approximately 500 microns. Thus, highly porous Hydroxyapatite scaffolds were produced using the polymer replication method as seen in Figure 1. With SEM observations, pore diameters ranging from 400 to 500 μm were observed. On the other hand, micropores of size lesser than micron werealso visualised in the pore walls and wall struts. The open as well as interconnected pore network was an essential factor for the scaffold to permit cell growth and the transportation of nutrients and metabolic waste. Gotz, et al. has reported that poresizes around 300 μm were suggested for implants due to improved new bone and capillary formation. Hollister, et al. [9] conducted in vivo studies on HAP scaffolds with pore diameters ranging between 400 μm and 1200 μm and inferred no significant difference in bone growth for scaffolds of all poresizes (Table 1).
Figure 1. Synthesised HAP Scaffold.
Table 1. Lattice constant.
Lattice Information: |
Phase name |
a(Aº) |
b(Aº) |
c(Aº) |
Alpha
(deg) |
Beta
(deg) |
Gamma
(deg) |
Vol
(Aº) |
Hydroxyapatite |
9.406674 |
9.406674 |
6.87543 |
90 |
90 |
120 |
527.083 |
The XRD patterns of the scaffolds prepared is shown in Figure 2. All of the peaks matched with the JCPDS pattern 09-0432 for HAP, which suggested that no other phases were present, as shown in Figure 3. All of the peaks were attributed to the HAP phase and no additional peaks were observed. The results indicated that the HAP did not decompose after sintering. The characteristic peaks at two theta 31.7°C corresponding to (211) diffraction became narrower and sharper for sintering temperature 1150°C. These data confirm that the major phase as Hydroxyapatite and absence of impurity such as calcium phosphates and calcium oxide are clearly identified.
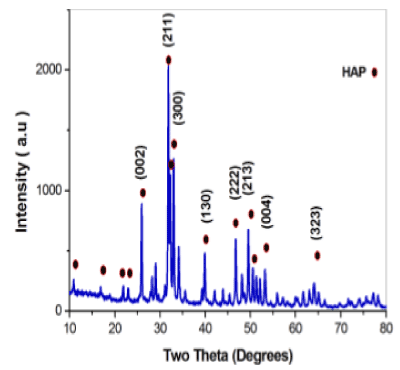
Figure 2. XRD pattern evenly distributed.
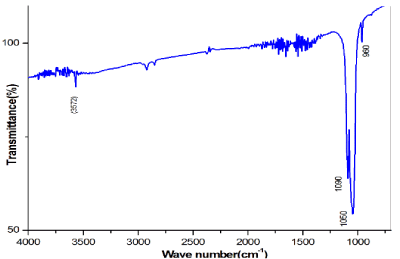
Figure 3. FTIR of Sintered Scaffold.
The FT-IR spectra of the synthesized HAP scaffold prepared using cellulose as a binding agent is shown in Figure 4. In the FTIR spectra, the bands at 3570 and 630 cm-1[7] were recognized to the hydroxyl stretching bands and bending bands of HAP, respectively. The broad absorption band from 3600 to 3300 cm-1[10] indicated the existence of the bending mode of absorbed water. The bands at 1093 and 1041 cm-1 were assigned antisymmetric ʋ3[PO43−] P-O stretching mode and the ʋ1 P-O symmetric stretching mode was detected at 962 cm-1 [9]. The bands at 603 and 569 cm-1 were attributed to components of the triply degenerate ʋ4 O-P-O bending modes (table 2).
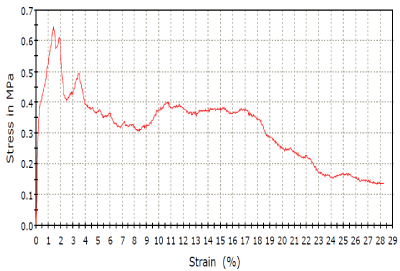
Figure 4. Stress – Strain analysis.
Table 2. Compressive Stress-Strain.
Area (mm2) |
Modulus (Automatic) (MPa) |
52.70670 |
160.31806 |
Compressive Strain at yield point (Zero slope) (mm/mm) |
Compressive Stress at yield point (Zero slope) (MPa) |
0.01430 |
0.6644 |
Compressive Stress (mm2) |
Extension at yield (Zero slope) (mm) |
0.47574 |
-11.009 |
The Scaffold prepared using cellulose showed high porosity of 75% with apparent density of 0.944g/cm3.It was able to tolerate a maximum stress 0.65 MPa. The mechanical properties are strongly subjective by apparent density [11]. In trabecular bone,the apparent density ranges from 0.14g/cm3 to 1.10g/cm3[11]. Porosity is based on the presence ofopen pores. It was found that mechanical properties varied with binders used. Moreover, a few studies [12] have reported that the decomposition of HAP would reduce its mechanical properties. Hence, a symmetry is to be maintained between the porosity and apparent density for precise purposes, since higher mechanical strength corresponds to higher density, while a high porosity provides asurrounding favourable for living organism.
In this work, porous scaffolds were prepared using a polymeric sponge template method using cellulose as a binding agent. A well-defined elongated cylindrical HAP crystal with negligible agglomeration was used to fabricate these scaffolds. The FESEM results exposed that the porous Hydroxyapatite scaffolds acquired macro pores and micro pores that emerges to be interconnected with a homogenous porous network. The scaffolds comprise pure crystalline hydroxy apatite phase and no additional phase were produced through the spongy technique as confirmed by XRD and FTIR. The scaffold prepared with cellulose in spite of high porosity (75%) appeared to report an apparent density of 0.944 g/cm3 comparable with trabecular bone (0.14 g/cm3 to 1.10 g/cm3). Thus, it is possible to produce porous scaffolds with varied porosity and density. Such scaffolds canfind its application for tissue engineering in non-load bearing applications or even as a vehicle for thedelivery of biological molecules. Currently, studies are being performed in order to incorporate collagen type I in these porous constructs, to improve their potential application.
This work was supported by DST – FIST under project number (SR-FIST-PSI-193-2014) Department of Physics, Sathyabama Institute of Science and Technology for providing the FT-IR and TGA facilities in characterizing the samples.
- Woodard JR, Hilldore AJ, Lan SK, Park CJ, Morgan AW, et al. (2007) The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials 28: 45-54. [Crossref]
- Itoh S, Kikuchi M, Takakuda K, Nagaoka K, Ko2021 Copyright OAT. All rights reservudy of a Novel Hydroxyapatite/Collagen (HAp/Col) Composite into Weight-Bearing Sites of Dogs. J Biomed Mater Res 63: 507-515. [Crossref]
- Sopyan I, Mel M, Ramesh S, Khalid KA (2007) Porous hydroxyapatite for artificial bone applications. Science and Technology of Advanced Materials 8: 116-123.
- Monmaturapoj N, Yatongchai C (2011) Influence of preparation method on hydroxyapatite porous scaffolds. Bull Mater Sci 34: 1733-1737.
- Tampieri A, Celotti G, Sprio S, Delcogliano A, Franzese S (2001) Porosity-graded hydroxyapatite ceramics to replace natural bone. Biomaterials 22: 1365-1370. [Crossref]
- Anita JL, Sundareswari M, Ravichandran K (2016) Porous hydroxyapatite scaffolds for orthopaedicand dentalapplications - the role of binders. Materials Today: Proceedings 3:1672-1677.
- Anita JL, Sundareswari M, Gill AS, Ravichandran K, Prabhkar JJ (2017) The Role of Cellulose in the Formulation of Interconnected Macro and Micoporous Biocompatible hydroxyapatite Scaffolds. Mechanics, Materials Science & Engineering P. 9.
- Anita JL, Sundareswari M, Ravichandran K (2018) Digest Journal of Nanomaterials and Biostructures 13: 235-243.
- Hollister SJ, Lin CY, Saito E, Lin CY, Schek RD, et al. (2005) Engineering craniofacial scaffolds. Orthod Craniofac Res 8: 162-173. [Crossref]
- Narbat MK, Orang F, Hashtjin MS, Goudarzi A (2006) Fabrication of Porous Hydroxyapatite-Gelatin Composite Scaffolds for Bone Tissue Engineering. Iranian Biomedical Journal 10: 215-223.
- Evans LA, Macey DJ, Webb J (1992) Calcium biomineralization in the radular teeth of the chiton, Acanthopleura hirtosa. Calcif Tissue Int 51: 78-82. [Crossref]
- Khalil KA, Kim WS, Kim HY (2007) Consolidation andmechanical properties ofnanostructured hydroxyapatite- (ZrO2 1 3 mol% Y2O3) bio-ceramics by highfrequency induction heatsintering. Mat Sci Eng A-Struct 456: 368-372.




