Abstract
Purpose: There have been studies aimed at determining hemodynamically significant of internal carotid artery (ICA) stenosis by blood flow distribution in precerebral arteries.
Materials and methods: We studied blood flow distribution in precerebral arteries by assessing flow velocity index in 56 patients with asymptomatic and symptomatic severe carotid stenosis.
Results: Decrease flow velocity index (FVI) in the ipsilateral stenosed ICA and increase FVI in the contralateral ICA were observed in most patients with ICA stenosis greater than 80%. Most of patients with ICA stenosis up to 80% did not manifest such changes. Additionally, in patients with ICA stenosis 90% or more, increase FVI in the ipsilateral vertebral artery was noted. Only in 8 patients out of 56 patients there was increase FVI in the ipsilateral external carotid artery with ophthalmic anastomosis. These patients had either bilateral ICA stenosis greater than 90% or the contralateral ICA occlusion, or a variation the circle of Willis. Another type of the precerebral blood flow distribution was observed in patients with multiple ICA and vertebral artery stenoses or variation the circle of Willis.
Conclusion: Thus, the results of the study confirm that peak systolic velocity is not informative enough to determine degree and hemodynamically significant of ICA stenosis. In order to assess hemodynamically significant ICA stenosis, it is possible to apply the precerebral blood flow distribution.
Keywords
carotid artery stenosis, cerebral blood flow, flow velocity index, hemodynamically significant, precerebral arteries
Introduction
Hemodynamically significant of internal carotid artery (ICA) stenosis is usually assessed by patterns of local hemodynamic changes in the area of ICA stenosis, which include: an increase peak systolic velocity (PSV), turbulence, pressure gradient, a decrease volumetric blood flow, changed spectrum in the pre- and post-stenotic segments [1-3]. Correlation of PSV and the degree of ICA stenosis was determined: the greater ICA stenosis, the higher the PSV. Nowadays, ICA stenosis is considered hemodynamically significant if PSV in the area of ICA stenosis (greater than 70%) is above 230 cm/s [1]. However, this criterion was determined over 30 years ago based on results of the first randomized NASCET study [4], when ultrasound methods just started their advance to the clinical practice and degree of ICA stenosis could only be assessed by local changes of blood flow. Currently, ultrasound methods have higher specificity and sensitivity, so their reliability is assured.
Usually, hemodynamically significant of ICA stenosis is determined by highly likely development of stroke in severe stenosis one of the precerebral arteries. The importance of this assessment is necessary for the timely surgical treatment of ICA stenosis and, therefore, reducing the risk of stroke. Believing that local changes in blood flow cannot serve as an accurate representation the state of cerebral hemodynamics and its disorders [5,6], many authors consider, that the criterion of hemodynamically significant ICA stenosis is not only increase PSV, but also the values of the blood flow velocity (BFV) in the poststenotic department, the final diastolic velocity in the area of stenosis and the state of collateral circulation. Thus, the researchers propose a multi-parametric approach in assessment of hemodynamically significant [5,7-10]. Some authors propose to determine hemodynamically significant ICA stenosis by the precerebral blood flow distribution [6,11]. It is assumed that the precerebral blood flow distribution can serve as a criterion in determining degree and hemodynamically significant ICA stenosis, and as indication for surgical intervention. At the same time, the most attention was focused on patients with symptomatic ICA stenosis, while primary prevention of stroke has dominant importance. So far, convincing data have not been received to prove a prognostic role the precerebral blood flow distribution and its regularities in conditions severe ICA stenosis. These criteria are not generally accepted and are not included in the current Guidelines for assessment hemodynamically significant ICA stenosis [12]. Meanwhile, assessment of precerebral blood flow can serve as an additional tool to ensure objective information about cerebral hemodynamics. Apart from that, there is another advantage of this approach – it can be used in patients with temporal acoustic window failure that prevents assessment of blood flow in the intracranial arteries and collateral circulation by transcranial Doppler sonography.
Purpose: To evaluate precerebral blood flow distribution in patients with hemodynamically significant severe internal carotid artery stenosis.
Materials and methods
56 patients (37 men, 19 women) with atherosclerotic severe ICA stenosis over 70% aged 49 to 85 years were studied. The degree of ICA stenosis was determined by duplex ultrasound (using the NASCET technique) and by computer or cerebral angiography. Duplex ultrasound and angiography results were the same. 30 patients were asymptomatic, 26 – symptomatic. There were no patients in the acute period of ischemic stroke), symptomatic patients were examined from 1 to 4 months after the last ischemic episode. Flow velocity index (FVI) in the precerebral arteries was determined by duplex ultrasound (GE Vivid e, USA) with 12 MHz linear probe and calculated as the product of arterial cross-sectional area and time-averaged mean velocity (TAMEAN) on the straight section, with Doppler angle no more than 60˚. BFV in the ipsilateral ICA was determined distally to stenosis in the laminar blood flow area [13,14]. Normal FVI values in the ICA ranged from 170 to 280 ml/min, in the vertebral artery (VA) – from 35 to 120 ml/min, in the external carotid artery (ECA) – from 80 to 190 ml/min [15-18]. BFV in the intracranial arteries was determined by transcranial Doppler sonography (MultiDop X, Germany) with 2 MHz transducers. Collateral supply via the ophthalmic artery was indicated by retrograde periorbital arteries and a pulsatility of the Doppler flow spectrum comparable with that of the intracranial arteries.
All patients were divided into 3 groups depending on degree of ICA stenosis: group I consisted of 21 patients with stenosis 70-79%, group II – 17 patients with stenosis 80-89% and group III – 18 patients with stenosis 90-99%.
Exclusion criteria were not atherosclerotic genesis of stenosis (n=5), individual intolerance of examination (n = 2) and severe cardiac failure or arrhythmia (n = 2).
The data was statistically processed in the standard statistical software (Statistica 12.0 for Windows, Excel), using parametric (Student's) and non-parametric (Kolmogorov-Smirnov) criteria. P <0.05 was statistically significant.
The patient's screening protocol was approved by the Ethics Board of the Russian Polenov Neurosurgical Institute (Protocol No. 1 dated 02.06.2010). All patients who took part in the clinical study signed a written voluntary informed consent. The study complied with the Declaration of Helsinki adopted by the World Medical Association. No animal studies or human experiments were conducted as part of this work.
Results
In 31 (62%) patients (all patients in group III, 10 – in group II and only 6 – in group I) a decrease FVI in the ipsilateral stenosed ICA was noted (Figure 1). However, in most patients of group I (14 patients) there was no decrease FVI in the ipsilateral ICA. In 6 cases, it was not possible to determine FVI in the ipsilateral ICA due to its tortuosity, prolonged atherosclerotic plaque or high bifurcation of the common carotid artery.
Increase FVI in the contralateral ICA was determined in 20 (48%) patients (11 patients in group III, 7 patients in group II, and only 2 patients in group I). Apart from that, these patients had an increase BFV in the contralateral anterior cerebral artery (88 ± 19 cm/s, p < 0.05). At the same time, most patients in group I (14 patients) did not have increase FVI in the contralateral ICA (Figure 1). In 5 cases, it was not possible to determine FVI in the contralateral ICA due to its tortuosity, prolonged atherosclerotic plaque or high bifurcation of the common carotid artery. 9 patients had contralateral ICA occlusion.
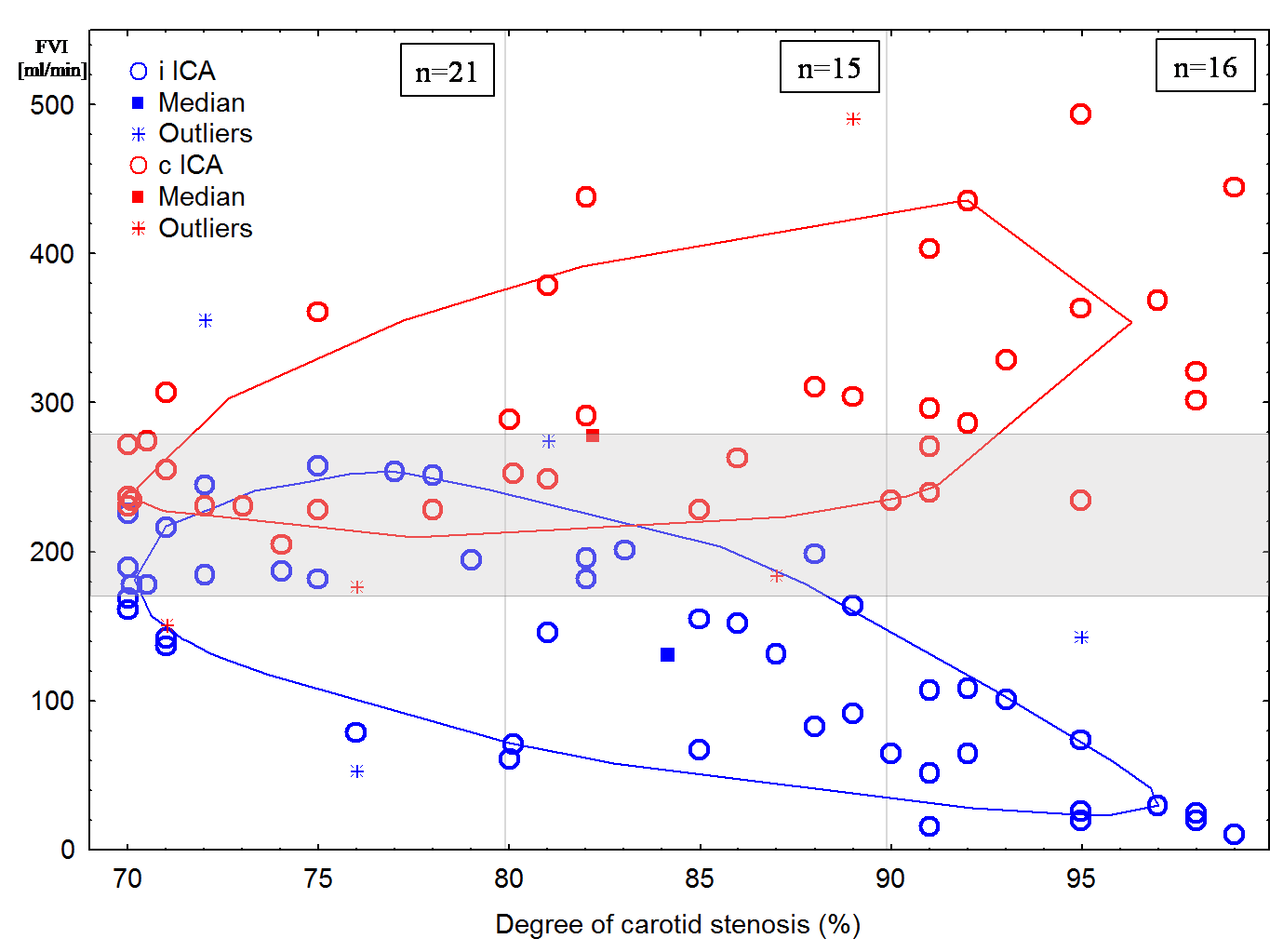
Figure 1: Distribution of the flow velocity index (FVI) in the ipsilateral (i ICA) and the contralateral internal carotid arteries (c ICA) depending on degree of stenosis. Normal values area is shaded.
Only 20 (11 patients in group III, 4 – in group II, and 5 – in group I) from 50 patients (in 6 cases it was not possible to determine FVI in the ipsilateral VA reliably due to its tortuosity, severe artery stenosis or occlusion) had increase FVI in the ipsilateral VA (Figure 2) and, correspondingly, increase BFV in the ipsilateral posterior cerebral artery (61 ± 14 cm/s, p < 0.05). At the same time, for most patients FVI in the ipsilateral VA remained within the normal range.
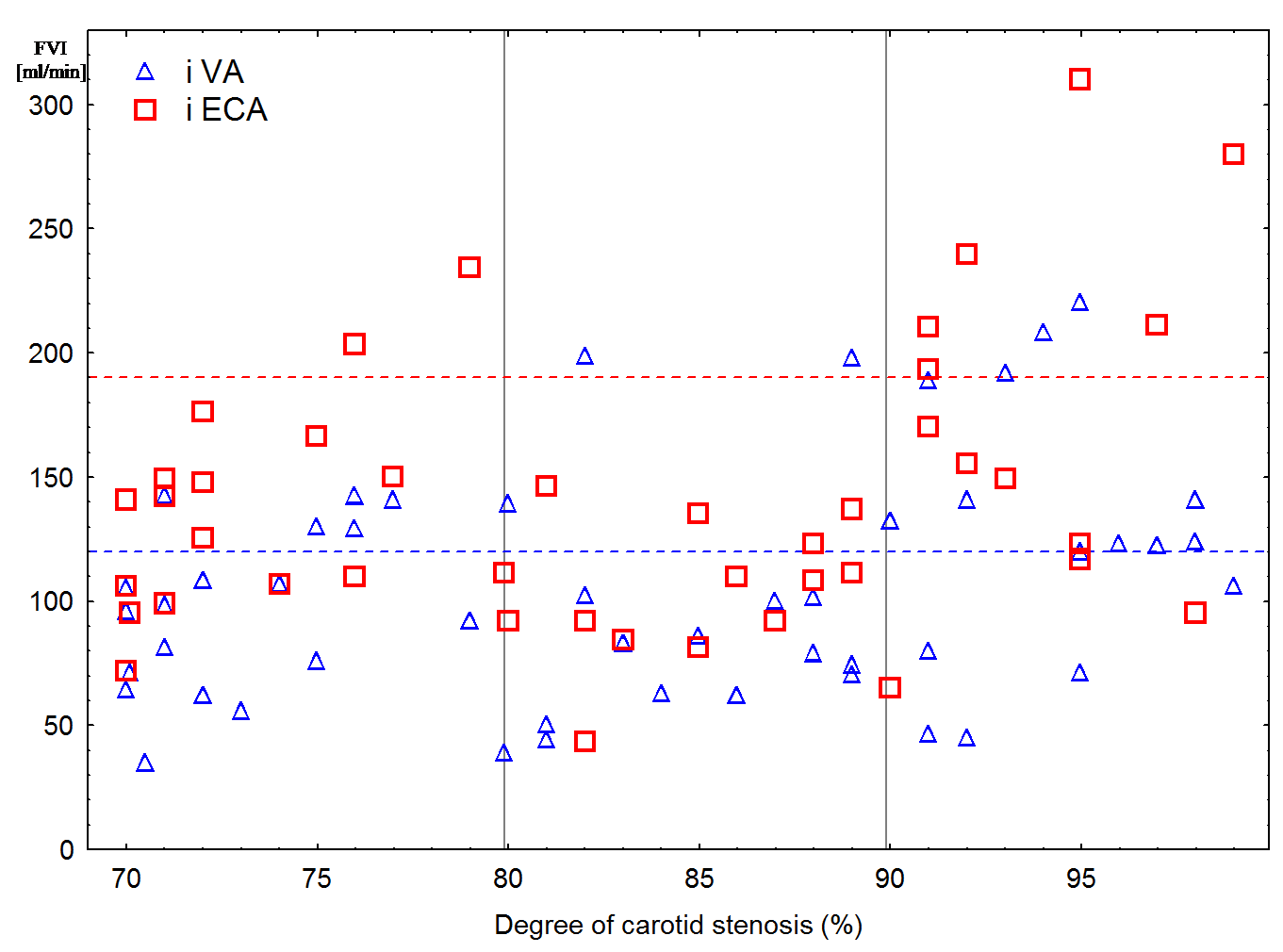
Figure 2: Scatter Plot of flow velocity index (FVI) in the ipsilateral vertebral (VA) and external carotid artery (ECA) depending on degree of stenosis. The upper limit of normal values is shown as a dotted line.
Only in 8 (6 patients in group III and 2 in group I) from 44 patients (in 12 cases it was not possible to determine FVI in the ipsilateral ECA reliably due to its tortuosity or atherosclerotic plaque), increase FVI in the ipsilateral ECA with ophthalmic anastomosis was noted (Figure 2). At the same time, these patients had either bilateral ICA stenosis greater than 90% or contralateral ICA occlusion, or variation the circle of Willis (CoW).
The duplex scanning results of the precerebral arteries in the patient with unilateral ICA stenosis over 90% and complete CoW are shown in Figure 3.
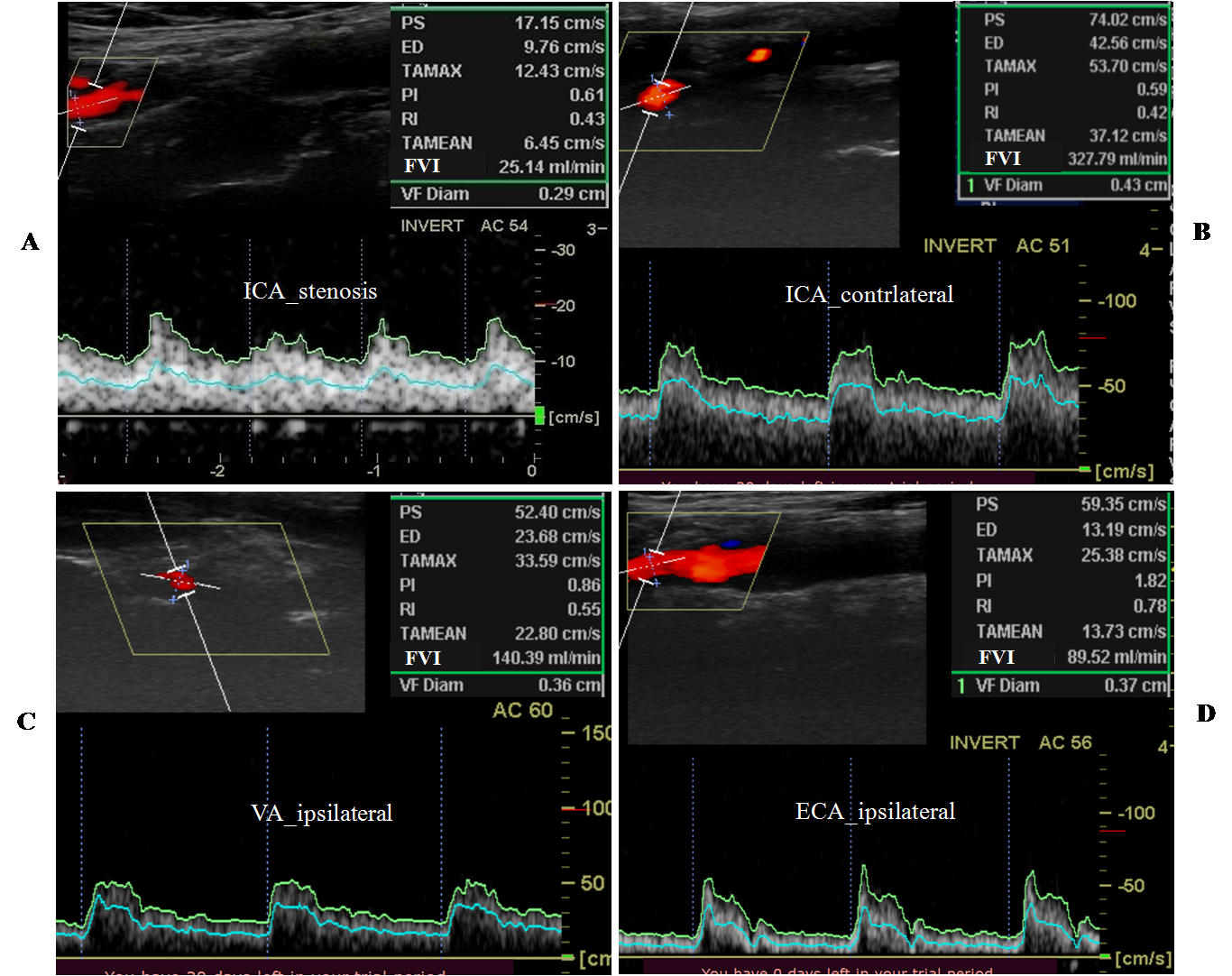
Figure 3: Duplex scanning results of the precerebral arteries in the 59-year-old patient with severe (97%) ICA stenosis. There was not contralateral ICA disease. The peak systolic velocity in the stenosis area was over 320 cm/s (which corresponds to stenosis greater than 90 %). ICA – internal carotid artery, VA – vertebral artery, ECA – external carotid artery. FVI – flow velocity index.
Figure 4 shows FVI mean values in the precerebral arteries. Only patients of groups II and III had significant decrease FVI in the ipsilateral ICA and increase FVI in the contralateral ICA. Most patients of group III had increase FVI in the ipsilateral VA. Significant increase FVI in the ipsilateral ECA was observed in patients’ group III only.
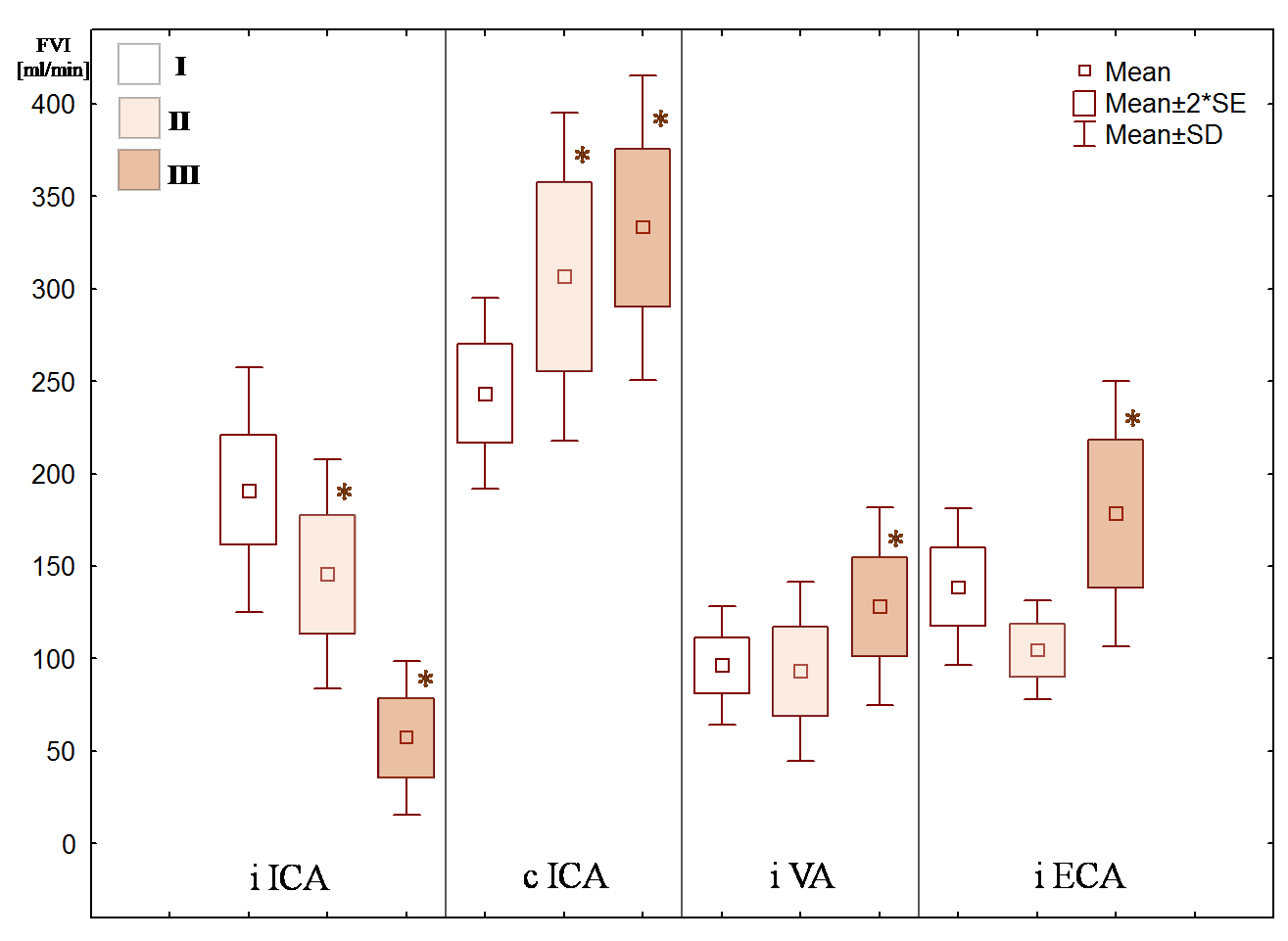
Figure 4: Flow velocity index (FVI) mean values in the precerebral arteries with different degree (%) of stenosis (group I-III): ICA – internal carotid artery, VA – vertebral artery, ECA – external carotid artery, i – ipsilateral, c – contralateral. * – statistical significance of variation from normal values, p < 0.05.
The dependence of FVI in the precerebral arteries for symptomatic and asymptomatic disease with different ICA stenosis degree was not revealed. The total FVI in the precerebral arteries in patients with asymptomatic and symptomatic disease amounted 606 ± 58 ml/min and 625 ± 65 ml/min, respectively (p>0.3) and did not relate to degree of ICA stenosis (p > 0.08).
Discussion
At present, when determining hemodynamically significant, most attention is focused on assessment of PSV in stenosed ICA [1,3,19,20]. However, it is often ignored, that other precerebral arteries take part in cerebral circulation. Local changes of blood flow in stenosed ICA do not reflect the compensatory capabilities of cerebral hemodynamics and state of collateral circulation [21]. Moreover, assessment of PSV can give false positive results in case of arterial tortuosity, calcified structure of atherosclerotic plaque, tandem stenosis and decreased cardiac output [3,22]. Many authors have developed their own criteria for hemodynamically significant ICA stenosis, forming a multiparametric approach for its determination. This approach, apart from assessment of local changes in hemodynamics, includes characteristics of atherosclerotic plaque, state of collateral blood flow and cerebral autoregulation [5,7-10,23-25]. Some authors argue that such factors as state of collateral blood flow and impaired cerebral autoregulation make a more reliable and objective criterion of hemodynamically significant. A change of cerebral hemodynamics distally to ICA stenosis directly determines stroke risk stratification [24,25].
Our results show that a significant decrease FVI in the ipsilateral ICA occurs with stenosis of 80 % or more, rather than the case of 70 % (Figure 4) as now stated in most guidelines [12]. The first of all, ICA 80 % stenosis is accompanied by increase FVI only in the contralateral ICA (Figure 4) with correspondingly increase BFV in the contralateral anterior cerebral artery. These changes indicate the anterior collateral pathway of the CoW [11,21,26-28].
At the same time, most patients with ICA stenosis greater than 90 % manifested increase FVI not only in the contralateral ICA, but also in the ipsilateral VA (Figure 2, 3) with correspondingly increase BFV in the ipsilateral posterior cerebral artery. Taking into account results of other authors, in patients with ICA stenosis greater than 90 %, collateral blood circulation is carried out not only the anterior collateral pathway, but also the posterior collateral pathway of the CoW necessarily [11,14,26,27]. Apparently, it is impossible to maintain cerebral perfusion in stenosis of ICA greater than 90 % when there is only one collateral pathway.
Besides, in case of ICA stenosis greater than 90 % (Figure 2) and the contralateral ICA occlusion or CoW variation (absence of one collateral pathways), there is also increase FVI in the ECA (Figure 4) with the functioning ophthalmic anastomosis, which is confirmed by other authors [6,11,21,28].
However, in ICA stenosis up to 80% there was a different picture. There was no significant change in the precerebral arteries in patients of group I (in the absence of bilateral ICA stenosis or vertebral artery stenosis). This fact confirms that assessment of hemodynamically significant ICA stenosis only by PSV is ambiguous and does not show real state of cerebral hemodynamics.
Patients with the precerebral arteries multiple stenoses and/or CoW variation manifest another variant of FVI distribution. In the case multiple ICA and/or VA stenoses, as well as CoW variation, decrease FVI in the ipsilateral ICA and increase FVI in the contralateral ICA can be observed with stenosis less than 80 %. In 6 patients of group I there was decrease FVI in the ipsilateral ICA and, vice versa, there were no changes FVI in 5 patients (however, there was a tendency to decrease) of group II (Figure 1). Similarly, with stenosis less than 90 %, there may be no decrease FVI in the ipsilateral ICA, no increase FVI in the contralateral ICA and the ipsilateral VA. In 2 patients of group I there was increase FVI in the contralateral ICA and while 8 patients of groups II-III showed no changes (Figure 1). In patients with bilateral ICA and VA stenoses or CoW variation, 8 patients of groups I–II had increase FVI in the ipsilateral VA and while 5 patients of group III had no changes FVI (Figure 1). Thus, the study of the precerebral blood flow distribution in these patients is a difficult task and does not always reveal patterns of changes in the precerebral arteries with unilateral ICA stenosis and complete CoW.
It should be noted that in the above-mentioned group in 2 patients with stenosis up to 80 %, causes of FVI «irregular» distribution in the precerebral arteries were not associated with multiple stenoses. One patient had decrease FVI in both ICA, the other — decrease FVI in the ipsilateral ICA and increase FVI in the contralateral ICA and the ipsilateral VA. The reasons for such changes remain unclear but may have cardiogenic nature.
Despite existing the precerebral blood flow distribution in severe ICA stenosis of one of the main arteries, total FVI remained within the normal range regardless of degree ICA stenosis. Volumetric blood flow was constant regardless of decrease of cerebral perfusion pressure on the side of severe ICA stenosis, which confirms that there is no impairment of cerebral autoregulation in most patients [11,14,15,17,18].
Conclusion
The results confirm that PSV is not informative enough to determine degree and hemodynamically significant ICA stenosis. Hemodynamically significant ICA stenosis can be determined by quantitative assessment FVI in the precerebral arteries. The more hemodynamically significant ICA stenosis is, the greater corresponding precerebral blood flow distribution is.
Precerebral blood flow distribution, together with local changes in the ICA stenosis area, characteristics of atherosclerotic plaque, assessment of cerebrovascular reserve and collateral blood flow, should be taken into account when selecting the most appropriate therapy for patients with ICA stenosis.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or
not-for-profit sectors.
Ethical approval
The patient's screening protocol was approved by the Ethics Board of the Russian Polenov Neurosurgical Institute (Protocol No. 1 dated 02.06.2010).
Informed consent
All patients who took part in the clinical study signed a written voluntary informed consent. The study complied with the Declaration of Helsinki adopted by the World Medical Association.
Author contributions
VS designed and conceptualized the study, analyzed the data and revised the manuscript for intellectual content. AN had major role in the acquisition of data, performed the statistical and machine learning analysis, interpreted the data, drafted the manuscript for intellectual content. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Acknowledgment
The authors would like to thank all of members of brain circulation pathology lab.
References
- Spencer M (1987) Hemodynamics of arterial stenosis. Ed. M. Spencer. Martinus Nijhoff Publishers, Dordrecht, 300.
- Taylor D, Strandness D (1987) Carotid artery duplex scanning. J Clin Ultrasound 15: 635–644.
- Grant EG, Benson CB, Moneta GL, Alexandrov AV, Baker JD, et al. (2003) Carotid artery stenosis: gray-scale and Doppler US diagnosis--Society of Radiologists in Ultrasound Consensus Conference. Radiology 229: 340–346. [Crossref]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators, Barnett HJM, Taylor DW, Haynes RB, Sackett DL, et al. (1991) Beneficial effect of carotid endarterectomy in symptomatic patients with high grade stenosis. N Engl J Med 325: 445–453. [Crossref]
- von Reutern G, Goertler M, Bornstein NM, Del Sette M, Evans DH, et al. (2012) Grading carotid stenosis using ultrasonic methods. Stroke 43: 916–921. [Crossref]
- Elwertowski M, Leszczyński J, Kaszczewski P, Lamparski K, Sin Yee Ho S, et al. (2018) The importance of blood flow volume in the brain-supplying arteries for the clinical management – the impact of collateral circulation. J Ultrason 18: 112–119. [Crossref]
- Serena J, Irimia P, Calleja S, Blanco M, Vivancos J, et al. (2013) Ultrasound measurement of carotid stenosis: recommendations from the Spanish Society of Neurosonology. Neurologia 28: 435–442. [Crossref]
- Arous EJ, Baril DT, Robinson WP, Aiello FA, Hevelone ND, et al. (2014) Institutional differences in carotid artery duplex diagnostic criteria result in significant variability in classification of carotid artery stenoses and likely lead to disparities in care. Circ Cardiovasc Qual Outcomes 7: 423–429. [Crossref]
- Klingelhofer J (2014) Ulrtasonography of carotid stenosis. IJCNMH 1: 11.
- Mozzini C, Roscia G, Casadei A, Cominacini L (2016) Searching the perfect ultrasonic classification in assessing carotid artery stenosis: comparison and remarks upon the existing ultrasound criteria. J Ultrasound 19: 83–90. [Crossref]
- Zarrinkoob L, Wеhlin A, Ambarki K, Birgander R, Eklund A, et al. (2019) Blood Flow Lateralization and Collateral Compensatory Mechanisms in Patients With Carotid Artery Stenosis. Stroke 50: 1081–1088. [Crossref]
- Aboyans V, Ricco JB, Bartelink ELM, Björck M, Brodmann M, et al. (2018) 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur Heart J 39: 763–816. [Crossref]
- Blanco P (2015) Volumetric blood flow measurement using Doppler ultrasound: concerns about the technique. J Ultrasound 18: 201–204. [Crossref]
- Zarrinkoob L, Ambarki K, Wåhlin A, Birgander R, Eklund A, et al. (2015) Blood flow distribution in cerebral arteries. J Cereb Blood Flow Metab 35: 648–654. [Crossref]
- Dörfler P, Puls I, Schliesser M, Mäurer M, Becker G (2000) Measurement of cerebral blood flow volume by extracranial sonography. J Cereb Blood Flow Metab 20: 269–271. [Crossref]
- Scheel P, Ruge C, Petruch UR, Schöning M (2000) Color Duplex Measurement of Cerebral Blood Flow Volume in Healthy Adults. Stroke 31: 147–150. [Crossref]
- Yazici B, Erdogmus B, Tugay A (2005) Cerebral blood flow measurements of the extracranial carotid and vertebral arteries with Doppler ultrasonography in healthy adults. Diagn Interv Radiol 11: 196–198. [Crossref]
- Amin-Hanjani S, Du X, Pandey DK, Thulborn KR, Charbel FT (2015) Effect of age and vascular anatomy on blood flow in major cerebral vessels. J Cereb Blood Flow Metab 35: 312–318. [Crossref]
- AbuRahma AF, Srivastava M, Stone PA, Mousa AY, Jain A, et al. (2011) Critical appraisal of the Carotid Duplex Consensus criteria in the diagnosis of carotid artery stenosis. J Vasc Surg 53: 53–59. [Crossref]
- Carnicelli AP, Stone JJ, Doyle A, Chowdhry A, Gillespie DL, et al. (2014) Predictive Multivariate Regression to Increase the Specificity of Carotid Duplex Ultrasound for High-grade Stenosis in Asymptomatic Patients. Ann Vasc Surg 28: 1548–1555. [Crossref]
- Fang H, Song B, Cheng B, Wong KS, Xu YM, et al. (2016) Compensatory patterns of collateral flow in stroke patients with unilateral and bilateral carotid stenosis. BMC Neurol 16: 39.
- Columbo JA, Suckow BD, Griffin CL, Cronenwett JL, Goodney PP, et al. (2017) Carotid endarterectomy should not be based on consensus statement duplex velocity criteria. J Vasc Surg 65: 1029–1038. [Crossref]
- Arning C, Widder B, von Reutern GM, Stiegler H, Görtler M (2010) Revision of DEGUM ultrasound criteria for grading internal carotid artery stenoses and transfer to NASCET measurement. Ultraschall Med 31: 251–257. [Crossref]
- Reinhard M, Müller T, Roth M, Guschlbauer B, Timmer J, et al. (2003) Bilateral severe carotid artery stenosis or occlusion - cerebral autoregulation dynamics and collateral flow patterns. Acta Neurochir (Wien) 145: 1053–1059. [Crossref]
- Semenyutin VB, Asaturyan GA, Nikiforova АA, Aliev VA, Panuntsev GK, et al. (2017) Predictive value of dynamic cerebral autoregulation assessment in surgical management of patients with high-grade carotid artery stenosis. Front Physiol 8: 872. [Crossref]
- Tan T, Schminke U, Lien L, Eicke BM, Tegeler CH (2002) Extracranial internal carotid artery occlusion: the role of common carotid artery volume flow. J Neuroimaging 12: 144–147. [Crossref]
- Shakur SF, Hrbac T, Alaraj A, Du X, Aletich VA, et al. (2014) Effects of extracranial carotid stenosis on intracranial blood flow. Stroke 45: 3427–3429. [Crossref]
- Yang F, Hsu P, Lin S (2016) Reduced Internal Carotid Artery Flow in Color-coded Carotid Duplex Sonography. Acta Neurol Taiwan 25: 136–147. [Crossref]




