Abstract
During this study, three horses suffering of tendinopathy or desmitis were treated by injection of tendon-derived mesenchymal stem cells at the lesion site. Two of them had previously received platelet-rich plasma (PRP) treatment that was found to be ineffective.
Few months after injection, tendon-derived mesenchymal stem cells (TDSCs) has effectively improved repair of the lesion. TDSCs show great ability to fill the lesion and to let a well homogenized tissue after recovery.
Although these results need to be confirmed by a standardized and randomized study, TDSCs still offer promising treatment for tendinopathies treatment.
Keywords
equine, tendon-derived mesenchymal stem cells, tendinopathies, desmitis, platelet-rich plasma
Abbreviations
SDFT: superficial digital flexor tendon; SL: suspensory ligament; DDFT: deep digital flexor tendon; MSCs: mesenchymal stem cells; TDSCs: tendon-derived mesenchymal stem cells; MHC-II: type II - major complex of histocompatibility; DPBS: Dulbecco’s Phosphate Buffered Saline; DMEM: Dulbecco's Modified Eagle Medium; FBS: Fetal Bovine Serum; DMSO: dimethyl-sulfoxide; PRP: platelet-rich plasma; DSL: distal sesamoid ligament
Introduction
Tendons and ligaments injuries are the most common musculoskeletal disorders to occur in all sport horses, with specific overstrain conditions which are overrepresented in racehorses whose tendons are operating close to their functional limits [1,2].
Most tendon injuries (97-99%) take place in the forelimb. Tendon lesions are reported to account for 43% of injuries that occur to horses in training, among which 33% are to the superficial digital flexor tendon (SDFT), 31% to the suspensory ligament (SL) and 17% to the deep digital flexor tendon (DDFT) [3].
Although tendons and ligaments have the ability to heal spontaneously with time, the repair tissue is usually biomechanically inferior. The healing process is composed of three overlapping phases: a short inflammatory phase, a proliferative phase when collagen fibrils are synthesized, and a modelling/maturation phase during which these are organized into tendon bundles [4]. Unfortunately, the proliferative and maturation phases lead to the formation of excessive fibrous tissue. The altered collagen composition (mostly type III collagen) and the production of a disorganized matrix results in impaired tendon elasticity, reducing performance and increasing the risk of re-injury and recurrent lameness [5].
Tendon/ligament injuries are clinically managed by conservative treatment, surgical interventions and regenerative approaches [2]. Conservative treatments often lead to the end of the animal’s career. Surgical treatments including bursoscopic debridement and desmotomy of annular ligaments are invasive and the results are often unsatisfactory [1,6]. These treatments often relieve the pain but also induce a higher rate of reinjury after only a few months.
Veterinary regenerative medicine is constantly evolving and is leading to new approaches for orthopaedic issues. Orthobiologics are a new paradigm for the treatment of chronic tendons and ligaments injuries. Among the biological substances used, mesenchymal stem cells (MSCs) are front-runners in the field of regenerative medicine [7].
Published studies demonstrated clinical improvement and integrity of impaired tissues confirming the efficacy of MSC therapy for the treatment of tendinopathies [8]. MSCs have the capacity to boost tendon healing by improving the tenogenic properties of resident cells [9].
The use of tendon-derived mesenchymal stem cells (TDSCs) showed promising outcomes comparatively to adipose and bone marrow-derived mesenchymal stem cells. The use of autologous TDSCs might be limited due to donor site morbidity [5,10,11].
Allogenic TDSCs are considered as low immunogenic cells due to their lack of expression of MHC-II and thus represent an interesting alternative [12,13].
A recent report demonstrated that MSCs isolated from post-mortem ligaments (i) are easy to collect, (ii) express stem cells markers and (iii) proliferate in vitro with a high capacity for differentiation. They are thus promising MSCs for the treatment of tendinopathies [14]. These stem cells are harvested and prepared with a non-expansive technique compared to others autologous sources for which sampling and preparation are invasive, take time and are costly.
This pre-clinical study was conducted with three specific objectives: (i) to confirm the injection technique and the protocol of cell preparation, (ii) to explore the potential adverse effects of this treatment and (iii) to confirm the capacity of TDSCs to improve tendon lesions healing using ultrasonographic and orthopaedic assessment techniques.
Material and Methods
Cells preparation
TDSCs were isolated from a four years old equine superficial digital flexor tendon within 48-72 hours post-mortem. The horse had died of a non-musculoskeletal disease. The preparation of the TDSCs was performed as described [14]. Briefly, tendons were recovered and split into small pieces. The samples were digested by collagenase 0,2% in Dulbecco’s Phosphate Buffered Saline (DPBS) during 18h. The filtered solution containing cells was centrifuged and the cells were seeded in a tissue culture-flask containing growth medium (Dulbecco's Modified Eagle Medium (DMEM) + Foetal Bovine Serum (FBS) 10% + penicillin-streptomycin 1% + amphotericin 1%). Cells passage was done upon reaching a confluence of 70-80%. After 4 passages (P4), TDSCs were stored in a cryopreservation solution containing 10% dimethyl-sulfoxide (DMSO) and 20% FBS diluted in DMEM and frozen in liquid nitrogen until use. Four days before injection, cells were thawed in DMEM heated at 37°C and placed back in culture medium. A few hours before treatment, cells were trypsinized and resuspended in a solution of DMEM (supplemented by penicillin-streptomycin 1% + amphotericin 1%).
Treatment
After local disinfection, under ultrasound guidance, a 1 mL solution containing 1x106 TDSCs in DMEM was injected at the injury site at one or two different points using a sterile 21 gauge needle. The limb was bandaged immediately after injection. Bandages were changed every two days and kept in place for two weeks. The rehabilitation program, based on clinical veterinarian experience and on literature design, is described in Figure 1 [2]. During this rehabilitation program the horses were controlled clinically and by ultrasound after one month and two months post-injection.
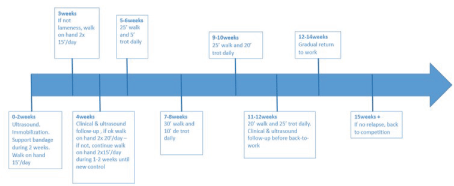
Figure 1. Rehabilitation program
Results
Case n°1
A 7 years-old gelding used for jumping was shown for a lameness graded 3/5 on the right hindlimb using the scoring scale from the American Association of Equine Practitioners. A desmitis of the proximal branch of the suspensory ligament was diagnosed. This horse was first treated with platelet-rich plasma (PRP) but only a partial improvement was observed 3 months after PRP injection (Figure 2A). In view of these unsatisfactory results, it was decided to treat him with two-point injections of TDSCs under ultrasound control. One month post-treatment, ultrasound imaging did not show significant improvement. A second TDSCs injection was then performed with the same approach. Six weeks later the ultrasound image showed clear improvement with disappearance of the hypoechogenic zone but a small hyperechogenic zone remained (Figure 2B). This zone progressively disappeared within 3 months post-injection (Figure 2C). During this time the horse came gradually back to work and the clinical evolution remains satisfactory four months after the second injection.
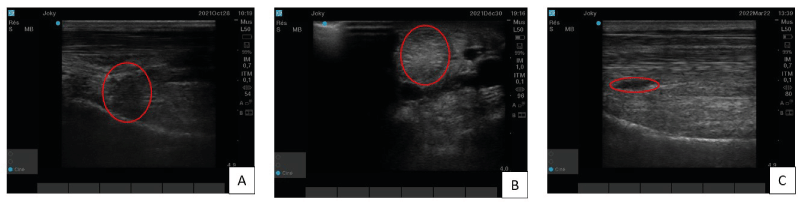
Figure 2. Case one’s proximal branch of the suspensory ligament ultrasound imaging : A) Longitudinal section, three months after PRP treatment,. In red circle, a hypoechogenic zone which is the sign of the ligament’s lesion. B) Transversal section, six weeks after second tendon-derived mesenchymal stem cells injection. In this image, the red circle is on the hyperechogenic zone. C) Longitudinal section, four months after tendon-derived mesenchymal stem cells second injection. This one, show a homogenous and linear organisation but in the red circle, a little hypoechogenic zone persists
Case n°2
This horse is a 15 years-old gelding used for unprofessional dressage. He was presented with a lameness graded 2/5 on the right forelimb. A desmitis of the lateral branch of the suspensory ligament was diagnosed (Figure 3A) and treated with PRP. The first results concluded to a return to work. Unfortunately, one year later, a reinjury (Figure 3B) appeared and it was decided to do a two-points TDSCs injection. One month after that injection an improvement could be shown by hypoechogenic zone decrease through ultrasound imaging, but as for case one a hyperechogenic zone appeared approximately 3 months after the treatment (Figure 3C) without any clinical impact. Six months after TDSCs injection the horse got back to work as the clinical and ultrasound evolution of the lesion were satisfactory with a disappearance of the hyperechogenic zone and a nice homogenization of the lateral SL branch (Figure 3D).
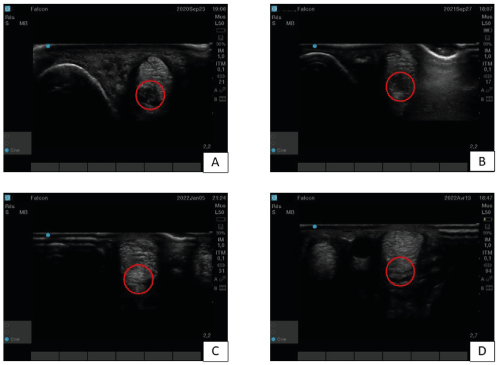
Figure 3. Case two’s lateral branch of suspensory ligament ultrasound imaging – A) Before treatment at diagnostic time. The red circle delineates a hypoechogenic zone, sign of the desmitis. B) One year after PRP treatment, the image is not really better. C) Three months after tendon-derived mesenchymal stem cells injection. The red circle delimits a hyperechogenic zone. D) Six months after tendon-derived mesenchymal stem cells injection. The image show a nice regeneration of the ligament, with homogenous and linear organization (red circle)
Case n°3
This horse is a ten years-old gelding that participates in trotting races. He was diagnosed with a distal sesamoid ligament (DSL) desmitis on the left forelimb (Figure 4A). The lameness was objectively graded 3/5. He received a one-point TDSCs injection under ultrasound control. During the recovery phase, the horse showed a constant clinical improvement and a progressive disappearance of the lesion as assessed by ultrasound. The hypoechogenic zone showed by Figure 4(A) seemed completely filled (Figure 4B). After 7 months, the horse was back to normal work and won a race 2 months after the end of the revalidation program.
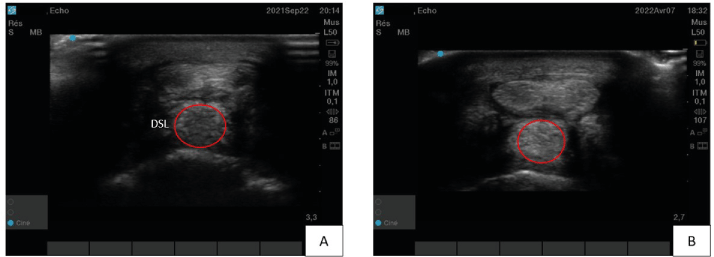
Figure 4. Case three’s distal sesamoid ligament (DSL) ultrasound imaging – A) Before treatment at diagnostic time. The red circle defines a non-homogenous and hypoechogenic zone (desmitis). B) Seven months after tendon-derived mesenchymal stem cells injection. The ligament is well repair with a homogenous and linear organization of the tissue (red circle)
Discussion
Combined with controlled rehabilitation, regenerative medicine has become an important treatment option for horses with tendons and ligaments lesions. The main injectable orthobiologic products currently used are stem cells and platelet-rich plasma (PRP). The principal therapeutic effect of PRP is due to the release of various growth factors (i) enhancing cell migration, proliferation and differentiation, (ii) improving extracellular matrix components synthesis and (iii) inducing angiogenesis. If clinical studies and several in vivo experiments demonstrate the benefit of PRP treatment in healing tendinous or ligament lesions, the lack of a large randomized controlled trial must be considered when interpreting the results of PRP treatments. [2] For this reason, the use of PRP is presently very controversial without any evidences of the efficacy of the treatment on a large population.
In case one and two, the PRP treatment did not give satisfactory results. The repair of the lesions seemed heterogeneous with a high risk of relapse. The horse of case two relapsed 12 months after PRP treatment.
The intralesional injection of TDSCs effectively improved repair of the lesion in both cases.
The TDSCs show great ability to fill the lesion and to form a homogeneous tissue after recovery. This ability is because these cells are isolated post-mortem from a hypoxic niche that selects the most robust cells. These cells are in better condition for proliferation and can synthetize type I collagen for regeneration. This important collagen synthesis is probably responsible for the appearance, generally three months after TDSCs treatment, of the hyperechogenic zone indicating fibrosis. This fibrotic tissue is reorganized during a second phase and leaves a homogenous tissue.
In none of these 3 cases did the TDSCs treatment induce any side effects.
The efficacy of treatment and the duration of the revalidation period is strictly correlated with the size of the lesion. The case one horse, due to the depth of the lesion, had to receive a second TDSCs injection. Despite this a small lesion persisted four months after this second injection.
Case three horse was treated with a single cell injection of TDSCs without prior PRP treatment and the results were promising. At the end of the revalidation program he won a trotting race (Mons, Belgium) but the hindsight is only of nine months.
To attest to the effectiveness of the TDSCs treatment, as for PRP, it is nevertheless essential to look at the treatment long term.
If TDSCs injection by itself or in combination of PRP certainly remains a promising and original treatment, these case reports need to be completed with a standardized and randomized clinical study to confirm the efficacy and the innocuity of the TDSCs on larger cohorts of animals.
Acknowledgement
We thanks Dr Eric Ghilain very much for his clinical participation in this study.
Conflict of interest
The authors declare no conflict of interest.
Financial Disclosure
The authors declare commercial interest for the treatment discussed in the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Humbach KE, Gutierrez-Nibeyro SD (2018) Desmotomy of the accessory ligament of the deep digital flexor tendon for treatment of chronic deep digital flexor tendinopathy in three Quarter Horses. Equine Veterinary Education 30: 538–544.
- Ortved KF (2018) Regenerative Medicine and Rehabilitation for Tendinous and Ligamentous Injuries in Sport Horses. Vet Clin North Am Equine Pract 34: 359–373. [Crossref]
- Thorpe CT, Clegg PD, Birch HL (2010) A review of tendon injury: Why is the equine superficial digital flexor tendon most at risk? Equine Vet J 42: 174-180. [Crossref]
- Lomas A, Ryan C, Sorushanova A, Shologu N, Sideri A, et al. (2015) The past, present and future in scaffold-based tendon treatments. Adv Drug Deliv Rev 84: 257–277. [Crossref]
- Shojaee A, Parham A (2019) Strategies of tenogenic differentiation of equine stem cells for tendon repair: Current status and challenges. Stem Cell Res Ther 10: 181. [Crossref]
- Smith MRW, Wright IM (2012) Endoscopic evaluation of the navicular bursa: Observations, treatment and outcome in 92 cases with identified pathology. Equine Vet J 44: 339–345 [Crossref]
- Dhillon M, Behera P, Patel S, Shetty V (2014) Orthobiologics and platelet rich plasma. Indian J Orthop 48: 1-9. [Crossref]
- Connard SS, Linardi RL, Even KM, Berglund AK, Schnabel LV, et al. (2021) Effects of continuous passage on the immunomodulatory properties of equine bone marrow-derived mesenchymal stem cells in vitro. Veterinary Immunology and Immunopathology p. 234.
- Costa-Almeida R, Calejo I, Gomes ME (2019) Mesenchymal stem cells empowering tendon regenerative therapies. Int J Mol Sci 20: 3002. [Crossref]
- Durgam SS, Stewart AA, Sivaguru M, Wagoner Johnson AJ, Stewart MC (2016) Tendon-derived progenitor cells improve healing of collagenase-induced flexor tendinitis. J Orthop Res 34: 2162-2171. [Crossref]
- Song H, Yin Z, Wu T, Li Y, Luo X, et al (2018) Enhanced Effect of Tendon Stem/Progenitor Cells Combined With Tendon-Derived Decellularized Extracellular Matrix on Tendon Regeneration. Cell Transplant 27: 1634-1643. [Crossref]
- Russo V, El Khatib M, Prencipe G, Citeroni M, Faydayer M, et al. (2022) Tendon Immune Regeneration: Insights on the Synergetic Role of Stem and Immune Cells during Tendon Regeneration. Cells 11: 434. [Crossref]
- Textor J, Clark L, Walker N, Aristizobal F, Kol A, et al. (2018) Allogeneic Stem Cells Alter Gene Expression and Improve Healing of Distal Limb Wounds in Horses. Stem Cells Transl Med 7: 98-108. [Crossref]
- Shikh Alsook MK, Gabriel A, Piret J, Waroux O, Tonus C, et al. (2015) Tissues from equine cadaver ligaments up to 72 hours of post-mortem: A promising reservoir of stem cells. Stem Cell Res Ther 6: 253. [Crossref]




