Polymers and heterocyclic compounds play an important role in pharmacy according to their functions as excipients and inert carriers of other pharmacological active compounds. This review article focused on recent advances in alternative pharmaceutical use of heterocycle and polymers as pharmacologically active agents known as polymeric drugs and heterocyclic drug compounds in both function types drug delivery and co-drug compounds, including the benefits of polymeric drugs that are associated with their macromolecular character and the ability of both to explore biologically relevant multivalence processes. We discuss the main therapeutic uses of polymeric drugs as well as heterocyclic compounds such as antimicrobials, anti-hyperkalaemia, and anticancer
polymer, anticancer drug, polydiacetylenes
Polymer as drug and drug carriers
It is desirable that the drug reaches its site of action at a particular concentration and that this therapeutic dose range remains constant over a sufficiently long period of time to the target. However, the action of pharmaceutical agents is effected by various factors, including their degradation, their interaction with non-target cells, and their inability to penetrate the body tissues according to their chemical nature.
For the above reasons, new formulations are being studied to achieve a greater pharmacological response; among these, polymeric systems of drug carriers are of high interest. These systems are an appropriate tool for time- and distribution-controlled drug delivery. The mechanisms in controlled release require polymers with a variety of physicochemical properties. So, several types of polymers have been tested as drug delivery systems, including Nano and micro-particles, dendrimers, Nano and micro-spheres, capsosomes, and micelles [1-4].
In all these systems, drugs can be encapsulated or conjugated in polymer matrices. These polymeric systems have been used for a variety of treatments such as antineoplastic activity, bacterial infections and inflammatory processes, sequestrant in addition to vaccine. It was also known that many cancer drugs are key heterocyclic compounds [5] . These compounds were designed as anti-cancer due to their being extremely common in nature, with numerous number cellular and mechanistic pathways for their interactions. There are variety of metabolic path ways associated with cellular cancer pathology which can be attributed to heterocyclic compounds. In this article we are going to introduce the most important heterocyclic and polymer compounds incorporated to cancer and other therapy in both areas the market and that which are in development in both areas of polymer and heterocyclic chemistry discussing their properties that make them valuable as drugs.
The slow release of drug using acrylic itaconic co- polymer
This study was published else were [6] and showed regular release of anti-biotic in vivo within a period of 14 days on 4 group of animals (rabbits). Polymer synthesis and conditions were illustrated below (Scheme 1).
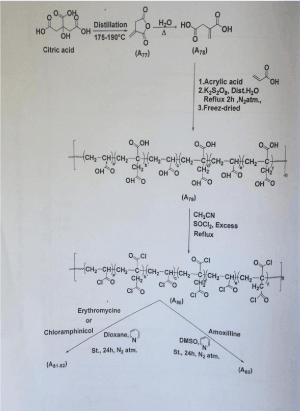
Scheme 1. synthesis of Acrylic itaconic co polymer loaded with antibiotics
Drug-Polymer Conjugates with Tuberculostatic Activity Based on Poly (N-Vinyl Pyrrolidone-alt-Itaconic Anhydride and Novel Amino acid Hydrazides was also investigated [7]. Polymer synthetic pathway can be seen below (Schemes 2, 3).
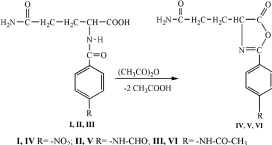
Scheme 2. The formation of amidizolonyl amino asci as a residue of the polymer
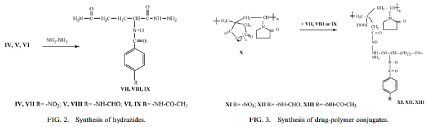
Scheme 3. showing the steps of preparation of the co poly (N-vinyl Pyrrolidone-alt-Itaconic Anhydride with amino acids
This polymer showed significant activities against Tuberculosis.
Usually polydiacetylenes acting as a bilayer similar to that of the cell wall which will use to diagnose a variety of common diseases, so if this polymer is functionalize with suitable moiety such as sugar group or lipid it will becomes bio sense due to collar change phenomena created by the interaction of this group with malarial toxins or any other events (Figure 1). The colour change is irreversible a can be used to diagnose several diseases as shown below (Figure 2).
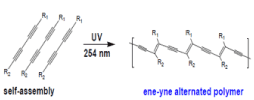
Figure 1. Polydiacetylene (PDA) synthesis via self-assembly and polymerization of diacetylene monomers
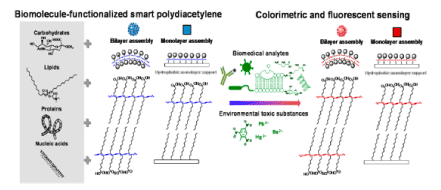
Figure 2. Biomolecule-functionalized polydiacetylene (PDA) based and environmental sensing
For example, GM1 and GT1b gangliosides, which are present on the surface of intestinal cells and at neuromuscular junctions, were utilized for colour-based detection of cholera toxin and botulinum neurotoxin . For this application 5% ganglioside lipid was used, since a higher concentration inhibits polymerization of the diacetylene assembly [8].
Brazilian researchers in October 2010 have synthesized 10,12 pentacosadiynoic acid (PCDA) +N-[(2-tetradecan amide)-ethyl]) ribonamide (TDER) vesicles to determine the colorimetric response induced by pathogenic bacteria (Staphylococcus aureus and Escherichia coli). The addition of bacterial supernatants caused a colorimetric transition in TDER/PCDA vesicles, even in diluted concentrations, indicating that chemical interactions occur between the vesicles and the released bacterial compounds. This study was important fort food packing and Food born bacteria. Japanese researchers in march 2016 studied imidazolyl Functionalized diacetylenes.
In their study, They report the first example of polydiacetylenes (PDAs), where the PDA-based system acts as both a sensing probe and killer for bacteria. The contact of imidazolium and imidazole-derived PDA with various bacterial strains including MRSA (methicillin-resistant Staphylococcus aureus) and ESBL-EC (extended-spectrum β-lactamase-producing Escherichia coli) results in a distinct blue-to-red colorimetric change of the solution as well as a rapid disruption of the bacterial membrane, which is demonstrated by transmission electron microscopy and confocal microscopy.
Zeta potential analysis supports that antibacterial activity of the PDA solution originates from an electrostatic interaction between the negatively charged bacterial cell surface and the positively charged polymers. These results suggest that the PDA has a great potential to carry out the dual roles of a probe and killer for bacteria as shown below (Figure 3).
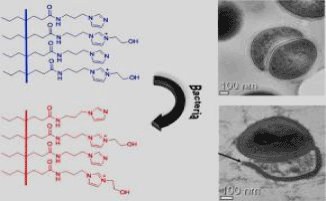
Figure 3. showing the effects of polydiactelyne functionalized with imidazoylgroup on bacteria cell membrane
Polymers as drugs
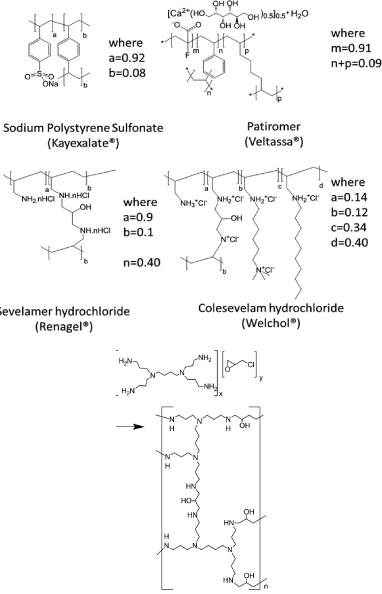
The first known type of binders Polymer is Sodium polystyrene sulfonate (Kayexalate VR , SPS) which is the first synthetic polymers to be widely used as a clinical sequestrant, its structure is shown in scheme 4 the salt of a polymeric acid, SPS is able to reversibly bind a range of cations, including potassium. Since potassium is the most abundant ion in the colon, and is reversibly absorbed in the lower GI tract, a Gi restricted polymeric agent with capability to bond potassium which may provide an effective means to reduce serum potassium (Elevated serum potassium).

Scheme 4.how is the synthetic steps of polystyrene sulfonate
(Hyperkalemia) is a significant concern for patients with CKD or cardiovascular conditions and can result in arrhythmia and finally sudden death [9]. SPS was approved by the FDA for the treatment of hyperkalemia in 1958 SPS which can be produced by the polymerization of styrene in presence of crosslinking agent divinylbenzene as shown below.
Sevelamer hydrochloride (Renagel VR ) was the first polymeric phosphate control and removal of excess phosphate is of benefit to patients with chronic kidney disease CKD where dialysis is unable to maintain safe phosphorus levels. Sevelamer limits the absorption of dietary phosphorus by binding phosphate in the intestine through ionic interaction with the polyamine polymer. Sevelamer is across linked form of poly(allylamine) containing primary and secondary aliphatic amine residues and was approved for the treatment of hyperphosphatemia by the FDA in 1998.The following Scheme 5 shows the synthetic lines for Sevelamer hydrochloride.
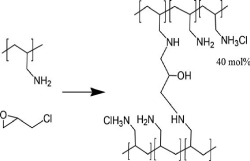
Scheme 5. Represent the formation of poly allylamine as a residue of sevelamer structure
Synthesis of Sevelamer Hydrochloride. Approximately 40 percent of the amine moieties are in the HCl form. Crosslinking degree is 10%.
The Structure of some commercially available polymer sequestrant drugs, were as follows:
Were sorbitol, which is frequently dosed with SPS as a laxative the risk of swelling of above drugs Leeds to some improvements to the above drudge polymers to increase of its capacity and reducing its swelling property sevelamer is changed into cross liked N,N, N,N-tetrakis (3-aminopropyl) butane-1,4-diamin (Schemes 6, 7) as illustrated below support the safety profile in clinical studies of up to 52 weeks it is approved for treatment of hyperphosphatemia by FDA in 1998.
Scheme 6. Showing the network formation of patiromer amine residue
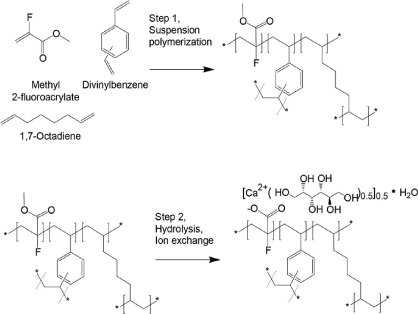
Scheme 7. Synthetic steps of Patiromer
Patiromer is a non-absorbed, potassium-sequestering polymer which is a crosslinked form of poly (fluoroacrylic acid).The fluorine substituent lowers the pKa of the acid group in patiromer compared to acrylic acid such that a higher proportion of acid groups are available for ion binding.
Suspension polymerization during patiromer manufacture allows for the generation of monodisperse uniform polymer particles, with spherical shape, controlled size distribution, and low swelling. The bead particles have a median diameter of around 100 µm. Patiromer was approved by the FDA for the treatment of hyperkalemia in 2015 based on clinical studies showing effective potassium lowering and acceptable safety profile in clinical studies of up to 52 weeks duration.
It is precisely because heterocycles are so prevalent in nature that they have become so important for anti-cancer drug design. Representing an extremely large cohort of molecules with such an unprecedented level of variability in terms of the interactions they can engage with, heterocycle-based compounds not surprisingly have formed the basis of drug therapies time and againg. As many enzymebinding pockets are redisposed to interacting with heterocyclic moieties, heterocycles are a good choice when designing molecules that will interact with targets and disrupt the biological pathways associated with cancer progression. Pathways related to cell growth and development are often targeted by such anti-cancer therapies. Moreover the relative ease by which heterocyclic rings can be modified with additional substituents allows them to cover a broad area of chemical space, further qualifying them as excellent starting points for anti-cancer drug development.
As a result of these factors, heterocyclic structures have long played a key role in anti-cancer drug design, featuring prominently in anti-cancer drug compounds currently available on the market.
Indeed, 65% of the anti-cancer drugs granted market approved by FDA between 2010-2015 form the basis of many of the anti-cancer agent currently in development today Nitrogen-based heterocycles.
Nitrogen-based heterocycles are of particular importance in anti-cancer drug design, featuring in almost three-quarters of the heterocyclic anticancer agents approved by the FDA between 2010 and 2015.
Nitrogen heterocycles, indoles are among the most valuable, in research demonstrated their ability to induce cell death in a number of cancer cell lines 2 During the last few decades, indole and its derivatives have been shown to modulate a number of biological pathways multiplicated in the progression of cancer.
These include the prevention of cell signalling normal cell cycle progression, tumour vascularisation and DNA repair, as well as the ability to induce cellular oxidative stress and cell death The most important early indole-based anticancer agents are vincristine and vinblastine are recognised for their tubulin polymerisation inhibition since the early-mid 1960s and both still of clinical importance today. Vincristine (Figure 4) is used as a combinatorial treatment for acute lymphoblastic leukaemia and both Hodgkin’s and- non-Hodgkin’s lymphoma [10], and so for oxygen heterocylles Cabazitaxel Trade name Jevtana its structure is as follows.
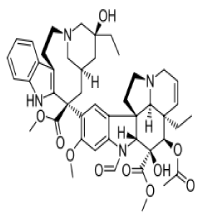
Figure 4. Used as a combinatorial treatment for acute lymphoblastic leukaemia and both Hodgkin’s and- non-Hodgkin’s lymphoma
While for chlro ,oxygen and nitrogen compounds according to the following scheme 8 and graphical curves(NCI report) showed remarkable results for these types of compounds.
The above investigation revealed that the studied compounds kill about 89-100% cancer cell (6-8 types) at the same time [11].
\
Scheme 8. Shows the synthetic steps of Amino chloropyrano oxazine compounds and the graphical results showing the effects of these compounds on different types of cancer cells
- Chun MK, Cho CS, Choi HK (2002) Mucoadhesive drug carrier based on interpolymer complex of poly(vinyl pyrrolidone) and poly(acrylic acid) prepared by template polymerization. J Control Release 81: 327-34. [Crossref]
- Vllalobos, Patricio, Charez, MariaI, Olguin, et al. (2012) The application of polymerized lipid vesicles as colorimetric biosensors for real-time detections of pathogens in drinking water Electronic Journal of Biotechnology 15.
- McKeating KS, Aube A, Masson JF (2016) Biosensors and nanobiosensors for therapeutic drug and response monitoring. Analyst 141: 429-449. [Crossref]
- Su H, Li S (2012) Nanomaterial-based biosensors for biological detections. Advanced Health care Technologies 3: 19-29.
- Mohammadian N, Zare K, Monajjemi M (2017) S-NICS investigation for heterocyclic antic cancer compounds. Orient J. of chemistry 33: 1595-1602.
- Al-Ajely MS, Qasim AY (2012) Synthesis of some streoregular polydiacetylenes and the slow release of drug using some linear polymers. Pakistan J. of chemistry 2: 142.
- Delia G, Mihaela N, Catalina L, Cristian P (2013) Drug-Polymer conjugates with tuberculostatic activity, based on poly (n-vinyl pyrrolidone-alt-itaconic anhydride) and novel aminoacid hydrazides. Polymer-Plastics Technology and Engineering 52: 1213-1219.
- Lee S, Cheng H, Chi M (2016) Sensing and antibacterial activity of imidazolium-based conjugated polydiacetylenes. Biosens Bioelectron 77: 1016-1019. [Crossref]
- Eunae Cho, Seunho Jung (2018) Biomolecule-Functionalized Smart Polydiacetylene for Biomedical and Environmental Sensing. Molecules 23: 107. [Crossref]
- Martins P, Jesus J, Santos S, Raposo LR, Roma-Rodrigues C, et al. (2015) Heterocyclic Anticancer compounds: Recent advances and the paradigm shift toward the use of nanomedicine toolbox. Molecules 20: 16891. [Crossref]
- Mohammad S. Al-Ajelyand, F.Thanoon Al-Abachi, Iraqi patent No. 2807 Awarded in (15/1/2000)












 \
\