Objective: Approximately half of inflammatory abdominal aortic aneurysms, defined by the prominent thickening adventitia, are immunoglobulin G4 (IgG4)-related diseases characterized by elevated serum IgG4 and IgG4-positive-plasmacytes infiltration. IgG4-related AAAs (IgG4-AAAs) exhibit vasculitis with elevated inflammatory marker levels. Matrix metalloproteinases (MMPs) degrade the extracellular matrix to destroy the aortic structures to progress aneurysm. This report examined pre- and postoperative serum MMPs and inflammatory marker levels to evaluate the prognosis of IgG4-AAAs after endovascular aortic repair (EVAR).
Methods: Among 25 patient with inflammatory abdominal aortic aneurysms (>2-mm-thick periaortic fibrosis) treated with EVAR, IgG4-AAA was diagnosed in 14 patients, and the remaining 11 patients were classified as non-IgG4-AAA. IgG4-AAAs were categorized into cases with increased (IgG4-AAA-up; n = 6) or decreased (IgG4-AAA-down; n=8) postoperative serum IgG4 levels. Before EVAR and after 24 months, we compared the serum MMP, inflammatory marker levels, periaortic fibrosis, and aneurysm diameter.
Results: IgG4-AAAs patients showed higher preoperative IL-6 levels, increased postoperative MMP-9 levels, and enlarged postoperative aneurysmal diameters compared with non-IgG4-AAA patients. Among IgG4-AAAs, IgG4-AAA-up exhibited higher preoperative MMP-9, higher preoperative monocytes and eosinophils, increased postoperative MMP-9 and IL-6, and larger aneurysm diameter than IgG4-AAA-down. All patients in IgG4-AAA-down showed shrunk preoperative aneurysm, although almost all patients in IgG4-AAA-up showed enlarged. In IgG4-AAA-up, IgG4/IgG ratio was significantly higher consistently before and after EVAR. MMP-9 was significantly correlated with IgG4 and IL-6. MMP-2 was not significantly different between patients with and without as well as between IgG4-AAA-up and IgG4-AAA-down.
Conclusion: Increased postoperative MMP-9 and IL-6 in IgG4-AAA may accelerate aneurysmal progression after EVAR. IgG4-AAA-up exhibited increased IL-6 levels and larger aneurysm diameters following EVAR than IgG4-AAA-down and were considered as the high-risk group with a tendency toward progression. Before surgery, a high IgG4/IgG ratio, high MMP-9 concentrations, a high ratio of monocytes and eosinophils might predict IgG4-AAA-up.
endovascular aneurysm repair, serum immunoglobulin G4, inflammatory aneurysm, matrix metalloproteinase, predictor
IgG4-related diseases (IgG4-RDs) are systemic conditions with mass formation or focal organ enlargement and are histologically attributed by an abundant infiltration of IgG4-positive plasmacytes in conjunction with massive fibrosis [1]. Patients often exhibit elevated serum IgG4 levels [1]. IgG4-related abdominal aortic aneurysms (IgG4-AAAs) predominantly affect the adventitia and are the most frequent site and form of IgG4-RDs in the vascular system [2,3]. It has been reported that IgG4-AAAs account for approximately half of the inflammatory abdominal aortic aneurysms (IAAAs) characterized by fibrous thickening adventitia [2,3].
Endovascular aortic repair (EVAR) has become the primary surgical treatment for the majority of patients with IAAAs because of its low invasiveness and difficulties associated with the surgery compared with open surgical repair (OSR) [4-6]. Being a relatively newly recognized disease, evidence regarding IgG4-AAA treatment is scarce and controversial. Some authors have mentioned that patients with IgG4-AAA who underwent OSR with or without steroid medication exhibited a satisfactory convalescence [7-11]. Others have described that patients with IgG4-AAAs were satisfied with a preferable prognosis after EVER, with reduced operative complication risks due to periaortic fibro-inflammatory tissue (perianeurysmal fibrosis; PAF) adhered to the surrounding organs, particularly with a combination of steroid medication [12,13]. However, several recent studies on patients with IgG4-AAA treated by EVAR have reported severe complications leading to death, such as aneurysmal enlargement to rupture or aortoduodenal fistula and aortic dissection [14-17]. Furthermore, another study, although with a small sample size, compared the prognosis of patients with IgG4-AAA without steroid medication between those treatment by EVAR and those treated by OSR and indicated that approximately half of patients with IgG4-AAA who underwent EVAR were at risk for persistent symptoms, PAF progression, and enlarged aneurysm sac, whereas those treated by OSR did not experience these complications [18]. Considering the severe outcomes of several patients with IgG4-AAA after EVAR [14-17], a preoperative criterion to predict them would be crucial to select the surgical procedures, and the following postoperative markers would need to be assessed to provide additional treatment for their early recovery. Interestingly, the literature reports that patients with IgG4-AAA who recovered well after EVAR were characterized with decreased serum IgG4 levels after EVAR [12,13], whereas patients with IgG4-AAA with several complications exhibited an increased serum IgG4 level following EVAR [14,15]. Therefore, we hypothesized that for some patients with IgG4-AAAs, persistent or recurrent inflammation associated with IgG4-RD would result in several problems following EVAR.
Inflammation and tissue remodeling cause structural alterations that characterize the formation of aneurysms [19]. These mechanisms involve several molecular mediators, including matrix metalloproteinases (MMPs) that degrade the extracellular matrix. MMPs are a family of 28 zinc-endopeptidases classified based on their structure or substrates as follows: collagenases, gelatinases, stromelysins, and membrane-bound MMPs. In particular, MMP-2 and MMP-9 digest denatured collagen (gelatin) and primarily play a vital role in the pathogenesis and progression of atherosclerotic AAA (aAAA) [20,21]. Serum levels of circulating MMP-2 and MMP-9 may be useful for detecting inflammatory activity in atherosclerosis and aneurysmal prognosis and in predicting cardiovascular risk [22]. Meanwhile, the production of MMPs in patients with IAAA has been investigated very rarely [23]. In addition, the association between MMPs and IgG4-AAAs has not yet been examined.
A recent report regarding IgG4-AAAs described that mesenchymal stromal cells and macrophages in the adventitia could be involved in the characteristic cytokine networks, in particular interleukin (IL)-6 [24]. It has also been reported that IL-6 promotes the infiltration of inflammatory cells and the modulation of the synthesis of positive acute-phase reactants and could mediate MMP expression [25,26]. We considered that patients with IgG4-AAAs exhibit a specific MMP expression pattern because of the peculiar composite immune cells and cytokine networks in the thickening adventitia of IgG4-AAAs.
In the present study, we examined the association between clinical parameters and serum levels of MMP-2, MMP-9, and inflammatory markers, including IL-6, in patients with IgG4-AAA compared with control patients with non-IgG4-related inflammatory abdominal aortic aneurysms (non-IgG4-AAAs) and aAAAs. Using these serum data, we further explored the predictors and/or the following markers of IgG4-AAA progression after EVAR.
Case selection
A total of 203 patients with AAA were treated by EVAR at the Kanazawa Medical Center between January 2008 and August 2017. IAAA was defined based on the PAF thickness of ≥2 mm, identified through imaging examinations as either the thickening of the aortic wall outside the ring of calcification (if present) or the late contrast enhancement of periaortic tissue that could not be interpreted as a part of the viscera [2,3]. Patients with other specific factors responsible for aneurysm formation, such as dissection by medial necrosis, infection, and large-vessel aortitis, were excluded. Finally, 25 (8.1%) patients with IAAAs were identified.
IgG4-RD cases were generally diagnosed according to the following comprehensive histological diagnostic criteria: severe sclerosing lymphoplasmacytic inflammation with storiform fibrosis, obliterative phlebitis, adventitial thickness of ≥2 mm, ≥50 IgG4-immunopositive plasmacytes per high power field (0.237 mm2), and IgG4/IgG ratio of ≥50% based on the immunohistochemical analysis of IgG4 [27]. Tissue samples for pathological examination were harvested from five patients with IAAA; one patient underwent open surgical biopsy of the periaortic fibrous tissue, one patient was diagnosed based on a biopsy of the retroperitoneal tissue, one patient was diagnosed based on a surgical specimen of a thoracic aortic aneurysm, and two patients were diagnosed based on pulmonary tissue samples. These five patients were considered as a definite group having IgG4-RD in accordance with the 2011 Comprehensive Diagnostic Criteria for IgG4-RD [28]. Three patients presented with autoimmune pancreatitis diagnosed based on characteristic radiological features without biopsy in accordance with the 2013 Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis [29]. Seven patients with thick PAF and high serum IgG4 levels (≥135 mg/dL) were diagnosed as a group with possible IgG4-RD [28]. These 14 patients were categorized as having IgG4-AAA (Figure 1). The remaining 11 patients with IAAA were classified as non-IgG4-AAA (Table 1). Between January 2012 and December 2012, there were 10 consecutive patients with aAAA with a PAF thickening of <2 mm and a serum IgG4 level of <135 mg/dL. Serum IgG4 levels before and after EVAR were compared to stratify patients with IgG4-AAA into two groups. Then, among IgG4-AAA group, patients with an increased serum IgG4 level after EVAR were defined as the IgG4-AAA-up subgroup (n=6), and those with a decreased serum IgG4 level after EVAR were defined as the IgG4-AAA-down subgroup (n=8) (Table 2).
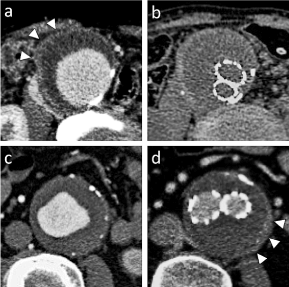
Figure 1. Enhanced computed tomography of the patients of IgG4-related abdominal aortic aneurysm. a, b; One patient showed gradual decreases in aneurysmal sac diameter and perianeurysmal fibrosis (PAF) after endovascular aneurysm repair (EVAR). Before EVAR, PAF was about 4mm thickening (arrow head)(a) and after 24 months EVAR, PAF was decreased to 1.5mm (b). c,d; Another one patient showed gradual increase in PAF and aneurysm sac diameter after EVAR. Before EVAR, PAF was thin (c), and after EVAR, PAF (arrow head) and aneurysmal sac diameter progressed (d)
Table 1. Data of the patients with IgG4-related abdominal aortic aneurysm, non-IgG4-related inflammatory abdominal aortic aneurysm and atherosclerotic abdominal aortic aneurysm
Group |
IgG4-AAA |
Non-IgG4-AAA |
aAAA |
Cases |
(n = 14) |
(n = 11) |
(n = 10) |
Gender (M/F) |
13 / 1 |
8 / 3 |
9 /1 |
Age (years) |
73.9 |
75.2 |
76.2 |
Presence of
other aneurysms |
9 (64%)
(TAA = 3, IAA = 5, CAA=1) |
5 (45%)
(TAA = 2, IAA = 3) |
4 (40%)
(TAA = 1, IAA =3) |
Risk factors of atherosclerosis
(smoking, HT, DM, HL) |
12 / 10 / 7 / 5
(86%/71%50%/36%) |
10 / 9 / 6 /5
(91%/82%/55%/45%) |
10 / 9 / 6 / 5
(100%/90%/60%/50%) |
Serum IgG4 (mg/dL) |
211 (76 - 573) |
30 (8 - 59) |
48 (12 - 88) |
CRP (mg/dL) |
0.9 (0.1 - 2.1) |
0.4 (0.1 - 8.0) |
0.3 (0.1 - 0.7) |
WBC (103/μL) |
6.9 (4.1 - 8.7) |
7.9 (4.5 - 19.5) |
6.2 (4.5 - 8.2) |
*Data are indicated as median (range)
IgG4-AAA, IgG4-related abdominal aortic aneurysm; non-IgG4-AAA, IgG4-related abdominal aortic aneurysm; aAAA, atherosclerotic abdominal aortic aneurysm; TAA, thoracic aortic aneurysm; IAA, iliac arterial aneurysm; CAA, coronary arterial aneurysm; HT, Hypertension; DM, Diabetes mellitus; HL, Hyperlipidemia; CRP, C-reactive protein; WBC, white blood cell.
Table 2. Data of the patients with IgG4-related abdominal aortic aneurysm increased postoperative serum IgG4 (IgG4-AAA-up) and IgG4-related abdominal aortic aneurysm decreased postoperative serum IgG4 (IgG4-AAA-down)
Subgroup |
IgG4-AAA-up |
IgG4-AAA-down |
|
Cases |
(n = 6) |
(n = 8) |
P-value |
Gender (M/F) |
5 / 1 |
8 / 0 |
0.880 |
Age (years) |
72.7 (61 - 83) |
75.8 (73 - 81) |
0.889 |
Presence of
other IgG4-RDs |
4 (67%)
(Lung = 1, Pancreas = 1,
Peritoneum = 2 |
4 (50%)
(Lung = 2, Pancreas = 2) |
0.937 |
Presence of
other aneurysms |
5 (83%)
(TAA = 1, IAA= 3, CAA = 1) |
4 (50%)
(TAA = 2, IAA= 2) |
0.468 |
Risk factors of atherosclerosis
(smoking, HT, DM, HL) |
5 / 5 / 4 / 3
(83%/83%/67%/50%) |
7 / 5 / 3 / 2
(88%/63%/38%/52%) |
0.581/ 0.797/
0.589/ 0.687 |
Allergy |
4 (67%) |
3 (33%) |
0.589 |
Autoimmune disease |
5 (83%) |
1 (11%) |
0.035 |
Serum IgG4 (mg/dL) |
192 (76 - 268) |
212 (143 - 573) |
0.093 |
CRP (mg/dL) |
0.6 (0.1 - 2.1) |
0.9 (0.2 - 2.0) |
0.699 |
WBC (103/μL) |
7.0 (5.3 - 8.0) |
6.9 (4.1 - 7.7) |
0.889 |
*Data are indicated as median (range).
IgG4-RD, IgG4-related disease; TAA, thoracic aortic aneurysm; IAA, iliac arterial aneurysm; CAA, coronary arterial aneurysm; HT, Hypertension; DM, Diabetes mellitus; HL, Hyperlipidemia; CRP, C-reactive protein; WBC, white blood cell |
Data collection
Patient data, including the diagnosis, age, gender, other aneurysms, and risk factors for atherosclerosis, were obtained from medical records. PAF thickness, aneurysm diameter, and aneurysm sac diameter were measured on semiannual enhanced computed tomography (CT) images with a slice thicknesses of 0.5 mm. CT scans were performed every 6 months following EVAR. Endoleaks were defined according to the criteria of the Society for Vascular Surgery [30]. Serum IgG level (normal range 820-1740 mg/dL), IgG4 level (normal value <135 mg/dL), IgE level (normal value <174 IU/mL), white blood cell (WBC) count, differential WBC count [normal range, monocytes (0%–12%), eosinophils (0%–8%), and basophils (0%–3%)], and C-reactive protein (CRP) levels (normal value <0.14 mg/dL) were examined at the time of EVAR and 24 months after EVAR. The levels of serum MMP-2 (Quantikine ELISA, Human MMP-2 Immunoassay, R&D Systems, Minneapolis, USA; normal range 300–500 ng/mL)[31], MMP-9 (Quantikine ELISA, Human MMP-9 Immunoassay; normal range <100 ng/mL)[31], and IL-6 (Human IL-6 Immunoassay 2nd Generation, Funakoshi, Tokyo, Japan; normal range <2.4 pg/mL) were measured using ELISA at the time of EVAR and approximately 24 months after EVAR. The difference (Δ) between the preoperative and postoperative values was calculated for each parameter.
Statistical analysis
The Kruskal–Wallis test was used for comparing continuous variables among the following three groups: IgG4-AAA, non-IgG4-AAA, and aAAA. The Mann–Whitney U test was used to compare variables between the IgG4-AAA-up and IgG4-AAA-down subgroups. The chi-square test of variance was used to analyze the incidence in each group. Spearman correlation coefficients were estimated to test the associations between continuous variables. The statistical significance was set at p<0.05. All analyses were performed using SPSS (version 20; IBM Corporation, Armonk, NY, USA).
The study protocol was approved by the Human Investigation Review Committee of Kanazawa Medical Center (no. 26–17) and conformed to the principles outlined in the Declaration of Helsinki and its later amendments. All patients provided informed consent for participation before the study.
Comparisons among the IgG4-AAA, non-IgG4-AAA, and aAAA groups
Figure 2 summarizes the results of comparison of each parameter among the IgG4-AAA, non-IgG4-AAA, and aAAA groups.
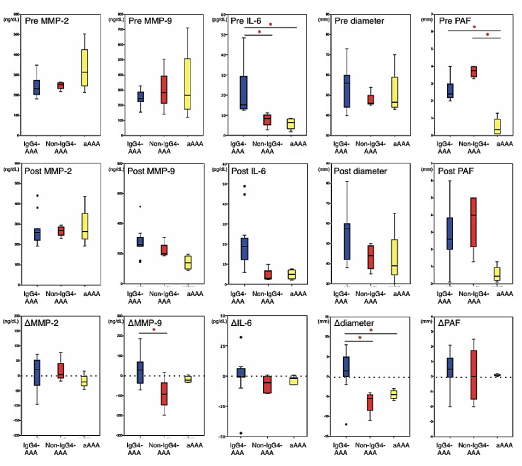
Figure 2. Box-and-whisker diagram of serumsdiological parameters in IgG4-related abdominal aortic aneurysm, non-IgG4-related inflammatory abdominal aortic aneurysm and atherosclerotic abdominal aortic aneurysm. Upper row show the preoperative values, middle row show the postoperative values of after 24 months EVAR, and lower row show the difference between the preoperative and postoperative values. Bottled lines of the lower row show the base line. Statistically significant differences between the groups are as indicated as *p<0.05
Before EVAR, the levels of MMP-9 (median, 249vs246 ng/mL; p=0.684) and MMP-2 (median, 226vs263 ng/mL; p=0.559) did not differ between the IgG4-AAA and non-IgG4-AAA groups. The IgG4-AAA group exhibited significantly higher IL-6 (median, 18.2 pg/mL) levels than the non-IgG4-AAA (median, 8.4 pg/mL, p=0.046) and aAAA (median, 5.4 pg/mL, p=0.032) groups. The CRP level was higher in the IgG4-AAA group (median, 1.1 mg/dL) than in the control groups (non-IgG4-AAA: median, 0.6 mg/dL; p=0.058 and aAAA: median, 0.3 mg/dL; p=0.001). No significant differences were observed in the WBC counts, WBC fractions, IgE levels, and aneurysm diameters among the three groups.
After EVAR, the IgG4-AAA group exhibited higher postoperative MMP-9 levels (median, 286 ng/mL) and larger aneurysm diameter (median, 56 mm) than the control groups, although these differences were not significant. There was also no significant difference in the levels of MMP-2, IL-6, IgE, CRP, WBC count, WBC fractions, and PAF thickness among the three groups. During the 24 months after EVAR, none of the patients developed endoleaks.
Regarding the changes between preoperative and postoperative values, ΔMMP-9 was significantly greater in the IgG4-AAA group (median, +33.1 ng/mL) than in the non-IgG4-AAA group (median, -90.8 ng/mL, p=0.036). Δdiameter in the IgG4-AAA group was significantly larger (median, +1.8 mm) than that in the other two groups. ΔMMP-2, ΔPAF, ΔIL-6, and ΔIgE showed no differences among the three groups.
In all patients included in this study, MMP-9 but not MMP-2 levels demonstrated a significant positive correlation with serum IgG4 (r=0.414, p=0.032) and IL-6 (r=0.432, p=0.041) levels. Both MMP-9 and MMP-2 levels exhibited no significant correlation with the aneurysm diameter, PAF thickness, CRP level, IgE level, WBC count, and WBC fractions.
Comparison between IgG4-AAA-up and IgG4-AAA-down subgroups among patients with IgG4-AAAs
The results of comparison of each parameter between the IgG4-AAA-up and IgG4-AAA-down subgroups are summarized in Figure 3.
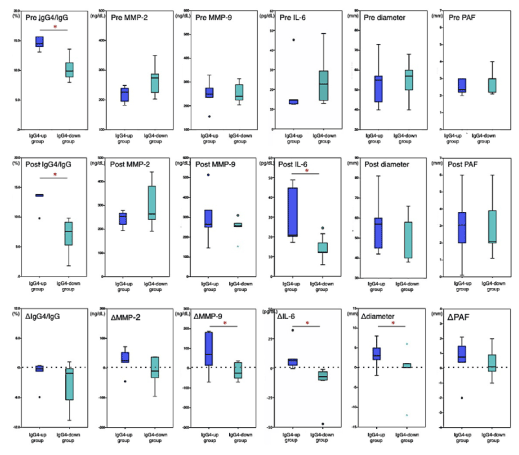
Figure 3. Box-and-whisker diagram for serum and radiological parameters in IgG4-related abdominal aortic aneurysm increased postoperative serum IgG4 (IgG4-AAA-up) and IgG4-related abdominal aortic aneurysm decreased postoperative serum IgG4 (IgG4-AAA-down). Upper row show the preoperative values, middle row show the postoperative values of after 24 months EVAR, and lower row show the difference between the preoperative and postoperative values. Bottled lines of the lower row show the base line. Statistically significant differences between the groups are as indicated as *p<0.05
Before EVAR, the serum IgG4/gG4 ratio was significantly higher in the IgG4-AAA-up subgroup (median, 14.5%, range 9.8%–23.5%) than in the IgG4-AAA-down subgroup (median, 9.8%, range 7.8%–13.2%; p = 0.007). The preoperative MMP-9 levels in the IgG4-AAA-up subgroup (median, 273 ng/mL) were higher than those after EVAR but were significantly different from those in the IgG4-AAA-down subgroup (median, 249 ng/mL, p=0.064). Regarding the differential WBC count, the ratios of monocytes (median, 11.6% vs 6.5%, p=0.042) and eosinophils (median, 7.7% vs 2.4%, p=0.034) were significantly higher in the IgG4-AAA-up subgroup than those in the IgG4-AAA-down subgroup. No significant difference was observed in the preoperative levels of IgG4, IgE, MMP-2, IL-6, and CRP or in the aortic aneurysm diameter and PAF thickness between the two subgroups.
After EVAR, the aneurysmal sac of almost all patients (5 of 6 patients) in the IgG4-AAA-up subgroup increased in size (Δdiameter median, +5.5 mm, range, from −1.5 to 12 mm; p=0.043), whereas the postoperative aneurysm sac of all patients in the IgG4-AAA-down subgroup decreased in size (Δdiameter median, −6.5 mm, range, from −12.1 to −6 mm). Patients in the IgG4-AAA-up subgroup exhibited significantly larger ΔMMP-9 (median, +70 vs. −25 ng/mL; p=0.035) and ΔIL-6 (median, +6.3 vs. −7.3 pg/mL; p=0.031) than those in the IgG4-AAA-down subgroup. Postoperative PAF was significantly thicker in the IgG4-AAA-up subgroup than in the IgG4-AAA-down subgroup (median, 4.3vs1.3 mm, p=0.02). Patients in the IgG4-AAA-up subgroup showed considerably higher postoperative IL-6 levels (median, 20.8vs12.2 pg/mL; p=0.003) and postoperative serum IgG4/gG4 ratio (median, 13.8% vs. 7.6%, p=0.006) than those in the IgG4-AAA-down subgroup. Postoperative MMP-9 levels (median, 322 vs 249 ng/mL, p=0.065) and serum IgG4 (median, 184 vs 122 mg/dL; p=0.055) and IgE (median, 325 vs 85.5 IU/mL; p=0.073) levels were rather higher in the IgG4-AAA-up subgroup than in the IgG4-AAA-down subgroup, although the differences were not statistically significant. There was also no significant difference between the two subgroups in terms of the postoperative data of other factors.
The major causes of aneurysmal sac enlargement after EVAR include various factors such as endoleaks, aneurysmal diameter, shape, and location [32]. However, local inflammation in the aneurysmal sac has received little attention as a potential explanation for complications caused due to EVAR [33]. In our study, the results of the comparison among the three groups of patients with IgG4-AAA, non-IgG4-AAA, and aAAA, the IgG4-AAA group exhibited features of increased serum MMP-9 levels and aneurysmal progression after EVAR. In particular, the IgG4-AAA-up subgroup had a propensity of progression of aneurysmal enlargement and PAF thickness after EVAR, but the IgG4-AAA-down subgroup did not, which is similar to several previous reports [12-15]. Therefore, we considered that local inflammation related to IgG4-RD itself in patients with IgG4-AAA would be concerned with the aneurysmal progression and the adventitial fibrosis.
In addition, the IgG4-AAA-up subgroup was associated with increased serum IL-6 levels compared with the IgG4-AAA-down subgroup. Moreover, serum MMP-9 levels exhibited a significant positive correlation with serum IL-6 and IgG4 levels. Previous reports have mentioned that the stromal cells distributed in the tunica media and the adventitia could produce both IL-6 and MMP-9, which are reciprocally activated [26, 34]. In addition, a recent report described that IL-6 production in numerous mesenchymal stromal cells and macrophages in the adventitia was the histological characteristic of IgG4-AAA [24,35]. Hence, we assumed that reflecting the higher activity of IgG4-RD in the IgG4-AAA-up subgroup, intensified IL-6 production would accelerate MMP-9 production. It has been reported that increasing IL-6 levels in patients with IgG4-AAA would be closely related to the aspect of vasculitis [24,25] and that particularly the IgG4-AAA-up subgroup may belong to the active vasculitis status.
Recently, several authors have discussed that baseline elevations in serum IgG4 and IgE levels as well as blood eosinophil counts before treatment could predict worsening of IgG4-RD after steroid medication [36-38]. A preoperative predictor of the outcome of IgG4-AAAs would be practically and clinically more significant, because the selection of the surgical procedure would be dependent on this aspect. In the present study, although the serum IgG4 levels before EVAR were similar between patients in the IgG4-AAA-up subgroup and those in the IgG4-AAA-down subgroup, the preoperative IgG4/IgG ratio in the IgG4-AAA-up subgroup was significantly higher than that in the IgG4-AAA-down subgroup. Interestingly, the IgG4/IgG ratio was consistently higher before and after EVAR in the IgG4-AAA-up subgroup; hence, the higher IgG4/IgG ratio might reflect the higher activity of IgG4-RD in patients with IgG4-AAAs.
Our results indicated that a high ratio of preoperative monocyte and eosinophil segments was also a characteristic feature of the IgG4-AAA-up subgroup, similar to that of previous reports on IgG4-RD [36]. Indeed, studies have shown that frequent eosinophil infiltration and M2 macrophage proliferation in the adventitia were one of the histopathological attributes of IgG4-AAAs [2,3,24]. Therefore, we considered that the eosinophil and monocyte counts reflect the local characteristic immune cell infiltration in IgG4-AAAs. However, it must be considered that patients with IgG4-RD often develop some allergies that could influence the WBC segments.
Preoperative MMP-9 levels may serve as a reference to identify the IgG4-AAA-up subgroup. Previous reports have suggested that postoperative serum IL-6 levels are a hallmark of relapse following aAAA treatment [33]. Although increased postoperative IL-6 levels and larger ΔIL-6 were characteristic of the IgG4-AAA-up subgroup, unlike our expectations, preoperative IL-6 levels were not useful in identifying patients in the IgG4-AAA-up subgroup.
Based on these findings, before surgery, a high serum IgG4/IgG ratio, high MMP-9 concentration, and high monocyte and eosinophil counts would be useful in determining the IgG4-AAA-up subgroup. Furthermore, for patients with IgG4-AAA with these hallmarks, we recommend OSR to prevent the progression of aneurysm [18]. This is because, as previously reported, OSR could provide complete recovery for patients with IgG4-AAA along with longer survival and fewer complications [7-11,18].
Our study has several limitations. First, an extremely small number of patients was included because of the single-center design, with limited postoperative serology and imaging data. An updated meta-analysis or a multicenter study would provide more reliable data in the future. In this study, we hypothesized that circulating plasma MMP levels would reflect MMP production in the local aortic tissue [39]. Further studies are required to confirm the histological MMP expression in the adventitia of patients with IgG4-AAAs to compare with controls. It is also necessary to assess other predictive markers for the prognosis of patients with IgG4-AAAs.
After EVAR, patients with IgG4-AAAs exhibited higher serum MMP-9 levels and larger aneurysm diameters than patients with non-IgG4-AAAs and aAAA controls. Increased postoperative MMP-9 and IL-6 levels in patients with IgG4-AAA may accelerate aneurysmal progression after EVAR and would be closely associated with the aspect of vasculitis of IgG4-AAAs. Among patients with IgG4-AAAs, after EVAR, those in the IgG4-AAA-up subgroup exhibited higher serum IL-6 levels, larger aneurysm diameters, and thicker PAF than patients in the IgG4-AAA-down subgroup. Therefore, this can be considered as the active state of IgG4-AAAs with a tendency toward progression. Before surgical treatment, a high IgG4/IgG ratio, high MMP-9 concentration, and high ratio of monocytes and eosinophils are indicators of the IgG4-AAA-up subgroup. For patients with IgG4-AAA with the abovementioned characteristics, it is recommended to select OSR or to follow up attentively to prevent complications.
This work was supported by Grant-in AUC for Scientific Research (C) (JSPS KAKENHI) Grant Number 17K10773.
The authors would like to thank Mr Yutaka Yamagishi, Mr Katsumasa Ishida, Ms Aiko Matsuda, Ms Youko Umehara, Ms Kaori Nakanishi, Mr Keisuke Yonemura and Mr Rei Kasashima for their special technical assistance.
The authors declare that they have no conflict of interest.
- Stone JH, Zen Y, Deshpande V (2012) IgG4-related disease. N Engl J Med 366: 539-551.
- Kasashima S, Zen Y, Kawashima A, Konishi K, Sasaki H, et al. (2008) Inflammatory abdominal aortic aneurysms: Close relationship to IgG4-relared periaortitis. Am J Surg Pathol 32:197-204.
- Kasashima S, Zen Y, Kawashima A, Endo M, Matsumoto Y, et al. (2009) A new clinicopathological entity of IgG4-related inflammatory abdominal aortic aneurysm. J Vasc Surg 49:1264-1271.
- Puchner S, Bucek RA, Loewe C, Hoelzenbein T, Kretschmer G, et al. (2006) Endovascular repair of
inflammatory aortic aneurysms: long-term results. Am J Roentgenol 186: 1144-1147.
- Coppi G, Rametta F, Aiello S, Saitta G, Gennai S, et al. (2010) Inflammatory abdominal aortic aneurysms endovascular repair into the long-term follow-up. Ann Vasc Surg 24: 1053-1059.
- Lee SH, Won JY, Lee DY, Kim IJ, Lee SJ, et al. (2015) Mid-term clinical outcomes and morphological changes after endovascular aneurysmal repair of inflammatory abdominal aortic aneurysms: a single-center experience. Acta Radiol 56: 304-311.
- Qian Q, Kashani KB, Miller DV (2009) Ruptured abdominal aortic aneurysm related to IgG4 periaortitis. N Engl J Med 361: 1121.
- Trinidad-Hernandez M, Duncan AA (2012) Contained ruptured paravisceral aortic aneurysm related to immunoglobulin G4 aortitis. Ann Vasc Surg 26: 108.
- Kan-O M, Kado Y, Sadanaga A, Tamiya S, Toyoshima S, et al. (2015) Immunoglobulin G4-related multiple vascular lesions successfully treated with a combination of open vascular surgery and corticosteroid therapy. J Vasc Surg 61: 1599-1603.
- Uchida T, Hamasaki A, Kuroda Y, Sadahiro M, Tamazawa M, et al. (2018) Immunoglobulin G subclass 4-related lymphoplasmacytic thoracic aortitis in a patient with acute Type A aortic dissection. Ann Thorac Cardiovasc Surg 24: 208-210.
- Lun Y, Jiang H, Xu D, Xin S, Zhang J (2018) Contained rupture of a common iliac aneurysm associated with immunoglobulin G4-related disease. J Vasc Surg 68: 1564-1565.
- Ikeda A, Mitomi K, Konishi T, Matsuzaki K, Jikuya T, et al. (2015) Endovascular repair of a false aneurysm developing from IgG4-related periaortitis during corticosteroid therapy. Ann Vasc Surg 29: 1452.
- Sakai K, Watanabe T, Yoshida T (2018) Endovascular treatment of immunoglobulin G4-related inflammatory abdominal aortic aneurysm. J Vasc Surg Cases Innov Tech 4:189-192.
- Sarac M, Marjanovic I, Bezmarevic M, Zoranovic U, Petrovic S, et al. (2012) An aortoduodenal fistula as a complication of immunoglobulin G4-related disease. World J Gastroenterol 18: 6164-6167.
- Kasashima S, Kawashima A, Kasashima F, Endo M, Matsumoto Y, et al. (2014) Immunoglobulin G4-related periaortitis complicated by aortic rupture and aortoduodenal fistula after endovascular AAA repair. J Endovasc Ther 21: 589-597.
- Luís M, Brites L, Fernandes B, Jesus D, Santiago T, et al. (2018) The many faces of IgG4-related disease: report of a case with inaugural recurrent aortic aneurism ruptures and literature review. Rheumatol Int 38: 1565-1570.
- Bezmarevic M, Marjanovic I, Sarac M (2014) What is the appropriate treatment of immunoglobulin G4-related vascular lesions? J Endovasc Ther 21: 598-600.
- Kasashima S, Kasashima F, Kawashima A, Endo M, Matsumoto Y, et al. (2017) Clinical outcomes after endovascular and open surgery repair of immunoglobulin G4-related or non-related inflammatory aortic aneurysms. J Endovasc Ther 24: 833-845.
- White JV, Haas K, Phillips S, Comerota AJ (1993) Adventitial elastolysis is a primary event in aneurysm formation. J Vasc Surg 17:371-381.
- Raffetto JD, Khalil RA (2008) Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol 75: 346-359.
- Benjamin MM, Khalil RA (2012) Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. Exp Suppl 103: 209-279.
- Chistiakov DA, Sobenin IA, Orekhov AN (2013) Vascular extracellular matrix in atherosclerosis. Cardiol Rev 21: 270-288.
- Kasashima F (1995) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in atherosclerotic aortic aneurysms. Juzen Med Soc 104: 216-229.
- Kasashima S, Kawashima A, Zen Y, Ozaki S, Kasashima F, et al. (2018) Upregulated interleukins (IL-6, IL-10, and IL-13) in immunoglobulin G4-Related aortic aneurysm patients. J Vasc Surg 67: 1248-1262.
- Szekanecz Z, Shah MR, Pearce WH, Koch AE (1994) Human atherosclerotic abdominal aortic aneurysms produce interleukin (IL)-6 and interferon-gamma but not IL-2 and IL-4: the possible role for IL-6 and interferon-gamma in vascular inflammation. Agents Actions 42: 159-162.
- Ijaz T, Tilton RG, Brasier AR (2016) Cytokine amplification and macrophage effector functions in aortic inflammation and abdominal aortic aneurysm formation. J Thorac Dis 8: 746-754.
- Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, et al. (2012) Consensus statement on the pathology of IgG4-related disease. Mod Pathol 25: 1181-1192.
- Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, et al. (2012) Comprehensive diagnostic criteria for IgG4-related disease. Mod Rheumatol 22: 21-30.
- Okazaki K, Kawa S, Kamisawa T, Ito T, Inui K, et al. (2013) Working Committee of the Japan Pancreas Society and the Research Committee for Intractable Pancreatic Disease supported by the Ministry of Health, Labour and Welfare of Japan. 2014. Amendment of the Japanese Consensus Guidelines for Autoimmune Pancreatitis, Concept and diagnosis of autoimmune pancreatitis. J Gastroenterol 49: 567-588.
- Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, et al. (2002) Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery, Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg 35: 1048-1060.
- Fujimoto N, Iwata K (1994) Enzyme immunoassays for matrix metalloproteinases and their inhibitors. Connective Tissue 26: 237-244.
- Schanzer A, Greenberg RK, Hevelone N, Pobinson WP, Eslami MH, et al. (2011) Predictors of abdominal aortic aneurysm sac enlargement after endovascular repair. Circulation 123: 2848-2855.
- Nessvi Otterhag S, Gottsäter A, Acosta S, Palmqvist B, Lindblad B. 2014. Inflammatory mediators after endovascular aortic aneurysm repair. Cytokine 70:151-155.
- Tieu BC, Lee C, Sun H, Lejeune W, Recinos A 3rd, et al. (2009) An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest 119: 3637-3651.
- Kasashima S, Kawashima A, Kasashima F, Endo M, Matsumoto Y, et al. (2018) Inflammatory features, including symptoms, increased serum interleukin-6, and C-reactive protein, in IgG4-related vascular diseases. Heart Vessels 33: 1471-1481.
- Wallace ZS, Mattoo H, Mahajan VS, Kulikova M, Lu L, et al. (2016) Baseline elevations in serum IgG4, IgE and blood eosinophil concentrations all predict IgG4-RD relapses independently. Rheumatol 55: 1000-1008.
- Culver EL, Sadler R, Bateman AC, Makuch M, Cargill T, et al. (2017) Increases in IgE, eosinophils, and mast Cells can be used in diagnosis and to predict relapse of IgG4-related disease. Clin Gastroenterol Hepatol 15: 1444-1452.
- Sasaki T, Akiyama M, Kaneko Y, Yasuoka H, Suzuki K, et al. (2018) Risk factors of relapse following glucocorticoid tapering in IgG4-related disease. Clin Exp Rheumatol 36: S186-S189.
- Li T, Jiang B, Li X, Sun H, Li XT, et al. (2018) Serum MMP-9 is a valuable biomarker for identification of abdominal and thoracic aortic aneurysm: a case-control study. BMC Cardiovas Disord 18: 202.



