A 55-year-old female with past medical history significant for secundum atrial septal defect (ASD) complicated by pulmonary hypertension (PHTN) and Eisenmenger’s Syndrome (ES), asthma, and allergic rhinitis who presented to Pennsylvania Hospital ICU on 01/23/2017 after right neck basal cell carcinoma excision with ENT surgery. Blood pressure was managed intraoperatively with a Swanz-Ganz (SG) catheter placement for pulmonary artery (PA) pressure monitoring and with vasopressors. PA pressures were measured as high as 80mmHg during the procedure, which worsened the patient’s hypoxia. Postoperatively, the patient was continued on vasopressors plus Flolan due to continued hypotension with associated worsening hypoxia. The patient was weaned off of support after she was hemodynamically stable and discharged to home on 5L nasal cannula (NC).
Eisenmenger’s Syndrome (ES), Congenital heart defects (CHDS), Atrial Septal Defect (ASD)
Eisenmenger’s Syndrome (ES) is defined by the triad of systemic-to-pulmonary congenital cardiovascular communication, pulmonary arterial hypertension, and cyanosis [1]. Modern advances, enhanced understanding of pediatric cardiology, and timely surgical intervention in the management of congenital heart defects (CHDS) has resulted in the decreased prevalence of ES by 50% [2]. An estimated 8% of patients with congenital defects will develop ES and 11% with CHDS plus left to right shunt physiology [3]. CHDS commonly associated with ES includes atrial septal defects (ASDs), atrioventricular septal defects, ventricular septal defects (VSDs) and patent ductus arteriosus (PDA). Most patients with ES survive for 20 to 30 years [1]. The survival of patients with pulmonary hypertension and ES into the 5th and 6th decades of life increase the likelihood of exposure to non-cardiac surgery [4]. Eisenmenger’s Syndrome is associated with perioperative complications including early and sudden postoperative death. Non-cardiac surgeries amongst ES patients carry a mortality risk as high as 30% [4,5].
Pathophysiology of ES starts with a systemic to pulmonary circulation from a CHD. At first there is a left to right shunting of blood due to increased compliance of the right side of the heart which causes right heart volume overload [1]. With time and increased pulmonary blood flow/pressure, the pulmonary vascular bed also remodels and injury occurs. The pulmonary vascular injury leads to a significant rise in pulmonary vascular resistance causing pulmonary arterial hypertension and reversal of the shunt to right to left [1]. Right to left shunting causes systemic hypoxia and erythrocytosis [1]. Once ES develops, closure of the ASD is not indicated because right ventricular failure worsens and risk of death increases [6]. Definitive treatment is combined lung and heart transplant.
A 55-year-old female with past medical history significant for secundum ASD diagnosed 20 years ago while the patient was pregnant complicated by PHTN, ES, asthma, and allergic rhinitis presented to Pennsylvania Hospital ICU on 01/23/2017 after right neck basal cell carcinoma excision with ENT surgery. Precautions were taken during the procedure due to concern that with elevated PA pressures, systemic hypotension may cause the patient’s right to left shunt to worsen and cause severe hypoxia. Patient’s blood pressure was managed intraoperatively with placement of a SG Catheter/PA pressure monitoring and vasopressin support. PA pressures were measured as high as 80mmHg during the procedure, which worsened patient’s hypoxia. Postoperatively, patient was continued on vasopressor support plus Flolan due to continued hypotension with associated worsening hypoxia. The patient was able to be extubated in the postsurgical area but was placed on neosynephrine, milrinone, vasopressin drip and Flolan due to an episode of hypotension with associated hypoxia. The patient was transferred to the ICU for management of hypoxia and ES.
Vitals at arrival to the ICU: Temperature-98.2F, Respiratory Rate-19, Blood Pressure-109/54mmHg on 175mcg/min of neosynephrine, milrinone 0.25mcg/kg/min, and vasopressin- 0.04units/min, Oxygen saturation -87% on Non rebreather.
On exam, the patient was not in distress, her neck had sterri strips on the right anterior neck with JP drain in place. Left side of the neck had SG catheter in place. Lungs were clear to auscultation bilaterally, with decreased breath sounds at the bases. Heart exam was notable for positive S1, fixed S2 with 4/6 systolic murmur left 2nd ICS, 3-5 intercostal space (ICS) midline 4/6 systolic flow murmur, right heart heave, point of maximal impulse (PMI) was displaced, positive jugular venous distention, and no pedal edema.
SG catheter readings were monitored (Table.1). Frequent arterial blood gases (ABG) demonstrated hypoxia with pulmonary arterial oxygen saturations ranging from 53-86%. Troponins were negative x3; chest X-ray noted markedly enlarged cardiac silhouette, dilated right atrium (RA) /right ventricle (RV) and enlarged PA consistent with her diagnoses (Figure.1). A Transesophageal Echocardiogram (TTE) noted ASD, increased PA pressures, enlarged RV, severe tricuspid regurgitation, and moderate pulmonary regurgitation (Figure.2). CT chest with IV contrast PE protocol demonstrated mild to moderate cardiomegaly with right heart enlargement, massive dilation of the main pulmonary outflow tract, PHTN secondary to known ES secondary to known ASD. Small pericardial/left pleural effusion and no pulmonary embolism were noted (Figure.3).
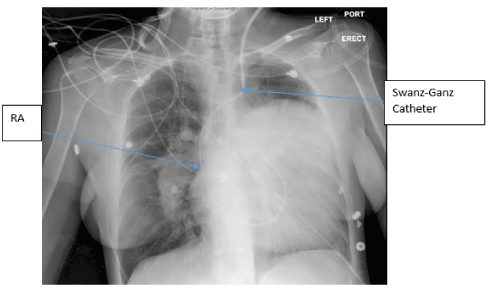
Figure 1. Chest X-ray- The left cardiac silhouette is severely enlarged occupying most of the left chest and extending well above the carinal level, at least partially due to a probably markedly enlarged left atrial appendage. The hilar vessels are enlarged, most conspicuous on the right, likely due to pulmonary artery enlargement secondary to pulmonary arterial hypertension. Left internal jugular Swan-Ganz catheter terminating in main pulmonary artery. Right Atrium (RA).
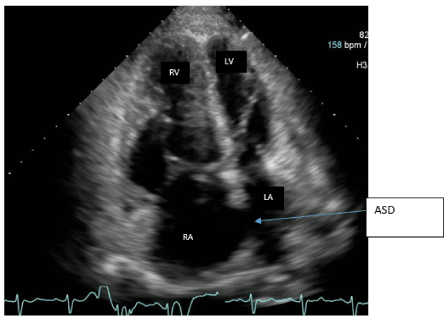
Figure 2. Four chamber views of TTE. Echo image demonstrates right atrial and right ventricular dilatation. ASD is seen between the right and left atrium. Right atrium (RA), Left atrium (LA), left ventricle (LV), Right ventricle (RV).
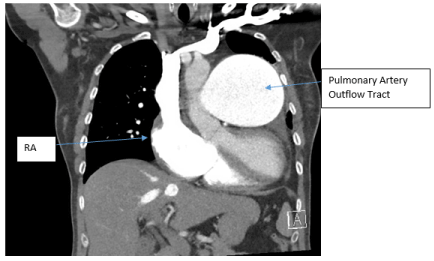
Figure 3. CT chest with IV contrast PE protocol. Mild to moderate cardiomegaly with right heart enlargement, massive dilation of the main pulmonary outflow tract, with peripheral pruning and scattered pulmonary arterial mural calcifications, consistent with severe long-standing pulmonary arterial hypertension secondary to known Eisenmenger’s Syndrome secondary to known atrial septal defect. Small pericardial/left pleural effusion and no pulmonary embolism was noted. Right atrium (RA).
Table 1. Swanz-Ganz pressure tracings
PA catheter Waveforms |
Normal Values |
Patients Values |
Cardiac Index (CO/ BSA) |
2.5-4L/min |
3.9L/min |
PA pressure |
Systolic 15-30
Diastolic 6-12
Mean 9-18 |
60/31(41) |
CVP |
2-6mmHg |
13mmHg |
Venous O2 saturation |
60-80% |
68% |
Follow up
The patient was successfully weaned off all vasopressor agents and Flolan. The patient was able to maintain her saturations on her home oxygen requirements of 5L NC. She was continued on her home anti-pulmonary hypertensive medications including Selexipag and Macitentan.
Understanding Eisenmenger’s physiology and anticipating the hemodynamic changes during the surgical intervention with targeted treatment could lead to a reduction in significant mortality. It is also important to consider the severity of pulmonary hypertension, right ventricular dysfunction, severity of tricuspid regurgitation, type and duration of surgery, other cardiac disease, and other comorbidities prior to any intervention [7].
The fundamental principles in perioperative management of patients with ES involve preventing systemic hypotension and avoiding elevation of pulmonary vascular resistance. An abrupt reduction in the systemic vascular resistance (SVR) will worsen the right to left shunting, leading to cyanosis, systemic hypoxemia, significant bradycardia, and cardiovascular collapse. Decreased SVR also leads to RV encroachment of the LV cavity causing diastolic impairment and decreased cardiac output. Therefore, measures should be in place to detect and manage alteration in hemodynamics during induction of anesthesia and surgery [3,4,7]. It is common for anesthetic induction to cause hypotension regardless of the induction agent used in ES patients. The use of vasopressor before and during anesthetic induction has been reported to reduce the incidence of hypotension. Therefore, it is generally recommended to use vasopressors during induction to maintain SVR and cardiac output.
Acute blood loss and loss of extra-cellular fluid during surgical procedures could exacerbate a right to left shunt. Volume overload could also cause right ventricular failure and increase right to left shunt. Therefore, careful monitoring of fluid balance and replacement is necessary for maintaining normo-tension is critical for effective management of this patient population2. Other potential risks during surgery associated with ES include cardiac arrhythmia, heart failure, hemoptysis, coagulation disorders (thromboembolism and bleeding), air embolism, hyperviscosity complication, brain abscess, cerebrovascular incidents, and renal dysfunction [7].
Several authors have proposed different techniques of monitoring ES patient intraoperatively. While some suggested the use of frequent blood pressure monitoring by non-invasive techniques, others advocate the combination of intra-arterial monitoring, central venous catheterization and use of Swan Ganz catheter in monitoring of PVR [6]. It is advisable to refer patients with ES to specialized centers capable of applying strategies aimed at the perioperative hemodynamic changes during surgical interventions [8-13].
As discussed in our case, close monitoring using standard tools or invasive monitoring, appropriate anesthetic use for induction, maintenance of SVR, oxygenation, pain control, use of vasopressors, and pulmonary therapies are important tools to consider in the perioperative management of ES.
- Vongpatanasin W, Brickner ME, Hillis LD, Lange RA (1998) The Eisenmenger syndrome in adults. Ann Intern Med 128: 745-755. [Crossref]
- Beghetti M, Galiè N (2009) Eisenmenger syndrome a clinical perspective in a new therapeutic era of pulmonary arterial hypertension. J Am Coll Cardiol 53: 733-740. [Crossref]
- Joyce JA (2006) Update for nurse anesthetists. Eisenmenger syndrome: an anesthetic conundrum. AANA J 74: 233-239. [Crossref]
- Bennett JM, Ehrenfeld JM, Markham L, Eagle SS. (2014) Anesthetic management and outcomes for patients with pulmonary hypertension and intracardiac shunts and Eisenmenger syndrome: a review of institutional experience. J Clin Anesth 26: 286-293. [Crossref]
- Nashat H, Kempny A, McCabe C, Price LC, Harries C, et al. (2017) Eisenmenger syndrome: current perspectives. Research Reports in Clinical Cardiology, 2 February Volume 2017:8 Pages 1-12.
- Foster JM, Jones RM2021 Copyright OAT. All rights reserv the Eisenmenger syndrome. Ann R Coll Surg Engl 66: 353-355. [Crossref]
- Ammash NM, Connolly HM, Abel MD, Warnes CA (1999) Noncardiac surgery in Eisenmenger syndrome. J Am Coll Cardiol 33: 222-227. [Crossref]
- Saito K, Toyama H, Ejima Y, Yamauchi M. (2016) Preoperative Assessment of the Impact of Positive Pressure Ventilation with Noninvasive Positive Pressure Ventilation in a Patient with Eisenmenger Syndrome: A Case Study. A A Case Rep 7: 193-195. [Crossref]
- Matsumoto Y, Shibuta S, Morita T, Iritakenishi T, Nishimura N et al. (2015) Transversusabdominis plane block for bilateral orchiopexy in an 8-year-old patient with Eisenmenger’s syndrome. JA Clinical Reports 1: 21.
- Burbridge, Mark A, Brodt, Jessica, Jaffe, et al. (2016) Ventriculoperitoneal Shunt Insertion Under Monitored Anesthesia Care in a Patient with Severe Pulmonary Hypertension. A&A Case Reports 7: 27–29.
- Kaemmerer H, Mebus S, Schulze-Neick I, Eicken A, Trindade PT, et al. (2010) The adult patient with Eisenmenger Syndrome: a medical Update after Dana Point Part I: epidemiology, clinical aspects and diagnostic options. Curr Cardiol Rev 6: 343-355. [Crossref]
- Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, et al.(2008) Guidelines for the Management of Adults with Congenital Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines on the management of adults with congenital heart disease). Circulation 118: 714. [Crossref]
- Kern M. (2017) Cardiac catheterization techniques: Normal hemodynamics. Uptodate. Accessed on 06/22/2017.



