Abstract
We present a case of 18yrs primigrada, who presented with sudden onset shortness of breath, fever and cough. She was 6 days postpartum after having undergone a cesarean due to pre-eclampsia. She was diagnosed heart failure, secondary to postpartum cardiomyopathy although pneumonia was her first working diagnosis on arrival to the emergency department. The patient was in remission after receiving chemotherapy a year ago for Hodgkins lymphoma, with no other known comorbidities.
Case scenario
An 18 year female patient presents to the emergency department (ED) with severe shortness of breath, productive cough and fever in the last few days. She was 6 days postpartum after her first pregnancy, delivered through cesarean section due to pre-eclampsia. She had Hodgkins lymphoma, treated a year ago with chemotherapy.
Her respiratory rate (RR) was 35/min, oxygen saturations (O2 sats) 62% (on room air), heart rate (HR) 135/min, blood pressure (BP) 200/100 mm Hg and temperature (Temp) 37.3 Celsius. There were bilateral crackles with bronchial breathing on auscultation. Patient also noticed a rash over her thigh areas on the day of presentation. There were no other findings on systemic examination.
She was started on oxygen through non-rebreathe bag followed by high flow oxygen & BiPAP, due to no improvement. She was initially treated on lines of hospital acquired pneumonia (HAP) with antibiotics and fluids. Later her chest X-ray and bed side Echocardiogram (ECHO) confirmed postpartum cardiomyopathy (PPCM). The patient was admitted in the coronary care unit (CCU) and discharged home in a stable condition after 21 days.
Introduction
The 2010 European Society of Cardiology defined PPCM as an idiopathic cardiomyopathy with development of heart failure (HF) towards the end of pregnancy or within five months following delivery, absence of another identifiable cause for the HF and a Left ventricular ejection fraction (LVEF) of less than 45% [1].
In some patients, LVEF of 45-50% may be diagnostic, if associated with chracteristic clinical features. Certain observational studies also suggest that patients with an earlier presentation of HF in pregnancy can also be a part of PPCM spectrum [2].
PPCM occurs worldwide with an incidence of approximately 1:4000 in the United States. Incidence data is not currently available in the Middle East and many other countries. The known risk factors include pre-existing cardiovascular disease, pre-eclampsia, multiple gestations and increasing maternal age. There is a varying incidence in different countries [3,4].
The etiology of the disease is not fully understood. It is considered to be mutifactorial with harmonal factors (prolactin), cytokines (tumour necrosis factor, interleukin-6), autoimmune response and genetic predisposition playing an intergral role towards a final common pathway, which leads to angiogenic imbalance [5-7].
Case progression
Our patient presented in the ED with sudden shortness of breath. She was initially diagnosed as respiratory tract infection in view of her recent discharge from the hospital. She was tachypneic on arrival and had oxygen saturations of 62% on air. She was given oxygen through the mask and first dose of IV antibiotic was prescribed. A full septic work including blood, urinary culture, viral multiplex (including Covid-19) was sent. A portable chest X-ray was also requested. The ECG (Figure 1) showed sinus tachycardia with no evidence of acute change. The blood tests showed leucocytosis and raised N terminal brain natriuretic pepetide (pro-BNP). Venous blood gas showed severe hypoxia with no acidosis. Chest X-ray (Figure 2) showed evidence of pulmonary edema and “infiltrates” suggestive of pneumonia. A bedside echocardiogram showed left ventricular ejection fraction (LVEF) of approximately 20-25%.
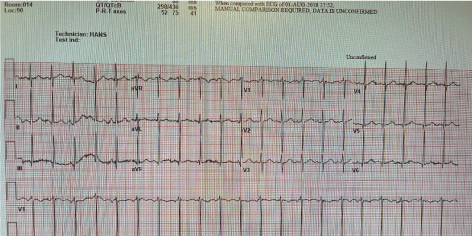
Figure 1. The ECG showed sinus tachycardia with no evidence of acute change.
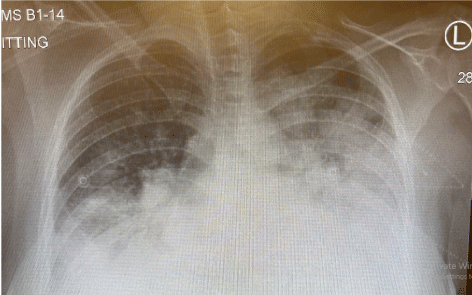
Figure 2. Chest X-ray showed evidence of pulmonary edema and “infiltrates” suggestive of pneumonia.
Hospital course
Patient was admitted under the cardiology service in a coronary care unit. She was treated on lines of pulmonary edema and also received a complete course of broad spectrum antibiotics for suspicion of pneumonia (significant leucocytosis, radiological findings). The patient’s blood pressure on arrival to ED was 200/100 mmHg. She needed IV antihypertensive to stabilise her BP in ED and during her hospital stay. Patient’s clinical condition showed a consistent trend of improvement over the period of next two weeks. During her stay, magnetic resonant imaging (MRI) of the heart ruled out myocarditis and showed a LVEF of 47% (attached video). During her regular assessment by the obstetrics team, no other gynaecological issues were found to be related to her presentation. The patient stayed admitted in the hospital for a total of 21days and was successfully discharged home on heart failure treatment. Patient’s follow up two months later showed consistent improvement in her clinical status with no further deterioration in her LVEF. She was advised by the gynaecology team to stay on contraception, till her heart condition stabilises further.
Diagnosis of PPCM
The diagnosis is mainly clinical at the time of the presentation with commonest presenting symptom being dyspnea. Most of the females present in the post partum period but a small proportion can present in the last few weeks of pregnancy. The latter group are likely to have a previous history of cardiomyopathy. Other symptoms include cough, hemoptysis, dependent edema and orthopnea [8-10].
Electrocardiogram is usually carried out to rule an ischemic event. Majority of the ECG findings will be sinus tachycardia.
Bedside echocardiogram is extremely uselful to check LVEF and screen for any other structural abnormalities, previously undetected [11].
Hematogical studies like Pro-BNP can be elevated. Troponin assay can be helpful to rule out myocardail injury.
Chest X-ray findings of pulmonary edema can “rule in” the diagnosis. It should be used with caution in the prepartum presentations due to risk of radiation exposure.
Magnetic resonance imaging, coronary artery catheterizationand endomyocardial biopsy can only be deployed in selected cases. Viral and bacterial cultures have limited diagnostic value [12,13].
Discussion
Several risk factors have been associated with the development of PPCM including: multiparity, extreme of maternal age, certain ethnicities, hypertensive disorders, and multiple gestations [1-3].
Hypertensive disorders of pregnancy (HDP) showed increased association with PPCM. A large cohort study done in Denmark between 1978 and 2012 showed a significant increased risk of PPCM in patients with HDP. Severe preeclampsia showed the highest relative risk among other disorders in the spectrum [8].
Another systematic review concluded pre-eclampsia as an important risk factor for developing PPCM worldwide. The cause is suggested to be due to the common anti-vascular pathophysiology that occurs in both situations [9].
Several studies have shown that the incidence of PPCM was higher among African American non-Hispanic descents and lower among east Asians, Europeans, and Hispanics. The highest incidence was amongst a tribe in Northern Nigeria, and it reached 1% [5-7,10].
Diagnosis of PPCM is easily missed because the clinical presentation is very vague and could mimic normal pregnancy symptoms and pneumonia, as in our reported case. Thus, PPCM needs a high index of suspicion.and should be a diagnosis of exclusion.
Management of PPCM is similar to the management of any other heart failure. Oxygenation, diuresis, nitrates & inotropic agents (if patients are presenting with evidence of low output state). Bromocriptine, antihypertensives, digoxin and cardiac resynchronization therapy (CRT) are individualized, according to each patient’s case.
Its important to note that the management of PPCM should be with the help of multidisciplinary team including obstetrics, cardiology, immunology, and pathology [5,11,12,15].
Prognosis of PPCM is variable depending on the presentation, level of ventricular impairment and risk factors. The lower the LVEF, worse is the long term outcome [13,14]. Other factors contributing to adverse outcomes include NYHA functional class, African ethnicity, multiparity and age (>35 years). The reported mortality for PPCM is around 10% at two years. The cause of death is usually ventricular arrythmia, pump failure, sudden death or thromboembolic events [15,16].
Pre-eclampsia was the main risk factor in our patient which led to PPCM. Our patient had received chemotherapy for Hodgkins lymphoma and was in remission at the time of presentation. Evidence of a direct correlation between Hodgkin lymphoma (HL) and PPCM has not been reported. A study done in Amsterdam on long term cardiotoxic effects of chemotherapy on HL patients found cardiomyopathy as one of the complications post treatment. Risk was variable depending on the modality and duration of treatment. However, the study population did not include pregnant ladies [17]. Direct correlation of HL predisposing to PPCM is not established.
References
- Baursache J, Konig T, Vander Meer P (2019) Physiopathology, diagnosis and management of peripartum cardiomyopathy. Eur J Heart Fail 21:827-843.
- Kodogo, Vitaris, Feriel Azibani, Karen Sliwa (2019) Role of pregnancy hormones and hormonal interaction on the maternal cardiovascular system: a literature review. Clin Res Cardiol 108:831-846.
- Stergiopoulos, Kathleen, Fabio V. Lima (2019) Peripartum cardiomyopathy-diagnosis, management, and long term implications. Trends Cardiovasc Med 29:164-173. [Crossref]
- Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, Regitz-Zagrosek V (2010) Heart Failure Association of the European Society of Cardiology Working Group on Peripartum Cardiomyopathy. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 12:767-778. [Crossref]
- Karaye, Kamilu M, Michael Y. Henein (2013) Peripartum cardiomyopathy: a review article. Int J Cardiol 164:33-38.
- Gunderson, Erica P, Croen LA, Chiang V, Cathleen K. Yoshida, Walton D, Alan SGo (2011) Epidemiology of peripartum cardiomyopathy: incidence, predictors, and outcomes. Obstet Gynecol 118:583-591.
- Okeke TC, Ezenyeaku CCT, Ikeako LC (2013) Peripartum cardiomyopathy. Ann Med Hea Sci Res 3:313-319.
- Behrens, Ida, Basit S, Lykke JA, Mattis F. Ranthe, Wohlfahrt J, Bundgaard H, Melbye M, Boyd HA (2019) Hypertensive disorders of pregnancy and peripartum cardiomyopathy: a nationwide cohort study. PloS one 14:e0211857.
- Bello, Natalie, Iliana S. Rendon H, Arany Z (2013) The relationship between pre-eclampsia and peripartum cardiomyopathy: a systematic review and meta-analysis. J Am Col Cardiol 62:1715-1723. [Crossref]
- Gentry, Mindy B, Dias JK, Luis A, Patel R, Thornton J, Reed GL (2010) African-American women have a higher risk for developing peripartum cardiomyopathy. J Am Col Cardiol 55:654-659.
- Bhattacharyya, Anirban, Basra SS, Sen P, Kar B (2012) Peripartum cardiomyopathy: a review. Tex Hea Ins J 39:8.
- Elkayam, Uri (2011) Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Col Cardiol 5:659-670. [Crossref]
- Martin, Sean, Short D, Wong CM, McLellan D (2013) A change of heart: case series of peripartum cardiomyopathy. Case rep obstet gynecol 563158. [Crossref]
- Kawthar, Abeer (2020) A Case of Peripartum Cardiomyopathy in Saudi Arabia. King Abdulaziz Uni-Med Sci 27:89-94.
- Tsang, Wendy, Bales AC, Lang RM (2015) Peripartum cardiomyopathy: treatment and prognosis. Up To Date [Accessed on April 07, 2016].
- Hilfiker‐Kleiner, Denise, Haghikia A, Masuko D, Nonhoff J, Held D, Libhaber E, Mark C. Petrie (2017) Outcome of subsequent pregnancies in patients with a history of peripartum cardiomyopathy. Eur J Heart Fail 19:1723-1728.
- Aleman, Berthe MP, Belt-Dusebout AWV, Bruin MLD, Veer MBV, Baaijens MHA, Boer JPD, Augustinus AMH (2007) Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 109:1878-1886.


