Abstract
Background: The relationship between vaccines and neuroinflammation have consistent molecular biology bases. In a recent paper we have already analyzed this kind of relationship.
Hypothesis: In this paper, we have gained additional evidence to support the link between vaccines and neuroinflammation. Furthermore, we found the molecular bases that support the link between HPV vaccines and certain adverse events (AEs). The peripheral proinflammatory cytokines (IL-1β, IL-6, and TNF-α), expressed after the injection of the vaccines can reach the brain and can cause neuroinflammation after microglia activation. After vaccine injection significant systemic immune activation may occur with signs suggesting reactive brain inflammation, such as acute crying, fever, restlessness and failure to eat. It is a warning of danger to the brain in front of which we should reflect before causing irreversible damage. We also hypothesized the existence of a post-vaccination inflammatory syndrome caused by the proinflammatory cytokines strongly expressed after HPV vaccine injections. In addition, the molecular explanation of the chronic pain that has affected many girls in the world, including the complex regional pain syndrome (CRPS) in Japanese girls.
Conclusion: All vaccines can cause neuroinflammation. HPV vaccines can cause a post-vaccination inflammatory syndrome characterized by chronic pain and neuroinflammation. In this case, the phenomena of central sensitization is responsible for all the symptoms associated with chronic pain. The strong expression of proinflammatory cytokines, secreted after HPV vaccinations, brings to process that can produce irreversible neurological results in HPV vaccinated girls.
Keywords
Post-vaccination inflammatory syndrome, Neuroinflammation, Microglia activation, vaccines and autism, ASD, Autism Spectrum Disorders, HPV Vaccines, HPV vaccines adverse events, HPV vaccines AEs, complex regional pain syndrome (CRPS) in Japanese girls, CRPS type I, Chronic pain, Central sensitization.
Introduction
Vaccines are an important health policy tool and have changed the history of infectious diseases. In recent years, the number of vaccines injected to infants have increased, and many doses are administered during the first year of life, when the immune system and the central nervous system have yet to complete their development. Moreover, the immune system and the brain are bonded for life, dependent upon each other in sickness and in health [1]. In addition, at the same time, each immunological challenge is a challenge for the brain, and each vaccination is a challenge for both. Each injection of vaccine, regardless of the type, is followed by the production of variable amounts of pro-inflammatory cytokines, which exert both local effects and at a distance from the production site.
Since the peripheral cytokines, produced after the injection of the vaccines, are able to reach the central nervous system, we have hypothesized, in our recent paper [2], that these cytokines can have effects on the microglia (macrophages of the central nervous system). Microglia are the primary responders to an immune challenge and the primary producers of cytokines and chemokines within the brain. Microglial activation is the initial cellular event that occurs during acute neuroinflammation [3]. Furthmore, timing of a developmental immune challenge can be critical in determining the long-term outcomes on brain and behavior [4], since cognitive function and immune function are inextricably linked [1].
Since the post-vaccination adverse events (AEs) are related to the different period of life (childhood or adolescence), and to the different nervous area involved (brain or spinal cord); in this article we will deal with the regressive form of ASDs and present our hypothesis of a new post-vaccination inflammatory syndrome triggered by HPV vaccines. In our discussion we will only manage with molecular biology and our work is not suitable for improbable comparisons with epidemiological studies on vaccines. In this case, our task will be to describe the biological plausibility that links vaccine injections to these two clinical entities. Therefore, we will also proceed with a review of the specific scientific literature published on the two topics to find the evidence that supports our scientific hypothesis.
Autism spectrum disorders (ASD) and neuroinflammation
In our previous paper [2], we discussed the immunological aspects in ASD and in this paper we report additional findings. ASD is a pervasive neurodevelopmental condition characterized by variable impairments in communication and social interaction as well as restricted interests and repetitive behaviors. In the case of regressive autism, children are born healthy, have apparently normal development, and have been reaching their developmental milestones (as clearly documented in their medical records) and suddenly develop autism-like symptoms shortly after receiving a schedule vaccine [5].
There is a growing body of work to support the role of inflammatory cytokines in ASD. An emerging focus of research into the etiology of ASD has suggested neuroinflammation as one of the major candidates underlying the biologica model [5]. Plasma levels of IL-1β, IL-6 and IL-8 were increased in children with ASD and correlated with regressive autism, as well as impaired communication and aberrant behavior [6,7,8]. Vargas [9] showed an active neuroinflammatory process in the cerebral cortex, white matter, and in the cerebellum of autistic patients. Immunocytochemichal studies showed marked activation of microglia [5].
Since brain and immune system are inextricably woven together, immune-activating events can influence the long-term trajectory and function of developmental processes [10]. At birth, the neonatal immune system faced the critical challenge of transferring from a sterile environment to a world filled whit pathogens, microbes, and toxins where it must effectively defend the newborn. Early life infection is not the only challenge that can activate the immune system and impact the developing brain and behavior [1], and vaccinations are important immune challenges.
1.1 Genes and Environment
Gene networks are involved in immune processes are overexpressed in the brain of individuals with ASD [11,12]. Human communication and patterns of behavior are governed by a large number of genes and by a complex orchestra-like communication between these genes which are formed and organized during the early stage of human fetal development [13].
There is an extreme vulnerability of the developing human brain to toxic exposure in the environment [14]. Moreover, the fetal brain can be a major target for some synthetic chemicals that can cause mutations and/or interrupt the well-orchestrated pattern of fetal brain development. Any environmental agents that interfere with fetal brain development can cause major adverse effects even after birth. Indeed, pollutants can induce genetic mutations in fetal brain cells [13]. These effects then depend on the time of exposure, on the composition of the contaminating chemical mixture and on the specific vulnerability of the fetus in that specific phase of development. For example, damage to a cellular progenitor can lead to a greater loss of a cellular component since the cell population, dependent on that ancestral progenitor, is missing. In addition, epigenetic modifications can make one type of brain progenitor neuron change to another type [15]. Epigenetic changes produce a change in phenotype without a change in genotype. Toxins, such as diesel exhaust, drugs such as morphine, amphetamines and alcohol can trigger TLR signaling [16]. Different environmental stimuli can trigger TLR signaling, either directly or indirectly via an alarmin pathway. HMGB1 is a ubiquitous component of chromatin which can be released by necrotic cells, and is actively secreted by cells undergoing an inflammatory challenge or biological stress. HMGB1 activates microglia in the brain via TLR4 [16].
1.2 Peripheral cytokines in ASD
Pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, appear to be at the forefront in the communication between the immune and the nervous system, playing dual roles in mediating physiological and neuroprotective roles in normal brain function or being detrimental and associated with brain diseases, especially when present at elevated concentrations [17]. Altered cytokine profiles have been consistently linked to ASD in children in the postnatal period [18]. Cytokines may influence behavior through effects on neurotransmitter function, neuroendocrine activity, neurogenesis, and alterations to brain circuitry [19]. Peripheral cytokine signals are thought to access the brain through three pathways: humoral, neural, and cellular [19,20]. The blood-brain barrier (BBB) has an energy-dependent, saturable, carrier-mediated transport system for cytokines, primarily IL-1, IL-6, and TNF-α [21,22]. When endothelial cells making up the BBB come into contact with these peripheral cytokines, they secrete various immune molecules into the brain parenchyma, including NO, prostaglandin E2, IL-1, and IL-6, all proinflammatory cytokines known to affect neurological function [23].
The entry of peripheral cytokines into the brain determines different effects. The brain recognizes cytokines such as the pro-inflammatory cytokines IL-1α, IL-1β, TNF-α, and IL-6 as molecular signals of sickness [24]. Elevated IL-1β and IL-6 have been associated with increased stereotypical behaviors. Normal levels of IL-1β and its IL-1ra receptor antagonist are necessary to achieve normal development and normal brain function. TNF-α is a central regulator of inflammation and is elevated in the cerebrospinal fluid of children with ASD [25].
1.3 Microglia
Microglia is associated with neurons, synapses and blood vessels. Microglia are multifunctional immune cells of the brain and are involved in the defense of neural parenchima, and in immune response in the brain. Microglia are also concentrated in sites of incomplete BBB function, such as the circumventricular organs (CVOs), or the organum vasculosum of the lamina terminalis, subcommissural organ, subfornical organ, area postrema, posterior pituitary, median eminence, pineal, and choroid plexus [26]. Amoeboid microglia remove inappropriate superflous axons and cellular debris. A recent study by [27] demonstrated a central role of microglia in synaptic pruning and circuit development in the developing embryonic brain.
Neuroglial activation and its innate immune responses have been reported to contribute to autism [28]. Microglial activation is reported to be present in autistic patients throughout their life (including the early period of development) and play a critical role in the development of autism [29]. Autopsy studies performed on autistic brains revealed marked activation of microglia [28] and sustained neurological inflammatory response due to microglial activation in cortical and subcortical white matter as well as in the cerebellum. Autistic brains expressed a wide array of pro-inflammatory cytokines.
On activation, microglial cells are known to secrete proinflammatory cytokines/chemokines and the presence of proinflammatory chemokines such as MCP-1 is attributed to the pathogenesis of autism by activated microglia or by recruiting monocyte/macrophages to the sites of cortical neuronal abnormalities [28]. Furthemore, excitotoxin glutamate released by activated microglia is also of prime concern as excess glutamate in the brain is deleterious to neurons and synaptic connections [30]. Microglia also exert cytotoxic effects through secretion of toxic factors such as nitric oxide (NO), reactive oxygen species (ROS) and cytokines [31].
1.4 Microglia primed
Microglial activation is quite rapid following systemic immune activation, usually within minutes and results in immunoexcitotoxicity. Upon stimulation, microglia produces also a vast array of cytokines, some of which are know to have neurotrophic and neuroprotective functions [31]. Indeed, IL-10 and TGF-β are known to have anti-inflammatory activities, IL-6 has also neurtrophic activity and pro-inflammatory activity [31].
Microglia can switch from a resting phenotype to a primed state by an initial immune stimulus that is not excessively intense. For example, a mild head injury or episode of hypoxia can switch microglia from its resting state to a functional condition in which the enzymes and genetic activation is upregulated, but the active immune molecules, primarily proinflammatory cytokines and chemokines, are not released [32]. With a second immune stimulus, these primed microglia began to release proinflammatory cytokines and chemokines in much higher concentrations than that of not primed microglia [32].
Systemic immune stimulation can prime brain microglia, which means that either subsequent brain disturbances or systemic immune activation would trigger a magnified immune response within the brain [32]. Immune events throughout life, exposure to neurotoxic metals, exposure to pesticides/ herbicides and fungicides, head injury, and other factors, can cause episodes associated with microglia priming and activation, leading to a progressive loss of neurons in the most vulnerable parts of the CNS, such as the hypothalamus, temporal lobes (hippocampus, striatal area, amygdala, and entorhinal cortex) and prefrontal cortex [32].
In the infant or small child, the priming event may come from a number of sources, such as vaccination of the mother during pregnancy or with intrauterine or early post birth infections [33,34]. In other instances, the priming event may occur with the first vaccine inoculation, usually at birth (hepatitis B). Once primed, subsequent vaccinations, especially within months of the previous inoculation, will trigger full microglial activation and in the developing brain can result in abnormal pathways development [35-38]. While natural infections can also produce this neurodestructive response, vaccinations produce higher levels of immune activation and the immune response can persist longer than natural infections – sometimes lasting years [32].
It is well-established that inflammation in the periphery can prompt immune responses in the brain [39]. Contrary to the long held assumption that immunological memory exists only in cells of the adaptive immune system, recent evidence has indicated that also myeloid cells display memory effects [40,41]. For example, certain immune stimuli training blood monocytes to generate enhanced immune responses to subsequent immune insults [42,43]. By contrast, other stimuli induce immune tolerance/suppression of inflammatory responses to subsequent stimuli [43,44].
Innate immune memory lasts for several days in vitro and for up to three months in circulating monocytes in vivo and is mediated by epigenetic reprogramming in cultured cells, with chromatin changes also apparent in vivo [43,45,46]. However, while training may be beneficial in the periphery, owing to enhanced pathogen elimination [47,48], and tolerance may be detrimental owing to higher rates of infection resulting from immune suppression [44], training promotes, while tolerance alleviates neuropathology [49].
In summary, innate immune memory is a vital mechanism of myeloid cell plasticity that occurs in response to environmental stimuli and alters subsequent immune responses [49]. Two types of immunological imprinting can be distinguished, training and tolerance. These are epigenetically mediated and enhance or suppress subsequent inflammation, respectively [49]. Peripherally applied inflammatory stimuli induce acute immune training and tolerance in the brain and lead to the differential epigenetic reprogramming of microglia that persists for at least six months [49]. Individual cytokines applied peripherally may also elicit immune memory effects in the brain [49].
1.5 Dangerous Oxidative Species
Many of the toxic substances secreted by microglia are reactive oxygen and nitrogen species (RONS) which include superoxide anion radical, hydrogen peroxide, nitric oxide, peroxynitrite, and nitrogen dioxide (Blaylock, 2004). Superoxide is produced by the enzyme NADPH oxidase. The RONS produced by microglia are capable of not only killing healthy neurons but they are also capable to cause oxidative stress and produce priming microglia for further activation (Kaur and Eng-Ang, 2012). Reactive nitrogen species are produced by nitric oxide synthase including the inducible isoform (iNOS) that is upregulated during microglia activation [31]. The iNOS is expressed only during inflammation by activated microglial cells under certain conditions such as hypoxic injury and infections [31]. Reactive nitrogen species are neurotoxic and are capable of damaging the vasculature and impairing the mitochondrial respiratory chain. NO has multiple roles in the brain, from regulation of blood flow to being a potent neurotoxin. These effects depend on the cellular source and the amount generated [31]. A simultaneous activation of iNOS and phagocyte NADPH oxidase in microglia leads to the formation of peroxynitrite which is an extremely powerful oxidizing agent [31].
2- Causality assessment of AEs (alias AEFI).
In the field of post-vaccination adverse reactions (AEs or AEFI), there are two major problems:
- The unbundled report system that is present in the vaccines data sheets.
2.1 The first problem: WHO
In January 2018, the WHO produces a document on how to catalog the adverse reactions that are indicated by the acronym AEFI. The WHO states: “Causality assessment is the systematic review of data about an AEFI case; it aims to determine the likelihood of a causal association between the event and the vaccine(s) received” [50]. It also specifies: “At the individual level it is usually not possible to establish a definite causal relationship between a particular AEFI and a particular vaccine on the basis of a single AEFI case report” [50]. Since all adverse reactions are case reports (because they occur in a single vaccinated individual), excluding them results in the consequent elimination of all post-vaccine AEs. Furthermore, the report cases form the series of reports that will never exist with this evaluation system that excludes the individual case reports.
A practical example shows that the reports of AEs do not end up on the reports of the regulatory agencies. These are two cases of transient neutropenia from MMRV vaccines (Measles, Mumps, Rubeola and Varicella) that have been published [51] after reporting to the Italian Medicines Agency [52], but it do not appear in the Agency Report [53].
2.2 The second problem: Vaccines Data Sheets
Taking as an example a vaccine widely used in Europe [54], we immediately notice that the adverse reactions are cataloged reporting the frequency of the single symptom, but there are no data on the combination of reactions in the same subject (GSK, 2018). Infanrix Hexa is indicated for primary and booster vaccination of infants and toddlers against diphtheria, tetanus, pertussis, hepatitis B, poliomyelitis and disease caused by Haemophilus influenzae type b. The following drug-related reported adverse reactions in clinical studies (data from more than 16,000 subjects) and during post-marketing surveillance (GSK, 2018).
Very Common Adverse Events (≥ 1/10 doses)
- Appetite lost.
- Crying abnormal and pain.
- Irritability.
- Fever ≥ 38°C.
The presence of all these symptoms in the same subject suggests a post-vaccination reactive brain inflammation produced by proinflammatory cytokines, secreted after vaccine injection.
2.3 Post-Vaccination Reactive Brain Inflammation
During the first two years of life, particularly in the winter months, the immune system is often engaged with several infectious challenges. These are immune stimulations added to the immune challenges, linked to the adoption of the vaccination schedule.
After vaccine injection, especially if multiple doses are given to a young child during a single office visit, significant systemic immune activation may occur with signs suggesting reactive brain inflammation, such as acute crying, fever, restlessness and failure to eat [32,36]. When this reaction takes place, it is necessary to suspend the vaccination schedule for at least 6 months to allow the innate immune system to “forget that it has become so little tolerant in the brain”. Otherwise, the neuroinflammation may produce serious damages especially if the microglia continues to be stressed by peripheral cytokines produced after each vaccination. It is a warning of danger to the brain and you can choose to continue the vaccination schedule (putting at risk the health of the small child) or, vice versa, stop with vaccinations to respect the principle of “primum non nocere”.
Post-Vaccination Inflammatory Syndrome: A new Syndrome.
Human papillomavirus vaccines (HPV Vaccines) are neither safe nor effective as claimed by so much scientific literature. These vaccines are anti-virus vaccines, but they are not anti-tumor vaccines [2], In our previous publication, we addressed the issues of the alleged safety and efficacy of these vaccines [2]. In this paper we will discuss the molecular biology that supports our hypothesis of a new post-vaccination inflammatory syndrome triggered by HPV vaccines.
Let us just remember that it was shown that vaccinated young women have had a higher prevalence of any HPV type infection (type with high and low risk for cancer), and a higher prevalence of virus infection with high risk of non-vaccine types, despite having a lower prevalence of vaccination types [55].
3.1 History of adverse reactions
In Japan, the period of HPV vaccination overlapped with the development of HPV vaccine-related symptoms in the vaccinated patients, including chronic regional pain syndrome (CRPS) and autonomic and cognitive dysfunctions [56]. Brinth [57] reported the characteristics of a number of patients with a syndrome of orthostatic intolerance, headache, fatigue, cognitive dysfunction, and neuropathic pain starting in close relation to HPV vaccination. The Lareb in the Netherlands, has received a substantial number of reports concerning long-lasting AEs after vaccination with Cervarix® [58,59].
3.2 HPV vaccines and pain
In the Cervarix Package insert [54] it is reported that: 20% of subjects were in pain, 20% of subjects had a sense of fatigue. In the Gardasil 4 Package insert [60] it is reported that: headache, fever, nausea, and dizziness; and local injection site reactions (pain, swelling, erythema, pruritus, and bruising) occurred after the administration of Gardasil. In the Gardasil 9 Package insert [61], pain is reported to be present in almost 90% of vaccinated girls.
3.3 HPV vaccines and pain: the molecular bases
Vaccination produces always inflammation. During inflammation, tissue resident and recruited immune cells secrete molecular mediators that act on the peripheral nerve terminals of nociceptor neurons to produce pain sensitization . Nociceptor peripheral nerve terminals possess receptors and ion channels that detect molecular mediators released during inflammation. Nociceptor neurons express receptors for immune cell-derived cytokines, lipids, proteases, and growth factors . High circulating plasma cytokine/chemokine levels were observed after the first dose of Gardasil 4® vaccine and the proinflammatory cytokines were elevated after the 1st and 3rd injection of the Cervarix® vaccine [62,63,64].
In summary, proinflammatory cytokines produced after vaccine injection are able to stimulate specific receptors that are present on nociceptor neurons. Indeed, Nociceptor neurons are also sensitized by TNF-α, IL-1β and IL-6 produced by mast cells, macrophages, and neutrophils [63]. All these proinflammatory cytokines are produced after vaccine injection.
3.4 Pain processing
Understanding pain processing is fundamental to identify the roots of post-vaccination inflammatory syndrome caused by HPV vaccines . This is a complicated path that begins with the expression of proinflammatory cytokines on the vaccine injection site, and then arrives at the somatosensory cortex.
3.5 Nociceptors
Physiological pain is initiated by specialized sensory nociceptor fibers which innervate peripheral tissues and are only activated by noxious stimuli [61]. The stimulation of nociceptors determines the onset of an action potential that is propagated along the axons of nociceptive Aδ and C fibres, through the dorsal root ganglion (DRG) to the axon terminals in the spinal cord dorsal horn [63]. A brief period of low frequency C-fibre stimulation, in the absence of nerve damage, is sufficient to activate microglia resulting in behavioural hyperalgesia [64].
Nociceptors by responding directly to cytokines can directly “sense” the immune response in inflamed tissue; essentially they are, therefore, not only noxious stimulus detectors, but also inflammation sensors [65]. Moreover, TNF-α is a key regulator of the inflammatory response and is involved in the increased production of proalgesic agents [64].
3.6 The second order neurons
The second order dorsal horn neurons, involved in pain circuitry, exist in two broadly characterised populations. After synapsing at the spinal cord, the second neuron travels in the spinal tracts, crosses the midline and runs up the spinothalamic tract to the thalamus where they synapse again and the next neuron travels to the somatosensory cortex. Here the impulses are processed in distinct areas, known collectively as the “pain matrix” so the nature of the pain can be perceived.
3.7 Neurophatic and inflammatory pain
Peripheral nerve injury activates spinal microglia. This leads to lasting changes in the properties of dorsal horn neurons that initiate central sensitization and the onset of neuropathic pain [66]. Vice versa, inflammatory pain is initiated by tissue damage/inflammation. Both are characterized by hypersensitivity at the site of damage and in adjacent normal tissue [67].
3.8 Chronic pain
It is now well established that chronic pain, such as inflammatory pain, neuropathic pain, and cancer pain, is an expression of neural plasticity, both in the peripheral nervous system as peripheral sensitization [68,69], and in the central nervous system (CNS) as central sensitization [69,70].
The rules of perception and pain management change in chronic pain. In fact, at peripheral level, nociceptors undergo sensitization and hyper-excitability (peripheral sensitization); while at the central level, excitatory synaptic transmission is increased in spinal cord, brainstem, and cortical neurons (central sensitization), caused by transcriptional, translational, and post-translational regulation [71].
3.9 Peripheral sensitisation
The International Association for the Study of Pain (IASP) definition of peripheral sensitisation is: “Increased responsiveness and reduced threshold of nociceptive neurons in the periphery to the stimulation of their receptive fields” [72]. Then, Peripheral sensitisation induces a hyperexcitability of afferent nociceptive neurons [63].
3.10 Central Sensitization
The IASP definition of central sensitisation is: “Increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input” [73]. Then, central sensitization refers to the amplification of pain by central nervous system mechanisms. On a cellular level, central sensitization results from multiple processes altering the functional status of nociceptive neurons [74]. Central sensitization increases response to pain sensation. Heightened sensitivity results in the perception of pain from non painful stimuli (allodynia) and greater pain than what one would be expected to get from normal painful stimuli (hyperalgesia).
3.11 Effects of Peripheral and central sensitization
While peripheral sensitization in nociceptors is essential for the development of chronic pain [75], and transition from acute pain to chronic pain [74], central sensitization regulates the chronicity of pain, causes the spread of pain beyond the site of injury, and influences the emotional and affective aspects of pain [62].
3.12 Spinal cord microglia
The spinal cord microglia, can respond to peripheral injuries that are distant from the spinal cord to produce neuroinflammation in the central nervous system [70]. Spinal glia activation is necessary and sufficient to induce neuropathic pain [74]. Astrocytes perform numerous critical functions such as neurotransmitter recycling, formation of the blood-brain barrier, regulation of extracellular ion concentration, and modulation of synaptic transmission, among many others [75].
3.13 Nociceptors activates microglia and astrocytes
In the case of strong and repetitive noxious stimuli, larger quantities and additional signaling molecules are released from the spinal terminals of nociceptive nerve fibers leading to the activation of microglia and astrocytes [76], and in some cases, to the degranulation of dural mast cells, to vasodilation, impairment of the blood–spinal cord barrier, and to the recruitment of T-cells to the spinal parenchyma [77]. This in turn causes the release of inflammatory mediators in the spinal cord, including chemokines and cytokines [76]. Hathway [63] had shown that a brief period of low frequency C-fibre stimulation, in the absence of nerve damage, is sufficient to activate microglia resulting in behavioural hyperalgesia.
3.14 Neuroinflammation in chronic pain
Neuroinflammation (in the peripheral and central nervous system) drives and manteined widespread chronic pain via central sensitization, which is a phenomenon of synaptic plasticity, and increased neuronal responsiveness in central pain pathways after painful insults [78]. A characteristic feature of neuroinflammation is the activation of glial cells, such as microglia and astrocytes, in the spinal cord and brain, leading to the release of proinflammatory cytokines and chemokines [78]. Sustained increase of cytokines and chemokines in the central nervous system also promotes chronic widespread pain that affects multiple body sites [78].
3.15 CRPS type I
Individuals without a confirmed nerve injury are classified as having CRPS type I, while in CRPS type II there is an associated and confirmed nerve injury. When pain arises in the absence of a nerve injury it is nociceptive pain. The term nociceptive pain is used to describe pain occurring with a normally functioning somatosensory nervous system to contrast with the abnormal function seen in neuropathic pain [71]. CRPS describes an array of painful conditions (nociceptive pain in CRPS type I) that are characterized by a continuing (spontaneous and/or evoked) limb pain that is seemingly disproportionate in time or degree to the usual course of any known trauma or other lesion. The pain is regional (not in a specific nerve territory or dermatome) and usually has a distal predominante [79]. Symptoms of CRPS-I include spontaneous pain (“burning” pain referred to the skin, and “aching” pain referred to deep tissues), and a variety of stimulus-evoked abnormal pain sensations, including mechano-hyperalgesia, mechano-allodynia, cold-allodynia and sometimes heat-hyperalgesia. Other symptoms include disorders of vasomotor and sudomotor regulation; trophic changes in skin, hair, nails, and bone, and dystonia and other motor abnormalities [80].
Thus, the most prominent mechanism appears to be the inflammatory process because all the classic signs of inflammation (oedema, redness, hyperthermia, and impaired function) are conspicuous in the early stages of CRPS [81]. High levels of the proinflammatory cytokines (TNF-α and IL-6) have been found in skin blister fluid of the affected limbs versus the unaffected limbs of CRPS patients [82]. In patients with CRPS, the levels of IL-1β and IL-6 were significantly increased in cerebrospinal fluid (CSF), compared to other subjects [83,84]. In the blood of subjects with painful neuropathy, TNF-α levels were doubled, compared to healthy subjects and those with non-painful neuropathy [85]. IL-1β can modulate the transmission of sensory neurons because it increases the release of substance P [86,87]. Thus, CRPS type I is associated with high levels of IL-1β and IL-6 in CSF, and high levels of TNF-α in the blood. Furthermore, these proinflammatory cytokines are strongly expressed after the injection of HPV vaccines.
Discussion
Each vaccine injection determines the mandatory intervention, at the injection site, of dendritic cells that are, at the same time, antigen presenting cells (APC) and tissue macrophages. Macrophages secrete pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, when activated. Furthemore, the immune competent cells are one of the largest sources of cytokines, that being capable to migrate in almost all tissues of the body, represent moving regulators of the local microenvironment [88]. Cytokines, together with neurotransmitters and hormones, are signaling molecules which have unique immunomodulatory functions. Virtually, they can influence every physiological system including neuroendocrine interactions, neurotransmitter metabolism and neuroplasticity, thereby affecting behavioral and cognitive functioning [89].
Each vaccine injection results in a strong expression of proinflammatory cytokines. Cytokines take center stage in orchestrating immune responses [90]. They act in most cases at shorter distances (with exceptions such as IL-1, IL-6 and TNF).
In a previous paper [2], we had hypothesized a link between vaccinations and neuroinflammation. The peripheral pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α), expressed after the injection of all vaccines, can reach the brain and can cause neuroinflammation after microglia activation. Elevated proinflammatory cytokines, particularly TNF-α, have been described in studies regarding the cytokines profile in autistic children. IL-1β represents a cytokine that controls the local pro-inflammatory cascade and thereby affects the balance between protective immunity and destructive inflammation. A subgroup of children with ASD have developed neuroinflammation. Several postmortem studies have confirmed the activation of microglia and neuroinflammation. A recent study shows the presence of aluminium in brain tissue in ASD. Aluminium was also found in microglia cells [91]. Aluminium from vaccines is redistributed to numerous organs including the brain, where it accumulates. Each vaccine adds to this tissue different levels of aluminium. Aluminum, like mercury, activates microglia leading to chronic brain inflammation and neurotoxicity.
Gardasil and Cervarix vaccines (Figure 1) contain aluminum, which activates caspase-1 enzyme, via NLRP3 inflammasome. The caspase-1 enzyme converts the pro-interleukins 1β and 18 in their active forms. IL-18 determines the production of IFN-γ. IL-1β represents a cytokine that controls the local pro-inflammatory cascade and contributes to activate the transcription factor NF-κB. The Cervarix adjuvant AS04 contains Aluminum Hydroxide and MPL. The second one stimulates the TLR4. Gardasil 4 vaccine is contaminated with foreign DNA in non-B conformation [92], which activates TLR9. TLRs act through the adapter protein MyD88 which acts increasing the activity of NFκB, which then increases the expression and secretion of IL-1β, IL-6 and TNF-α (for references see [2]. Thus, there is a strong immune stimulation and a strong production of pro-inflammatory cytokines, including IL-1β IL-6 and TNF-α, which are capable of exerting effects at a distance from the production site.
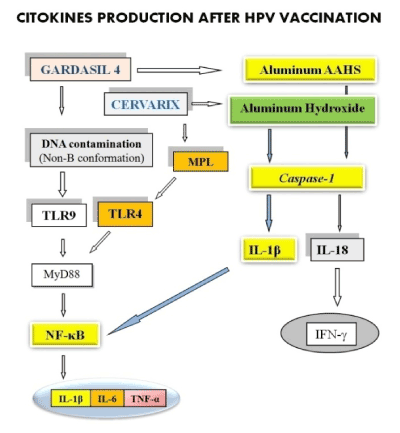
Figure 1. Gardasil and Cervarix vaccines contain aluminum, which activates caspase-1 enzyme, via NLRP3 inflammasome. The caspase-1 enzyme converts the pro-interleukins 1β (IL-1β) and 18 in their active forms. TLRs act through the adapter protein MyD88 that acts by increasing the activity of NF-κB. Both, the IL-1β and the activation of the toll-like receptors (TLR) 4 and 9, determines the activation of the transcription factor NF-κB, which then increases the expression and secretion of IL-1β, IL-6 and TNF-α.
All adjuvants modulated a common set of 168 genes and promoted antigen-presenting cell recruitment. Alum regulated 312 genes [93]. A number of in vitro experiments [94] have shown that alum activates the NLRP3 inflammasome in macrophages which, in turn, activates caspase-1 and consequent production of interleukin- (IL-) 1β (IL-1β). Upon activation, members of the Nod-Like Receptors (NLR) family, such as NLRP3, form complexes with ASC and pro-caspase-1. The complex formed by these molecules is referred to as the inflammasome. The NLRP3 inflammasome is activated by a number of materials, including alum. Whatever the cause of inflammasome activation, the consequences include production of active caspase-1 thus conversion of inactive precursor cytokines of the IL-1 family, including IL-1β, IL18 and IL-33, to their active forms [95].
In summary, the aluminum salts injected as vaccine adjuvants are taken by the innate immunity cells (especially from dendritic cells), they engage a receptor called NLR (NLRP3), which together with other proteins is organized into an intracellular macromolecular complex that activates the enzyme caspase-1. This enzyme converts pro-IL-1β and pro-IL-18 into their active forms (IL-1β, and IL-18). The role of caspase-1 is not limited to the conversion of pro IL-1β to IL-1β alone, but it strongly affects the secretion of proinflammatory cytokines: IL1β, IL-1α, IL-6, TNF-α, IL-18 and IFN-γ. IL-1 is a primary regulator of inflammatory and immune responses. Via its type I receptor it activates specific protein kinases, including the nuclear factor kappa-light-chainenhancer (NF-κB) inducing kinase (NIK) and three distinct mitogenactivated protein (MAP) kinase cascades. These modulate a number of transcription factors including NF-κB, AP1 and CREB, each of which regulate a plethora of immediate early genes central to the inflammatory response [96]. Therefore, each injection of the vaccine produces a proinflammatory response. An immune response to the vaccine antigens (to the quantities currently present in the vaccines), is non-possible without a pro-inflammatory response, which is produced by adjuvants.
In (Figure 2), the mechanism of action of the aluminum is always represented, but a new anti-meningococcal B vaccine produces the activation of the TLR- 2 and 4. The OMV vesicles contain lipoproteins that activate the TLR2, and LPS that activate TLR4. The strong production of peripheral pro-inflammatory cytokines is capable of producing microglia activation and neuroinflammation.
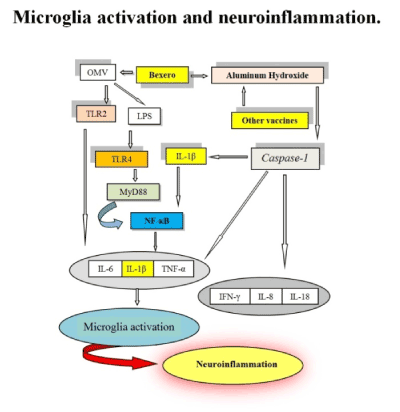
Figure 2. The mechanism of action of the aluminum is always represented, but a new anti-meningococcal B vaccine produces the activation of the TLR- 2 and 4. The OMV vesicles contain lipoproteins that activate the TLR2, and LPS that activate TLR4. The strong production of peripheral pro-inflammatory cytokines is capable of producing microglia activation and neuroinflammation.
On the right side of (Figure 3), you can see that the peripheral proinflammatory cytokines, expressed after the injection of vaccines, can reach the brain and, apart from neuroinflammation, can cause a post-vaccination inflammatory syndrome [97], as in the case of HPV vaccines. If a neuroinflammation is present, it could be followed by autoimmune reactions and neurodegeneration. Peripheral cytokines can produce primed microglia and the inflammatory phenotype M1 which participates in the neuroinflammation. The neuroinflammation increases production of pro-inflammatory cytokines and it activates astrocytes, it produces an oxidative stress and increases the production of prostaglandins in the brain. Oxidative stress and astrocytes activation cause a rupture of the BBB that eases the entry of the T and B lymphocytes in the brain. Oxidative stress also produces damage to self-antigens and may help to produce autoimmunity and neurodegenerative diseases for references see [2].
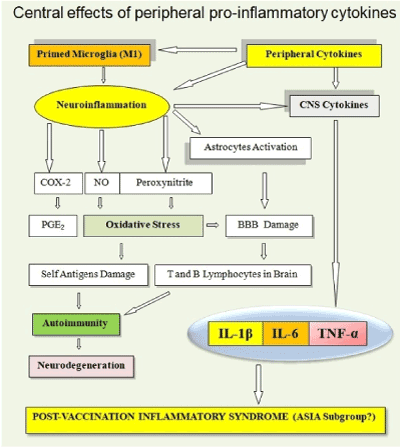
Figure 3. Peripheral pro-inflammatory cytokines, expressed after the injection of vaccines, can reach the brain and, apart from neuroinflammation, can cause a post-vaccination inflammatory syndrome, as in the case of HPV vaccines.
As for the relationship between vaccines and pain, it is now evident that the pro-inflammatory response to the injections of the HPV vaccines is identical, under the common cytokines substrate, to the inflammatory profile of the CRPS type I. Certainly, individual predisposition and other possible interfering factors have determined who should get sick and who does not, while expressing (both categories of subjects) high levels of proinflammatory cytokines after injections of HPV vaccines [2], As is evident, in the case of HPV vaccines, the pain is initially caused by the strong production of proinflammatory cytokines (such as IL-1β, IL-6, and TNF-α), which are followed by the phenomena of peripheral and central sensitization associated with signs of neuroinflammation in the CNS (elevated cytokines in CSF, such as IL-1β and IL-6).
Figure 4 shows the two mechanisms (haematic and neural) which can lead to neuroinflammation, in some girls, after the injection of HPV vaccines.
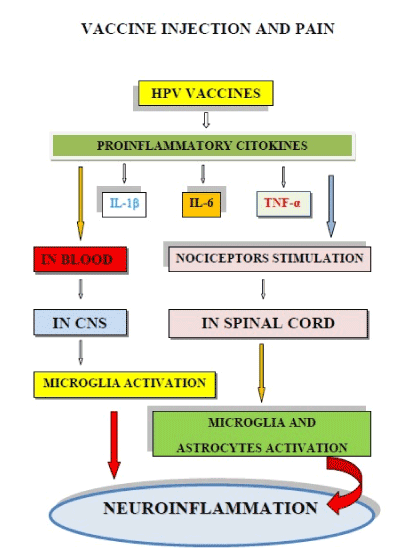
Figure 4. Injections of HPV vaccines can produce microglia activation and neuroinflammation. The strong expression of pro-inflammatory cytokines (such as IL-1β, IL-6 and TNF-α), can activate microglia via blood and/or through nociceptors which activate microglia and astrocytes in the spinal cord. The double activation pathway of microglia can produce neuroinflammation.
Finally, (Figure 5) demonstrates how an abnormal response of nociceptors, to cytokines produced after injections of HPV vaccines, can produce the peripheral and central sensitization phenomena, that are present in chronic pain, including the signs and symptoms of CRPS type I that in Japan was reported as an HPV vaccines AEs.
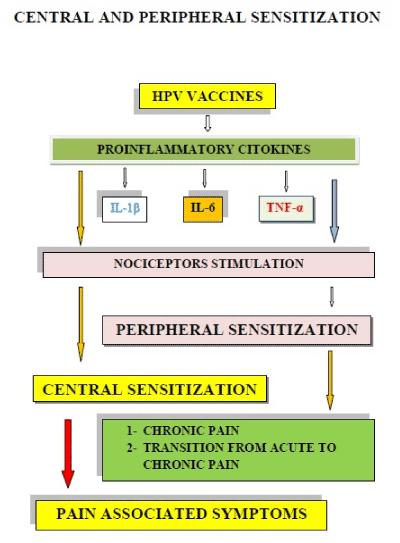
Figure 5. An abnormal nociceptor response to cytokines produced after HPV vaccine injections can produce peripheral and central sensitization phenomena. This new condition explains the mechanisms of chronic pain and produces the symptoms associated with it.
Conclusion
The existence of extensive lines of communication between the nervous system and immune system represents a fundamental principle underlying neuroinflammation. Immune memory in the brain is an important modifier of neuropathology. Systemic inflammation generates signals that communicate with the brain and lead to changes in metabolism and behavior, with microglia assuming a pro-inflammatory phenotype. Two types of immunological imprinting can be distinguished: Training and tolerance. These are epigenetically mediated and enhance or suppress subsequent inflammation respectively.
The molecular mechanisms presented here demonstrate how peripheral cytokines, expressed after vaccination, can cause neuroinflammation in some subjects, after microglia activation, depending on the immunogenetic background and the innate immune memory. The effects produced by the activation of the microglia, and the subsequent neuroinflammation, are diversified according to age: before the first two years of life they can contribute to producing ASD (in some subjects with ASD there is neuroinflammation and aluminum accumulation in the brain); while a different neurological symptomatology can arise in girls vaccinated with HPV vaccines. Indeed, the proinflammatory cytokines expressed after HPV vaccine injections can cause neuroinflammation and chronic pain, and we hypothesize that the aforementioned cytokines are capable of producing a post-vaccination inflammatory syndrome in which chronic pain and neuroinflammation are practically always present.
In all girls mentioned in the book “The HPV vaccine on trail” [98], the chronic pain is always present and highly debilitating. Furthermore, many girls present the signs and symptoms of central sensitization with the associated psychic and motor symptoms (Table 1). Finally, in Japanese girls, the period of human papillomavirus vaccination considerably overlapped with that of unique post-vaccination symptom development (symptoms including chronic regional pain syndrome and autonomic and cognitive dysfunctions in the vaccinated patients).
Table 1. Symptoms and signs produced by Central Sensitization (Smith, 2010).
Central Sensitization. |
Malignant process of up-regulation, pain be getting more pain, becoming autonomous. |
Effects |
|
Secondary Hyperalgesia |
Reduced threshold, Hyperpathia, Paresthesia, Numbness. |
Modality Effects |
Allodynia. |
Sympathetic System |
Dysautonomia, Persistent hyperactivation, Paradoxical hyporeactivity to stress, Psychological problem. |
Autonomic effects |
Hyperhydrosis, Arousal/Non-arousal, Local Changes, Neurogenic edema, Temperature changes, Hypoalgesia; onset of stress- induced hyperalgesia, Vascular changes, Trophic changes, Hair and Nails problems. |
Movement Effects |
Difficulty in initiation, maintenance, and precision of small movements. Weakness, Dystonia, Decreased range of motion, Tremor, Spasm, myclonic jerks, Neglect-like syndrome, Pathophysiology of complex regional pain syndrome (CRPS). |
Systemic Effects |
Sleep disturbance, Fatigue, Circadian Rhythm disruption, Development of Additional Pain Syndromes, Sickness Behavior. |
Psychological effects |
Fear, Anger, Social login, Pain Behaviors, Motivation, helplessness effects, Depressive effects, Preoccupation with pain, and body self. |
In this paper, we have provided the explanation, in terms of molecular biology, to the epidemiological observations published by [99], and we have shown that the symptoms presented by girls with AEs have a molecular basis and clinical entities well known to the scientific world (CRPS type I, and central sensitization).
The reading of table 1 crash the alibi of the denialist scientists, because the girls suffering from these HPV vaccines AEs have all symptoms of the central sensitization [100] that is produced, by these injections, with the molecular mechanisms described by us in figure 4 and figure 5.
To further confirm the role of proinflammatory cytokines in the neurological diseases, we list, in (Table 2), some neurological syndromes with the associated proinflammatory cytokines, such as: Pathological pain [100], Peripheral neuropatic pain [101], Hyperalgesia [102], CRPS [103,104,105], Chronic fatigue [106,107], CFS/ME [108,109], and POTS [110,111].
Table 2. Neurologic clinic syndromes and proinflammatory cytokines.
Syndrome |
Proinflammatory cytokines |
|
IL-1β |
TNF-α |
IL-6 |
IFN-γ |
IL-8 |
Pathological pain |
Yes |
Yes |
Yes |
Yes |
|
Peripheral neuropatic pain |
Yes |
Yes |
|
|
|
Hyperalgesia |
Yes |
|
|
|
|
CRPS |
Yes |
Yes |
Yes |
|
Yes |
Chronic fatigue |
Yes |
Yes |
Yes |
|
|
CFS/ME |
Yes |
Yes |
|
|
|
POTS |
|
|
Yes |
|
|
Funding Information
This work has not received any funding and publication costs are paid by the authors of this paper.
Author’s Contributions
Giannotta N., has conducted the bibliographic research and prepared the part of the paper that deals with pain. Furthermore, he prepared the figures included in the paper.
Giannotta G., has studied neuroinflammation and prepared the part of molecular biology, in particular he studied the relationship between vaccines and neuroinflammation.
Conflict of Interest
The authors declare that they have no conflict of interests.
Ethics
We believe that this paper provides a clear contribution in understanding the molecular biology of vaccines, and for this reason we believe it would not create any ethical problem after its publication.
References
- Schwarz JM, Bilbo SD (2012) Future directions to understanding immune function and brain development. In: The immune system and the developing brain, Morgan & Claypool Life Science pp: 81-83.
- Giannotta G and Giannotta N (2018) Vaccines and Neuroinflammation. Int J Pub Healthand Safe 3: 163
- Schwarz JM, Bilbo SD (2012) Microglia are immune cells of the brain. In: The immune system and the developing brain, Morgan & Claypool Life Science pp: 13-17.
- Schwarz JM, Bilbo SD (2012) Commonly used models of early life immune activation in the rodent. In: The immune system and the developing brain, Morgan & Claypool Life Science pp: 33-40.
- Bagasra O, Heggen C (2018) Vaccines and autism. In: Autism and Environmental Factors, Wiley Blackwell pp: 261-286.
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, et al. (2010) Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 25: 40-45. [Crossref]
- Hagberg H and Mallard C (2005) Effect of inflammation on central nervous system development and vulnerability: review. Curr Opin Neurol 18: 117-123. [Crossref]
- Tsilioni I, Taliou A, Francis K, Theoharides TC (2015) Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl Psychiatry 5: [Crossref]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA (2005) Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57: 67-81. [Crossref]
- Schwarz JM, Bilbo SD (2012) Introduction. In: The immune system and the developing brain, Morgan & Claypool Life Science pp: 1-2.
- Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, et al. (2011) Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474: 380-384. [Crossref]
- Voineagu I and Eapen V (2013) Converging pathways in autism spectrum disorders: Interplay between synaptic dysfunction and immune responses. Front Hum Neurosci 7: 738. [Crossref]
- Bagasra O, Heggen C (2018) Introduction to autism Spectrum disorders. In: Autism and Environmental Factors, Wiley Blackwell pp: 1-50.
- Bagasra O, Heggen C (2018) Autism and exposure to environmental chemicals. In: Autism and Environmental Factors, Wiley Blackwell pp: 169-233.
- Bagasra O, Heggen C (2018) Maternal Twins and male gender bias in autism spectrum disorders. In: Autism and Environmental Factors, Wiley Blackwell pp: 143-167.
- Schwarz JM, Bilbo SD (2012e) Environmental triggers of TLR activation: long-term programming of brain and behavior. In: The immune system and the developing brain, Morgan & Claypool Life Science pp: 73-79.
- Strunecka A, Blaylock RL, Patocka J, Strunecky O (2018) Immunoexcitotoxicity as the central mechanism of etiopathology and treatment of autism spectrum disorders: A possible role of fluoride and aluminum. Surg Neurol Internat 9: 74. [Crossref]
- Goines P and Van de Water J (2010) The immune system’s role in the biology of autism. Curr Opin Neurol 23: 111-117. [Crossref]
- Capuron L and Miller AH (2011) Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther 130: 226-238. [Crossref]
- Masi A, Glozier N, Dale R, Guastella AJ (2017) The Immune System, Cytokines, and Biomarkers in Autism Spectrum Disorder. Neurosci Bull 33: 194-204. [Crossref]
- Banks WA and Kastin AJ (1991) Blood to brain transport of interleukin links the immune and central nervous system. Life Sci 48: 117-121. [Crossref]
- Gutierrez EG, Banks WA, Kastin AJ (1993) Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol 47: 169-176. [Crossref]
- Fabry Z, Fitzsimmons KM, Herlein JA, Moninger TO, Dobbs MB, et al. (1993) Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J Neuroimmunol 47: 23-34. [Crossref]
- Dantzer R (2009) Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am 29: 247-264. [Crossref]
- Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M (2007) Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol 36: 361-365. [Crossref]
- Bechmann I, Galea I, Perry VP (2002) What is the blood-brain barrier (not)? Trends Immunol 28: 5-11. [Crossref]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, et al. (2011) Synaptic pruning by microglia is necessary for normal brain development. Science 333: 1456-1458. [Crossref]
- Pardo CA, Vargas DL, Zimmerman AW (2005) Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry 17: 485-495. [Crossref]
- Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, et al. (2010) Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry 68: 368-376. [Crossref]
- http://www.jpands.org/vol9no2/blaylock.pdf
- http://www.novapublishers.org/catalog/product_info.php?products_id=24279
- Blaylock RL (2013) Immunology primer for neurosurgeons and neurologists part 2: Innate brain immunity. Surg Neurol Int 4: 118. [Crossref]
- http://www.chemtrailsaustralia.com/uploads/3/4/2/1/3421517/aluminum-blaylock.pdf
- Blaylock RL and Strunecka A (2009) Immune-glutamatergic dysfunction as a central mechanism of the autism spectrum disorders. Curr Med Chem 16: 157-170. [Crossref]
- Aarum J, Sandberg K, Budd-Haeberlein SL, Persson MAA (2003) Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci U S A 100: 15983-15988. [Crossref]
- https://www.ncbi.nlm.nih.gov/pubmed/19043938
- https://www.ncbi.nlm.nih.gov/pubmed/19161050
- Schlett K (2006) Glutamate as a modulator of embryonic and adult neurogenesis. Curr Top Med Chem 6: 949-960. [Crossref]
- Perry VH, Cunningham C, Holmes C (2007) Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol 7: 161-167. [Crossref]
- Netea MG, Latz E, Mills KH, O’neill LA (2015) Innate immune memory: A paradigm shift in understanding host defense. Nat Immunol 16: 675-679. [Crossref]
- Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, et al. (2016) Trained immunity: A program of innate immune memory in health and disease. Science 352: [Crossref]
- Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, et al. (2014) mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345: [Crossref]
- Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, et al. (2014) Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science 345: [Crossref]
- Biswas SK and Lopez-Collazo E (2009) Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol 30: 475-487. [Crossref]
- Kleinnijenhuis J, Quintin J, Preijers F, Joosten LA, Ifrim DC, et al. (2012) Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 109: 17537-17542. [Crossref]
- Novakovic B, Habibi E, Wang SY, Arts R, Davar R, et al. (2016) β-Glucan Reverses the Epigenetic State of LPS-Induced Immunological Tolerance. Cell 167: 1354-1368.e14. [Crossref]
- Arts RJ, Moorlag SJ, Novakovic B, Li Y, Wang SY, et al. (2018) BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe 23: 89-100. [Crossref]
- Kaufman E, Sanz J, Dunn JL, Khan N, Mendonça LE, et al. (2018) BCG educates hematopoietic stem cells to generate protective innate immunity against tuberculosis. Cell 172: 176-190. [Crossref]
- Wendeln AC, Degenhardt K, Kaurani L, Gertig M, Ulas T, et al. (2018) Innate immune memory in the brain shapes neurological disease hallmarks. Nature 556: 332-338. [Crossref]
- https://www.who.int/vaccine_safety/publications/gvs_aefi/en/
- Giannotta G (2018) MMRV Vaccine Associated Transient Neutropenia: Description of Two Cases. J Clin Case Rep 8: 1069.
- AIFA vaccines report.
- http://www.aifa.gov.it/sites/default/files/Rapporto_Vaccini_2017_vers._acc.pdf
- https://gskpro.com/content/dam/global/hcpportal/en_MT/PDF/Homepage/Products/productlisting/infanrix-Hexa/Infanrix-hexa-(Feb_2018).pdf
- Guo F, Hirth JM, Berenson AB (2015) Comparison of HPV prevalence between HPV-vaccinated and non-vaccinated young adult women (20-26 years). Hum Vacc Immunother 11: 2337-2344. [Crossref]
- Ozawa K, Hineno A, Kinoshita T, Ishihara S, Ikeda SI (2017) Suspected adverse effects after human papillomavirus vaccination: A temporal relationship between vaccine administration and the appearance of symptoms in Japan. Drug Saf 40: 1219-1229. [Crossref]
- https://pdfs.semanticscholar.org/4151/aa8ac4091eb0301cce520c997cd44c8c70bc.pdf
- https://www.lareb.nl/media/2999/cervarix_31102016.pdf
- Cervarix Package insert
- Gardasil 4 Package insert
- Gardasil 9 Package insert
- https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM240436.pdf
- https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm111263.pdf
- https://www.fda.gov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm426457.pdf
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, et al. (2008) Nociceptors are interleukin-1beta sensors. J Neurosci 28: 14062-14073. [Crossref]
- Pinho-Ribeiro FA, Verri WA, Chiu IM (2016) Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation. Trends Immunol 38: 5-19. [Crossref]
- Herrin DM, Coates EE, Costner PJ, Kemp TJ, Nason MC, et al. (2014) Comparison of adaptive and innate immune responses induced by licensed vaccines for Human Papillomavirus. Hum Vacc Immunother 10: 3446-3454. [Crossref]
- Woolf CJ and Salter MW (2000) Neuronal Plasticity: Increasing the Gain in Pain. Science 288: 1765-1769. [Crossref]
- https://www.clinexprheumatol.org/abstract.asp?a=12194
- Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, Fitzgerald M (2009) Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain 144: 110-118. [Crossref]
- Xu Q and Yaksh TL (2011) A brief comparison of the pathophysiology of inflammatory versus neuropathic pain. Curr Opin Anaesthesiol 24: 400-407. [Crossref]
- Biggs JE, Lu VB, Stebbing MJ, Balasubramanyan S, Smith PA (2010) Is BDNF sufficient for information transfer between microglia and dorsal horn neurons during the onset of central sensitization? Mol Pain 6: 44. [Crossref]
- Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 39: 267-284. [Crosref]
- Gold MS and Gebhart GF (2010) Nociceptor sensitization in pain pathogenesis. Nat Med 16: 1248-1257. [Crossref]
- Ji RR, Kohno T, Moore KA, and Woolf CJ (2003) Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 26: 696-705. [Crossref]
- Kuner R (2010) Central mechanisms of pathological pain. Nat Med 16: 1258-1266. [Crossref]
- Ji RR, Berta T, Nedergaard M (2013) Glia and pain: is chronic pain a gliopathy? Pain 154: S10–S28. [Crossref]
- https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698#Centralsensitization
- Fleming KC and Volcheck MM (2015) Central sensitization syndrome and the initial evaluation of a patient with fibromyalgia: a review. Rambam Maimonides Med J 6: e0020. [Crossref]
- Reichling DB and Levine JD (2009) Critical role of nociceptor plasticity in chronic pain. Trends Neurosci 32: 611-618. [Crossref]
- https://www.painphysicianjournal.com/current/pdf?article=MTM1NA%3D%3D&journal=55
- Ji RR, Chamessian A, Zhang YQ (2016) Pain regulation by non-neuronal cells and inflammation. Science 354: 572-577. [Crossref]
- http://oxfordre.com/neuroscience/view/10.1093/acrefore/9780190264086.001.0001/acrefore-9780190264086-e-56?print=pdf
- Xanthos DN and Sandkühler J (2014) Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci 15: 43-53. [Crossref]
- Ji RR, Nackley A, Huh Y, Terrando N, Maixner W (2018) Neuroinflammation and Central Sensitization in Chronic and Widespread Pain. Anesthesiology 129: 343-366. [Crossref]
- Sebastin SJ (2011) Complex regional pain syndrome. Indian J Plast Surg 44: 298-307. [Crossref]
- Veldman PH, Reynen HM, Arntz IE, Goris RJ (1993) Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet 342: 1012-1016. [Crossref]
- https://s3.amazonaws.com/rdcms-iasp/files/production/public/Content/ContentFolders/Publications2/FreeBooks/Guide_to_Pain_Management_in_Low-Resource_Settings.pdf
- Huygen FJ, De Bruijn AG, De Bruin MT, Groeneweg JG, Klein J, et al. (2002) Evidence for local inflammation in complex regional pain syndrome type 1. Mediators Inflamm 11: 47-51. [Crossref]
- Alexander GM, Van Rijn MA, Van Hilten JJ, Perreault MJ, Schwartzman RJ (2005) Changes in cerebrospinal fluid levels of pro-inflammatory cytokines in CRPS. Pain 116: 213-219. [Crossref]
- Parkitny L, McAuley JH, Di Pietro F, Stanton TR, O’Connell NE, et al. (2013) Inflammation in complex regional pain syndrome: a systematic review and meta-analysis. Neurology 80: 106-117. [Crossref]
- Taha R and Blaise GA (2012) Update on the pathogenesis of complex regional pain syndrome: Role of oxidative stress. Can J Anaesth 59: 875-881. [Crossref]
- Inoue A, Ikoma K, Morioka N, Kumagai K, Hashimoto T, et al. (1999) Interleukin1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. J Neurochem 73: 2206-2213. [Crossref]
- Malcangio M, Bowery NG, Flower RJ, Perretti M (1996) Effect of interleukin1beta on the release of substance P from rat isolated spinal cord. Eur J Pharmacol 299: 113-118. [Crossref]
- https://www.elsevier.com/books/the-cytokines-of-the-immune-system/dembic/978-0-12-419998-9
- Di Benedetto S, Müller L, Wenger E, Düzel S, Pawelec G (2017) Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neurosci Biobehav Rev 75: 114-128. [Crossref]
- https://www.springer.com/in/book/9783709118887
- Mold M, Umar D, King A, Exley C (2018) Aluminium in brain tissue in autism. J Trace Elem Med Biol 46: 76-82. [Crossref]
- https://www.vaccinssansaluminium.org/wp-content/uploads/2015/09/SH-Lee.pdf
- Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F (2008) Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci USA 105: 10501-10506. [Crossref]
- McKee AS, Munks MW, MacLeod MK, Fleenor CJ, Van Rooijen N (2009) Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol 183: 4403-4414. [Crossref]
- Pétrilli V, Dostert C, Muruve DA, Tschopp J (2007) The inflammasome: A danger sensing complex triggering innate immunity. Curr Opin Immunol 19: 615-622. [Crossref]
- Stylianou E and Saklatvala J (1998) Interleukin-1. Int J Biochem Cell Biol 30: 1075-1079.
- https://autoimmunity.kenes.com/Documents/Autoimmunity%202016%20Program%20Book%20FINAL.pdf.
- https://www.skyhorsepublishing.com/9781510710801/the-hpv-vaccine-on-trial/
- https://www.slideshare.net/drmbsmith/central-sensitization-in-chronic-pain
- Zhang JM and An J (2007) Cytokines, inflammation and pain. Int Anesthesiol Clin 45: 27-37. [Crossref]
- Clark AK, Old EA, Malcangio M (2013) Neuropathic pain and cytokines: Current perspectives. J Pain Res 6: 803-814. [Crossref]
- Fletcher MA, Zeng XR, Barnes Z, Levis S, Klimas NG (2009) Plasma cytokines in women with chronic fatigue syndrome. J Transl Med 7: 96. [Crossref]
- Maes M, Twisk FN, Kubera M, Ringel K (2012) Evidence for inflammation and activation of cell-mediated immunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): Increased interleukin-1, tumor necrosis factor-α, PMN-elastase, lysozyme and neopterin. J Affect Disord 136: 933-939. [Crossref]
- Okamoto LE, Raj SR, Gamboa A, Shibao CA, Arnold AC, et al. (2015) Sympathetic activation is associated with increased IL-6, but not CRP in the absence of obesity: lessons from postural tachycardia syndrome and obesity. Am J Physiol Heart Circ Physiol 309: H2098-H2107. [Crossref]





