Background: Iodine deficiency remains a foremost public health problem in the developing world. The goiter rate is an important indicator to assess the long-term impact of iodine-deficiency disorders. Ethiopia is a country with high prevalence of goiter and its consequences continue to affect a large number of its population mostly school-aged children who are the future generations of the country. The aim of this study was to assess the prevalence of goiter and its determinants among children with an Implication for designing family centered prevention strategy in Southern Ethiopia.
Methods: A school-based cross-sectional study was conducted on 416 children from January 02, to February 02, 2019. A structured interviewer-administered questionnaire was used to collect data. The data was entered and analysed using SPSS version 20. Bivariate and multivariable logistic regression analysis was used for identifying the determinants of goiter.
Results: The overall prevalence of goiter was 37% [95%CI: 32.5, 41.7] with grade 1, 33.2% [95%CI: 28.8, 37.9] and grade 2, 3.8% [95%CI: 2.4, 6.2].Children from governmental school [AOR=2.00], children who had a family history of goiter [AOR=2.72], mother perceived the physical effects of ID [AOR=1.88], use of non-iodized salt [AOR=2.67], non-employing family advice about iodine salt usage [AOR=5.24], consumption of cabbage in a week [AOR=2.49] were the determinants of goiter.
Conclusions: The prevalence of goiter in this study was high, suggesting a public health problem among school-age children in southern Ethiopia. An integrated, highly acceptable and cost-effective goiter prevention approaches with an organized control of marketing non-iodized salt needed to be applied at all levels. Moreover, we need to give due attention to design family-centered goiter prevention strategy in resource-limited countries.
goiter prevalence, school age children, goiter grading, family-centered prevention
AOR: Adjusted Odds Ratio; ID: Iodine Deficiency; IDD: Iodine Deficiency Disorders; WHO-World Health Organization.
Iodine deficiency disorder is one of the most prevalent micronutrient deficiencies in developing countries including Ethiopia. Iodine Deficiency (ID) is considered as the most common preventable cause of mental retardation and responsible for decreased resistance against infections, poor school performance, and lack of physical strength of the child [1]. Furthermore, iodine deficiency disorders can result in endemic goiter, hypothyroidism, congenital anomalies, and cretinism [2].
The World Health Organization estimates that approximately 37% of school-age children; 285 million, and 1.88 billion people worldwide remain at risk of insufficient iodine intake and approximately a third of the world’s population lives in areas with some iodine deficiency [3]. In Ethiopia, iodine deficiency has remained a public health problem among school children for many decades, around 28 million people suffer from goiter, and more than 35 million people are at risk of iodine deficiency. The national total goiter weighted prevalence rate among children aged 6 to 12 years was 39.9% [4-5].
Goiter is the most noticeable manifestation of iodine-deficiency disorders. Goiter rate is useful to assess the long-term impact of iodine-deficiency disorders [6]. Goiter in school children is the best indicator of IDDs in the community and it is recommended that a total goiter prevalence of 5% or more in school children should be used to signal the presence of a public health problem [7-9]. Iodine deficiency is mainly caused by low iodine content in the diet, arising from low iodine levels in the soil, water, or crops. In addition, the consumption of goitrogenic substances containing food items, like cassava and millet. Compared to other population segments, pregnant mothers and school children are the most vulnerable groups for iodine deficiency [9,10].
Ethiopia has been exerted efforts to eradicate iodine deficiency by recognizing the extent of iodine deficiency and the disorders that could emanate from it, by an acceptable strategy of universal iodization of salt and designed micronutrient guideline nevertheless significant changes have not been attained [11]. Thus, this study aimed to assess the prevalence of goiter and its determinants among school-age children in Southern Ethiopia.
Study design setting and period: Prevalence of goiter and its determinants a survey part of a prospective follow up study on Iodine deficiency disorder and consequences among school age children in Hossana Province, Southern Ethiopia which was started in 2019. The study was conducted in primary schools of Hossana province. Hossana is 232 km far from Addis Ababa, the capital of Ethiopia. In the province, there are 11 governmental and 38 non-governmental primary schools. This study is primary goiter prevalence survey which was conducted from January 02 to February 02, 2019.
Population of the study: Source population comprised all children aged 6-12 who are students in primary schools of Hossana Town. Study population consisted of sampled children aged 6-12 who are students in primary schools of Hossana Province of southern Ethiopia. Students aged 6-12 who were critically ill or sick at the time of data collection were excluded from this study.
Sample size and sampling procedure: Sample size was computed based on the following assumptions: prevalence of goiter in school age children in Robe District, Oromia Region, Southeast Ethiopia which was 43.5 % [12], 95% confidence level, marginal of error 5% and 10% non- response rate, the final sample size was 416. Stratified sampling technique was employed. The schools were stratified in two, governmental and private schools. Three governmental & six private primary schools were selected using a lottery method (simple random sampling technique) and then one section per grade was selected through simple random sampling technique. Finally, to reach the study participants systematic random sampling techniques was employed using the lists of students.
Data collection procedure and quality control: Structured interviewer administered questionnaire was used to collect which is adapted from WHO and after reviewing different literatures of similar studies. To ensure quality of data, pre-test was done among school age children in primary school of Belesa on 10% of the total sample size and necessary modification was made after the pre-test. The data was collected by 6 nurses with diploma qualification with supervision of 2 nurses with qualification of Bachelor of Science. Training was given for data collectors and supervisors for 3 days. Supervision was done at the spot by investigators and supervisors.
Measurement: The inspection and palpation of the thyroid gland provides information about the size (enlargement), consistency, and surface of the thyroid. As a result, physical examination was performed by trained public health officers to determine the size of the thyroid gland. Goiter was clinically defined according to the WHO criteria: grade-0 (no palpable goiter), grade-1 (palpable and visible goiter with extended neck), and grade-2 (visible goiter with the head in normal position) [9].
Data analysis: After coding, data was entered and analysed using SPSS version 20. Descriptive statistic was carried out to compute frequencies, percentages and figures. To identify factors associated with of goiter binary logistic regressions was applied and the variables (p ≤0.2) found to have association with the outcome variable then entered into multivariable logistic regression analysis was used to control confounding factors. Finally, the variables which have significant association were identified on the basis of p-value ≤ 0.05 and AOR, with 95% Cl.
Socio-demographic characteristics
A total of 416 school-age children were included in this study with a response rate of 100%. The mean age of study participants was 9.9 years with SD ± 1.5 years. More than half of respondents 224 (53.8%), were female and 273 (65.6%) were in the age group 10-12 years. 286 (68.8%) enrolled in governmental schools and nearly three-fourth; 301 (72.4%) students were between grades 1 and 3. Two hundred eighty (67.3%) of the children’s mothers were housewives, 185 (44.5%) had followed primary education and the majority (92.5%) were married. Concerning children’s fathers; 157 (37.7%) were self-employed and 166 (39.9%) had followed secondary education. With regard to the source of information; 89 (15.8%) and 107 (25.7%) were television and radio respectively. Regarding topography, 398 (95.7%) live in plain land.
Health care and goiter related characteristics
Majority (94.2%) used tap water as main source of water. Three hundred forty-five (82.9%) mothers had less than six children and 245(58.9%) respondents had less than six family size. About 70% of the respondent had family history of goiter. Majority of the mothers (87.3%) have sufficient knowledge on the health effects of iodine deficiency. Mothers stated that loss of educational ability (35.8%), physical problem (69.0%), abortion (22.6%) and stigma (4.8%) are the perceived health effects of iodine deficiency.
Food and dietary characteristics
The current study revealed that 92 (22.1%) had used iodized salt and 273(65.6%) had used mixed (iodized & non-iodized) to cook their food at their home. Two hundred forty-eight (59%) mothers stated that their source of information on the use of iodized salt at home were health professionals. Only 28(6.7%) respondents had fed children fish or sardines. More than three fourth mothers (77.6%) had fed their children cabbage and 249(59.9%) mothers had fed their children cabbage less than 2 times per week. With regard to cassava conception, only 5(1.2%) mothers had fed their children cassava (Table 1).
Table 1. Food and dietary characteristics of school age children and their parents in primary schools of Hossana Province, Southern Ethiopia 2020 (n= 416)
Variables |
Frequency |
Percent (%) |
Type of salt used for cooking |
Iodized salt |
92 |
22.1 |
Non iodized salt |
59 |
14.2 |
Mixing two types |
273 |
65.6 |
Source of information about use of iodized salt |
Health professional |
248 |
59.6 |
Mass medias/TV and radio |
226 |
54.3 |
Friends |
40 |
9.6 |
Family |
35 |
8.4 |
I don’t know |
14 |
3.4 |
Feeding of fish or sardines for child |
Yes |
28 |
6.7 |
No |
388 |
93.3 |
Frequency of fish consumption per week |
Not consumed |
389 |
93.5 |
Less than 2 times |
18 |
4.3 |
Two and more times |
9 |
2.2 |
Feeding of cabbage for child |
Yes |
323 |
77.6 |
No |
93 |
22.4 |
Frequency of cabbage consumption per week |
Not consumed |
93 |
22.4 |
Less than 2 times |
249 |
59.9 |
Two and more times |
74 |
17.8 |
Feeding of cassava for child |
Yes |
5 |
1.2 |
No |
411 |
98.8 |
Frequency of cassava consumption per week |
Not consumed |
411 |
98.8 |
Less than 2 times |
5 |
1.2 |
Prevalence of goiter among school age children
The prevalence of goiter was 37% [95%CI: 32.5, 41.7] among school-age children aged 6-12 years at primary schools of Hossana province southern Ethiopia. From all school children with goiter, grade 1 and grade 2 were 33.2% [95%CI: 28.8, 37.9] and 3.8% [95%CI: 2.4, 6.2] respectively. Children with goiter were 29.1% in governmental schools (Figure 1).
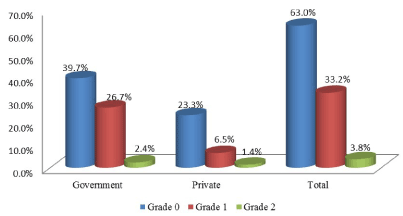
Figure 1. Goiter grade and school types among children at primary schools of Hossana province, Southern Ethiopia, 2020
Determinants of goiter among school age children
In current study; school type, family history of goiter, mother perceived physical effects of ID, type of salt used at home, source of information from family, consumption of cabbage were identified as determinants of goiter. School type was one of significant factor with goiter in the study area. The odds of developing goiter among students enrolled in governmental schools were 2.00 times higher than those enrolled in private schools [AOR=2.00; 95%Cl; 1.21-3.33].
The other risk factor which significantly associated with goiter was family history of goiter. The odds of developing goiter among students who had family history of goiter were 2.72 times higher than those with no family history of goiter [AOR=2.72; 95% Cl; 1.69- 4.38]. Mothers perceived a physical effect of ID was the other factor that was significantly associated with goiter. The odds of developing goiter among children whose mother’s perceived physical effects of ID were 1.88 times higher than those who did not perceive the physical of effects ID [AOR=1.88; 95% Cl; 1.16- 3.03].
The odds of developing goiter among families who didn’t use non iodized salt were 2.67 times higher than their counterpart [AOR=2.67; 95% Cl; 1.21- 5.88]. The odds of developing goiter among children whose mother had not received advice from families about use of iodized salt were 5.24 times higher than those who had advice received from families [AOR= 5.24; 95% Cl; 1.85- 14.87]. The frequency of cabbage consumption in a week among school age children was also found to be significantly associated with goiter. The odds of developing goiter among children who consumed cabbage once in a week were 2.49 times higher than their counterpart [AOR= 2.49; 95% Cl; 1.34- 4.64] (Table 2).
Table 2. Determinants of goiter among school age children at primary schools of Hossana province, Southern Ethiopia, 2020 (n=416)
Explanatory Variables |
Goiter status |
COR 95% CI |
AOR 95% CI |
P-Value |
Yes |
No |
School Type |
Governmental |
121(42.3) |
165(57.7) |
2.16(1.36,3.41) |
2.00(1.21,3.33) |
0.007* |
Private |
33(25.4 |
97(74.6) |
1 |
1 |
|
Family Hx of Goiter |
Yes |
72(58.1) |
52(41.9) |
3.55(2.29,5.49) |
2.72(1.69,4.38) |
<0.001* |
No |
82(28.1) |
210(71.9) |
1 |
1 |
|
Perceived physical effects of ID |
No |
63(48.8) |
66(51.2) |
2.06(1.34,3.15) |
1.88(1.16,3.03) |
0.010* |
Yes |
91(31.7) |
196(68.3) |
1 |
1 |
|
Type of salt used for cooking |
Iodized salt |
24(24.7) |
73(75.3) |
1 |
1 |
|
Non iodized salt |
31(53.4) |
27(46.6) |
3.49(1.75,6.98) |
2.67(1.21,5.88) |
0.015* |
Mixing two types |
99(37.9) |
162(62.1) |
1.86(1.10,3.14) |
1.76(0.99,3.14) |
0.056 |
Received Advice about iodized salt use from family |
No |
149(39.1) |
232(60.9) |
3.85(1.46,10.2) |
5.24(1.85,14.87) |
0.002* |
Yes |
5(14.3) |
30(85.7) |
1 |
1 |
|
Frequency of cabbage consumption per week |
Not consumed |
18(19.4) |
75(80.6) |
1 |
1 |
|
One times |
104(41.8) |
145(58.2) |
2.99(1.69,5.29) |
2.49(1.34,4.64) |
0.004* |
Two and more times |
32(43.2) |
42(56.8) |
3.18(1.59,6.33) |
2.04(0.95,4.39) |
0.068 |
*Statistically significant at p-value < 0.05
The prevalence of goiter among school-age children aged 6-12 years at primary schools of Hossana province was 37% [95%CI: 32.5, 41.7]. The goiter prevalence in this study was higher than a study conducted in China (8.8%) [13], in Sub-Himalayan Darjeeling District of West Bengal, India (8.7%) [14], and another study in Pauri, Uttarakhand district of India (16.8%) [15]. However, goiter prevalence in this study was lower than findings from studies carried out in Robe Oromia, (43.5%) [12], Tigray (71.4%) [16], Jimma (59.1%) [17], and Womberma, Burie, northwest Ethiopia (54 %) [18].
According to the current study from all school children with grade 1 and grade 2 goiter were 33.2% [95%CI: 28.8, 37.9] and 3.8% [95%CI: 2.4, 6.2] respectively. The prevalence of grade 1 goiter is almost comparable with study in Robe district (31.3%) [12] and ShebeSenbo district, (35.2%) [17]. The discrepancy could be attributed to socio-demographic, economic status, altitude, the difference in the utilization of iodized salt, food choice, and a difference in sample characteristics of the studies. It is known that WHO recommends a total goiter rate of 5% or more in primary school children of 6 to 12 years of age be used to signal the presence of a public health problem [7].
In this study, school type was one of the significant factors with goiter. The odds of developing goiter among students enrolled in governmental schools were 2.00 times higher than those enrolled in private schools. The finding might be due to the fact that those students who enrolled in government schools are from families from low socioeconomic status and their parents cannot afford to purchase iodized salt every month. This finding was supported by a study in Dabat District, northwest Ethiopia [19].
The other risk factor which was significantly associated with goiter was family history. The odds of developing goiter among students who had a family history of goiter were 3-fold higher than those with no family history of goiter. This finding is similar to studies [20-22]. Thus, children with family history of goiter imply genetic or hereditary factors may play a great role for causation of goiter [23,24].
The mother’s perceived physical effect of ID was the other factor that was significantly associated with goiter. The odds of developing goiter among children whose mothers perceived physical effects of ID were 2 times higher than those mothers not perceived it. This finding was supported by a study done in Gamo-Gofa Zone southern Ethiopia [25] and Dabat District, northwest Ethiopia [19]. This could be due to the fact that having perception on the health, cognitive and social effects of goiter and iodine deficiency help parents to protect their children and restrict all their family members from consuming non- iodized salt intake, goitrogen, and other goiter enhancing practices. The odds of developing goiter among families who don’t use non-iodized salt were 2.67 times higher than their counterparts. This is similar to studies from Germany and Italy [26, 27] and studies in Ethiopia [28-31]. This might be due to the fact that frequent use of iodized salt will avert the occurrence of goiter through fulfilling iodine demand required by the body.
On the other hand, the odds of developing goiter among children whose mother had not received advice from families about the use of iodized salt were 5.24 times higher than those who had advice received from families. This might be due to the fact that mothers who will not get advice about iodized salt are not motivated to use it. Hence, this will lead their children to get goiter.
The frequency of cabbage consumption in a week among school-age children was also found to be significantly associated with goiter. The odds of developing goiter among children who consumed cabbage once in a week were 2.49 times higher than their counterpart. This finding is similar with a study in Shebe Senbo District, Jimma [17], Anchar district of Eastern Ethiopia [30] and Gamo-Gofa zone Southern Ethiopia, southern Ethiopia, and Leku town, Southern Ethiopia [25,28,31] in which there is a significant association between the frequency of cabbage intake and the development of goiter. This might be due to the fact that cabbage comprises thiocyanate and isothiocyanate that inhibit iodine uptake by the thyroid follicular cells and also blocks the thyroid peroxidase enzyme. Besides, the iodination of thyroglobulin protein will be impaired, resulting in poor thyroxine production and enlargement of the thyroid gland [5, 29]. In this study, since it is a preliminary survey part of the cohort study, findings from lab measurements: urinary iodine, TSH level, and salt iodization level will be included in the next publications.
The prevalence of goiter in this study was high, suggesting a public health problem among school-age children in the study area. School type, family history of goiter, mother perceived the physical effects of ID, type of salt used at home, mother received advice about the use of iodized salt, cabbage consumption was identified as determinants of goiter. An integrated, highly acceptable, and cost-effective goiter prevention approaches with an organized control of marketing non-iodized salt needed to be applied at all levels. Likewise, parents need to feed their children foods enriched with iodized salt and avoiding goitrogenic foods to protect the future generation from goiter and other iodine deficiency disorders. Moreover, we need to give due attention to design family-centered goiter prevention strategy in southern Ethiopia and other resource-limited countries.
Approval for this study was provided by the Wachemo University, collage of medicine and health sciences research and community service review committee. Permission to assess students was obtained from schools and main data was obtained from mothers or care givers of children at their residence after obtaining written consent.
The datasets developed and/or analysed during the current study are available from the first author or from the corresponding author on reasonable request.
The authors declare that they have no competing interests
Wachemo University funded the research and it is open for the researchers to publish the manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to thank study participants, schools, and Education administrators of Hossana province and Wachemo University.
- WHO (2013) Urinary iodine concentrations for determining iodine status deficiency in populations. Vitamin and Mineral Nutrition Information System. Geneva: World Health Organization. Available from: https://www.who.int/vmnis/indicators/urinaryiodine/en/
- Zimmermann MB, Jooste PL, Pandav CS (2008) Iodine-deficiency disorders. Lancet 372:1251-62. [Crossref]
- Andersson M, Karumbunathan V, Zimmermann MB (2012) Global iodine status in 2011 and trends over the past decade. J Nutr 142:744–50. [Crossref]
- Dawit S, Seifu H, Carl KL Martin EK, Patrick K (2010) Post-production Losses in Iodine Concentration of Salt Hamper the control of Iodine Deficiency Disorders: A Case study in Northern Ethiopia. J Health Popul Nutr 28: 238-244. [Crossref]
- Abuye C, Berhane Y, Akalu G, Getahun Z, Ersumo T (2007) Prevalence of goiter in children 6 to 12 years of age in Ethiopia. Food Nutr Bull 28: 391-398. [Crossref]
- Delange F (1985) Adaptation to iodine deficiency during growth: etiopathogenesis of endemic goiter and cretinism. In: Delange F, Fisher D, Malvaux P, eds. Pediatric thyroidology. Basel: S. Karger, 295–326.
- Keith P, West Jr. (2013) Iodine Deficiencies Iodine Deficiency Disorders (IDDs). Most preventable cause of mental retardation in the world.
- WHO (2014) Salt reduction and iodine fortification strategies in public health. World Health Organization. Available from: https://www.who.int/nutrition/publications/publichealth_saltreduc_iodine_fortification/en/
- WHO, UNICEF, ICCIDD (2007) Assessment of iodine deficiency disorders and monitoring their elimination: a guide for programme managers. 3rd ed. Geneva: World Health Organization. Available from: https://apps.who.int/iris/handle/10665/43781
- De Benoist B, McLean E, Andersson M, Rogers L (2008) Iodine deficiency in 2007: global progress since 2003. Food Nutr Bull 29: 195–202. [Crossref]
- Federal Ministry of Health Family Health Department. Ethiopian National Guidelines for Control and Prevention of Micronutrient Deficiencies. 2004.
- Hailu S, Wubishet M, Woldie H, Tariku A (2016) Iodine deficiency and associated factors among school children in Robe, Oromia Regional State: A cross-sectional study in Ethiopia. Arch Public Health 74:46. [Crossref]
- Liu Y, Huang H, Zeng J, Sun C (2013) Thyroid volume, goiter prevalence, and selenium levels in an iodine-sufficient area: a cross-sectional study. BMC Public Health 13:1153. [Crossref]
- Biswas AB, Das DK, Chakraborty I, Kumar A, Puran B, et al. (2014) Goiter Prevalence, Urinary Iodine, and Salt Iodization Level in Sub-Himalayan Darjeeling District of West Bengal, India. Indian J Public Health 58: 129-33. [Crossref]
- Kapil U, Pandey RM, Prakash S, Sareen N, Bhadoria AS (2014) Iodine Deficiency Status amongst School Children in Pauri, Uttarakhand. Indian Pediatr 51: 569-70. [Crossref]
- Kidane T, Woldegebriel A (2006) Prevalence of Iodine deficiency disorder in a highland district in Tigray. Ethiop J Health Dev 20: 58-59
- Mezgebu Y, Mossie A, Rajesh PN, Beyene G (2012) Prevalence and severity of iodine deficiency disorder among children 6-12 years of age in shebe senbo district, jimma zone, southwest Ethiopia. Ethiop J Health Sci 22: 196-204. [Crossref]
- Aweke K, Adamu B, Girmay A, Yohannes T, Alemnesh Z, et al. (2014) Iodine Deficiency Disorders (IDD) in Burie and Womberma Districts, West Gojjam, Ethiopia. Afr J Food Agric Nutr Dev 14: 9167–80.
- Abebe Z, Gebeye E, Tariku A (2017) Poor dietary diversity, wealth status and use of un-iodized salt are associated with goiter among school children: a cross-sectional study in Ethiopia. BMC Public Health 17: 44. [Crossref]
- Mesele M, Degu G, Gebrehiwot H (2014) Prevalence and associated factors of goiter among rural children aged 6–12 years old in Northwest Ethiopia, cross -sectional study. BMC Public Health 14: 1–8. [Crossref]
- Tigabu E, Bekele K, Dachew B (2017) Prevalence of goiter and associated factors among schoolchildren in northeast Ethiopia. Epidemiol Health 39: 1–5. [Crossref]
- Bekele A, Adilo MT (2019) Prevalence of goiter and its associated factors among primary school children in Chole District, Arsi Zone, Ethiopia: a cross-sectional study. BMC Nutr 5: 5. [Crossref]
- Bottcher Y, Eszlinger M, Tonjes A, Paschke R (2005) The genetics of euthyroid familial goiter. Trends Endocrinol Metab 16: 314-9. [Crossref]
- Vijay Panicker (2011) Genetics of Thyroid Function and Disease. Clin Biochem Rev 32: 165-75. [Crossref]
- Kebede DL, Adinew YM (2015) Predictors of Goiter among School Children in Southwest Ethiopia: Case-Control Study. J Nutr Food Sci 5: 368.
- Farahati J, Wegscheider K, Christ K, Gilman E, Oing W (2006) Gender-specifc determinants of goiter. Biol Trace Elem Res 113: 223–30. [Crossref]
- Busnardo B, Nacamulli D, Frigato F, Vianello-Dri A, De Vido D, et al. (2003) Normal values for thyroid ultrasonography, goiter prevalence and urinary iodine concentration in schoolchildren of the Veneto Region, Italy. J Endocrinol Investig 26: 991–6. [Crossref]
- Wolka E, Shiferaw S, Biadgilign S (2014) Epidemiological study of risk factors for goiter among primary school children in southern Ethiopia. Food Nutr Bull 35: 20-7. [Crossref]
- Amar KC, Sanjukta M, Dishari L, Smritiratan T (2004) Goitrogenic content of Indian cyanogenic plant foods & their in vitro anti-thyroidal activity. Indian J Med Res 119: 180-185. [Crossref]
- Muktar M, Roba KT, Mengistie B, Gebremichael B, Tessema AB, et al. (2019) Goiter and its associated factors among primary school children aged 6-12 years in Anchar district, Eastern Ethiopia. PLoS One 14: e0214927. [Crossref]
- Hibstu DT, Tesfaye DJ (2017) Epidemiology of goiter and its predictors samong school age children in Leku town, Southern Ethiopia. Curr Pediatr Res 21: 620–626.
Editorial Information
Editor-in-Chief
Article Type
Research Article
Publication history
Received date: May 05, 2020
Accepted date: May 18, 2020
Published date: May 28, 2020
Copyright
©2020 Asfaw MM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Asfaw MM, Gebremariam BM et al. (2020) Prevalence of goiter and its determinants among children in Southern Ethiopia: An implication for designing family-centered prevention strategy. 3: DOI:10.15761/PMCH.1000143.

