Introduction
Platelet-Rich Plasma (PRP) and Amnion-derived fluid are both regenerative medicine treatments that are widely used to treat various orthopedic and wound care conditions. While regenerative medicine therapies have become more popular in recent times, the specific mechanisms of healing are still being explored. The current hypothesis is that the major therapeutic benefits of regenerative medicine treatments come from the paracrine action of trophic factors contained in significant concentrations which signal, among many things, the endogenous progenitor cells to begin proliferation and healing [1, 2, 3].
Platelet-Rich Plasma
PRP is a growth factor-rich autologous treatment derived from a blood draw. A patient’s blood is spun down using centrifugation, a desired fraction is then extracted and further processed into PRP (Figure 1). It is often activated immediately prior to clinical use with thrombin and calcium to release desirable growth factors [4]. Because there are multiple ways to extract PRP from a patient, several different ways to activate it, and different technicians producing the PRP, the growth factor profile for a given sample can vary, even when the samples come from the same patient [4]. Additionally, because PRP requires processing steps which must be performed on freshly isolated blood that is taken from the patient, the patient must be retained while their treatment is being prepared and processed from their own blood draw; adding time to the procedure and decreasing overall throughput in a clinical setting.
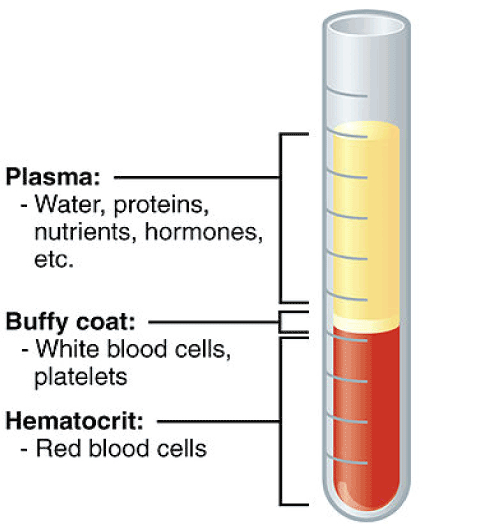
Figure 1: Diagram of the separation of the parts of blood with the layers separated based on size. The buffy coat is where the platelets are drawn from, then centrifuged again to create PRP.
Once platelets are activated, they release alpha granules, which contain growth factors that play a role in mitosis, cellular differentiation, and tissue vascularization [5,6]. Some of these growth factors have significant clinical advantages in promoting cellular activity and tissue remodeling and are considered desirable for numerous clinical therapies. While it has been reported that a variety of different growth factors and cytokines are released from activated platelets [7], there are a few key chemical mediators that have been reported to play a significant role in healing processes [8]. They include platelet derived growth factor (PDGF), transforming growth factor beta (TGF-β), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and epidermal growth factor (EGF) [5,6,9]. TGF-β is an immunoregulatory cytokine that contributes to the suppression of the inflammatory response, VEGF is a growth factor that is associated with increasing vascular permeability, angiogenesis, and also has anti-apoptotic functions [3]. PDGF, bFGF, and EGF are cellular mitogens that promote growth in a wide array of cell types, and serve an important role in the regenerative process associated with PRP [10].
PRP has been used for both wound care and orthopedic applications. It is currently recognized as an effective treatment for diabetic wounds [10], surgical wounds [9], and musculoskeletal injuries [11]. There have been reports of generally positive patient outcomes with a very low occurrence rate of adverse events, and while there have not been a large number of randomized clinical trials performed for some of the desired applications, such as ligament and tendon repair [12], the application of PRP for other conditions such as osteoarthritis has definitively yielded better results than alternatives such as hyaluronic acid [13] and corticosteroids [14].
PRP, however, is subject to several limitations. There is a significant amount of variability in growth factor profiles from donor to donor, which translates to a lack of consistency in clinical outcomes and therapeutic potential [9]. Additionally, the concentrations of certain factors such as EGF, TGF- β, PDGF-AB, PDGF-BB and IGF-1 have been found to decrease as patient age increases (negatively correlated with patient age) [9,15]. Furthermore, sex differences have been reported in the literature which add an additional variable to consider when harvesting PRP from older patients. For example, higher concentrations of these growth factors were found in female subjects [14].
However, the clinical successes of PRP afford support to the claim that paracrine action is the primary mechanism of healing when it comes to regenerative medicine therapies, as it is a treatment that is used only for the associated factors that are released; it does not contain cells that would be thought to hone in on damaged or degenerate areas, fuse, differentiate, or proliferate in order to regenerate tissue [16].
Amnion-derived fluid
Amnion-derived fluid is a conditioned media that is created during the growth and culture of cells extracted from the amnion, the innermost layer of the placenta. After a placenta is acquired, the amnion is mechanically separated from the rest of the placenta, and washed several times. After being thoroughly washed, the amnion is enzymatically digested to cleave the bonds holding the desired cells from the membrane [17]. These cells are then collected after isolating them from the remainder of the fluid via centrifugation. As the cells grow in culture, they release a similar milieu of growth factors and cytokines that mimic the previous in utero environment. These growth factors and cytokines condition the growth media they inhabit, while adhering to the bottom surface of the culture flask. The conditioned media, also known as amnion-derived fluid, is then collected and used as a treatment for a similar range of clinical conditions as PRP.
Amnion-derived fluid also contains with a wide range of growth factors, including VEGF, bFGF, EGF, TGF-β, among many others [18,19,20]. The mechanism of action for amnion-derived fluid is also believed not to be cell-based, similar to PRP, and acts by signaling endogenous resident cells to reduce chronic inflammation and promote regrowth of new tissue [21,22]. Due to the source of the tissue being the amniotic membrane, which is an immune-privileged tissue, the derived fluid also has a very low occurrence of any type of immune related adverse events [2]. Amnion-derived fluid is delivered as a ready-to-use, off-the-shelf product which does not require processing time in the clinic, nor does it require any additional procedures like blood collection from the patient, because it is an allogeneic treatment (Figure 2).
Figure 2: A vial containing AxoBioFluid®, a ready to use off-the-self product. AxoBioFluid® is an amnion-derived fluid created for homologous use.
Discussion
There exist several differences between PRP and amnion-derived fluid that are relevant to discuss when considering their use for therapeutic effectiveness or clinical efficiency.
First, because of the nature of cell culture and product manufacturing, the growth factor profile of amnion-derived fluid is more consistent, and is not as severely affected by donor-based biological variability as PRP due to the ability to quality control the product both during cell culture and in evaluating the end product. Additionally, the separate processing of amnion-derived fluid reduces chair time for the patient and processing time for the practitioner, without requiring the practitioner to purchase processing equipment, which increases throughput for the medical facility. Furthermore, the production of a PRP product depends significantly on the standard operating procedures that are used. Many clinics have unique or proprietary protocols that must be followed to successfully create a therapeutically-active PRP [23]. This introduces additional variables to consider in the clinic when different technicians perform the blood draw, subsequent centrifugations, and PRP harvest.
Differences aside, however, both treatments operate on the principle of growth factor-mediated therapeutic action rather than the previous understanding that cell homing, attachment, differentiation, and proliferation were the primary methods of action. It would be expected for both PRP and amnion-derived fluid to perform similarly due to the wide range of similarities between the mechanisms of action and the associated growth factor profiles.
References
- Hofer, Heidi R, Tuan RS (2016) Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Research and Therapy 7: 131.
- Madrigal, Marialaura, Rao KS, Riordan NH (2014) A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. Journal of Translational Medicine 12: 260.
- Vokurka J (2016) Concentrations of growth factors in platelet-rich plasma and platelet-rich fibrin in a rabbit model. Veterinarni Medicina 61: 567-570.
- Eppley, Barry L, Woodell JE, Higgins J (2004) Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plastic and Reconstructive Surgery 114: 1502-1508.
- Lana, Jose F (2014) latelet rich plasma and its growth factors: the state of the art. Platelet-Rich Plasma Springer Berlin Heidelberg 3: 1-59.
- Textor, Jamie (2014) Platelet-rich plasma (PRP) as a therapeutic agent: platelet biology, growth factors and a review of the literature. Platelet-Rich Plasma Springer Berlin Heidelberg 4: 61-94.
- Boswell, Stacie G (2012) Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy: The Journal of Arthroscopic & Related Surgery 28: 429-439.
- Evanson JR, Guyton MK, Oliver DL, Hire JM, Topolski RL, et al. (2014) Gender and age differences in growth factor concentrations from platelet-rich plasma in adults. Military Medicine 179: 799-805
- Picard, Frederic (2015) The growing evidence for the use of platelet-rich plasma on diabetic chronic wounds: A review and a proposal for a new standard care. Wound Repair and Regeneration 23: 638-643.
- Sampson, Steven, Gerhardt M, Mandelbaum B (2008) Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Current Reviews in Musculoskeletal Medicine 1: 165-174.
- Taylor, Drew W (2011) A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clinical Journal of Sport Medicine 21: 344-352.
- Kanchanatawan, Wichan (2016) Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surgery, Sports Traumatology, Arthroscopy 24: 1665-1677.
- Shen, Longxiang (2017) The temporal effect of platelet-rich plasma on pain and physical function in the treatment of knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. Journal of Orthopaedic Surgery and Research 12: 16.
- Cho HS, Song IH, Park S-Y, Sung MC, Ahn M-W, et al. (2011) Individual Variation in Growth Factor Concentrations in Platelet-rich Plasma and Its Influence on Human Mesenchymal Stem Cells. The Korean Journal of Laboratory Medicine 31:212-218.
- Bernstein, Erica D, Murasko DM (1998) Effect of age on cytokine production in humans. Age 21: 137-151.
- Alsousou J (2009) The biology of platelet-rich plasma and its application in trauma and orthopaedic surgery. Bone and Joint Journal 91: 987-996.
- Marta M (2016) Isolation, Culture, and Phenotypic Characterization of Mesenchymal Stromal Cells from the Amniotic Membrane of the Human Term Placenta. Mesenchymal Stem Cells: Methods and Protocols 3: 233-244.
- NJ Koizumi Growth factor mRNA and protein in preserved human amniotic membrane. Current Eye Research 20: 173-177.
- Marvin, Keith W (2002) Expression of angiogenic and neurotrophic factors in the human amnion and choriodecidua. American Journal of Obstetrics and Gynecology 187: 728-734.
- Yang, Shu (2013) Conditioned medium from human amniotic epithelial cells may induce the differentiation of human umbilical cord blood mesenchymal stem cells into dopaminergic neuron-like cells. Journal of Neuroscience Research 91: 978-986.
- Sartore, Saverio (2005) Amniotic mesenchymal cells autotransplanted in a porcine model of cardiac ischemia do not differentiate to cardiogenic phenotypes. European Journal of Cardio-Thoracic Surgery 28: 677-684.
- Hodgkinson, Conrad P (2016) Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology. Circulation Research 118: 95-107.
- Roukis, Thomas S, Zgonis T, Tiernan B (2006) Autologous platelet-rich plasma for wound and osseous healing: a review of the literature and commercially available products. Advances in Therapy 23: 218-237.


