Abstract
Prominent corneal nerves are related to neurofibromatosis, multiple endocrine neoplasia (MEN) type 2B, MEN-2A syndrome, Leprosy (Hansen’s Disease), and similar systemic disorders. This report describes a 41-year-old patient presenting with blocked gland orifices in the upper and lower eyelids, light conjunctival hyperemia, a millet seed-sized semitransparent neoplasm on the nasal limbus suggestive of neuromas, and prominent corneal nerves. In vivo confocal microscopy (IVCM) revealed structural alterations—namely a prominent hyperreflective and thickened nerve plexus—in both eyes. This patient may represent a discrete subgroup termed pure mucosal neuroma syndrome (MNS), which presents with the characteristic appearance of MEN2B but without RET gene mutations.
Keywords
Prominent corneal nerves, in vivo confocal microscopy (IVCM), neurofibromatosis, pure mucosal neuroma syndrome (MNS)
Case presentation
A 41-year-old patient presented to the ophthalmology clinic with the complaint of itchy eyes and irritation, without blurred vision. The patient’s uncorrected visual acuity was logMAR visual acuity 0.0 OD and logMAR visual acuity 0.0 OS, with normal ocular alignment. The patient’s medical and surgical history was unremarkable. Slit-lamp examination in both eyes revealed mild blocked gland orifices in the upper and lower eyelids, light conjunctival hyperemia, and a millet seed-sized semitransparent neoplasm on the nasal limbus suggestive of neuromas (Figure 1), without eversion and thickening of the upper or lower eyelid. In addition, extremely thickened, off-white, irregular branching filaments were noted bilaterally in the previous 2/3 part of the midcorneal stroma throughout the center and periphery of the cornea, varying in length (Figure 2). In addition, a limbal-like neoplasm was seen on the inferior conjunctival fornix of the left eye (Figure 1). The rest of the findings of the intraocular examination were unremarkable; intraocular pressure and tear film breakup time (BUT) were normal in both eyes. The patient also had thick lips, a premaxillary protrusion (overbite), and café au lait spots on the back of the left forearm.
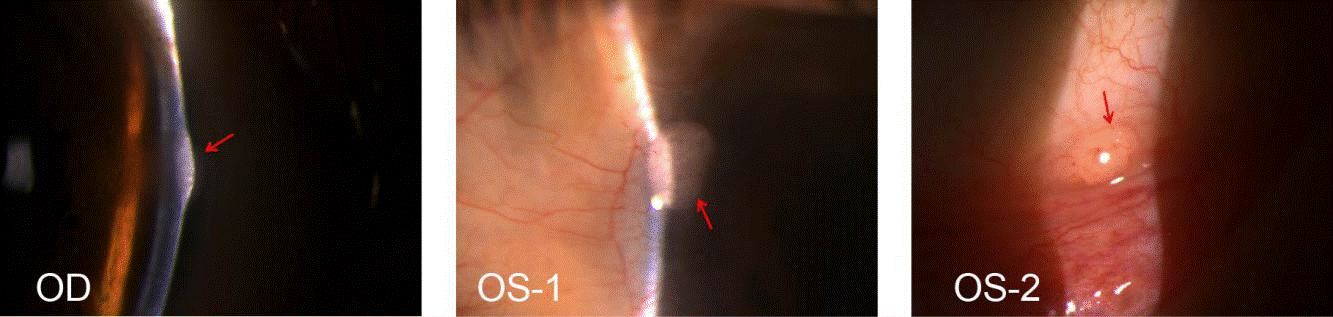
Figure 1. Slit-lamp images of semitranslucent neoplasm suggestive of neuromas(OD and OS, red arrowh).OS-1, Millet seed-size semitransparent neoplasm on nasal corneoscleral limbus of left eye, with ingrowing blood vessels. OS-2, Neoplasm on the inferior conjunctival fornix of left eye, (2mm*1.5mm size)
In vivo confocal microscopy (IVCM) is a non-invasive method used to assess the living corneal nerve morphology in both physiological and pathological states. IVCM was performed on the cornea with the Heidelberg retinal tomograph Ⅲ Rostock cornea module. Corneal IVCM images of the patient revealed a normal endothelium, with a sparse hyperreflective and thickened nerve plexus in both eyes (Figure 3). IVCM images of neuromas showed a disorganized bundle of nerves composed of elementary hyperreflective nerves with bifurcations, loops, and dilations (Figure 4).
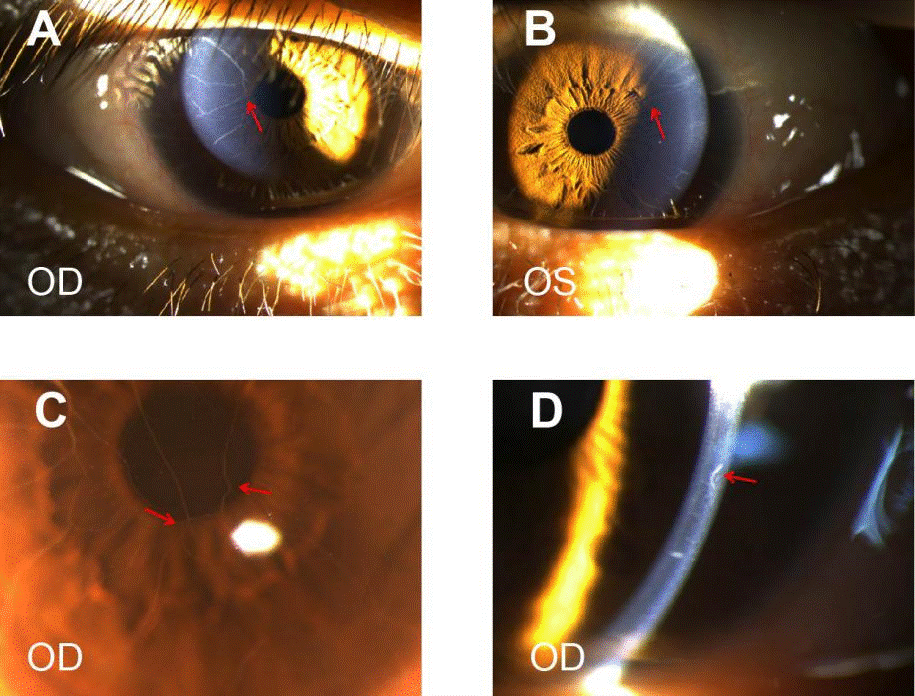
Figure 2. Corneal images. A B and C, Sharply marginated 2-4 branches of filaments extending onto the central cornea from corneoscleral limbus, partial interfitting (red arrow). The closer to the center of the cornea, the thinner the branch. D, prominent corneal nerves (magnification ×25).
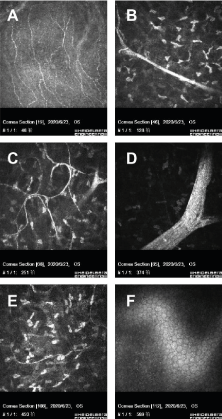
Figure 3. In vivo confocal microscopic images of cornea. No obvious abnormalities were found in the corneal pterygoid cells and basal cells. A, corneal sparse subbasal nerve plexus. B, hypertrophic nerves in anterior stroma. C, A few thin nerve fibers forming loops and showing nodular dilatations. Thickened main corneal nerve trunk appearing as a bright, hyperreflective structure, Penetrating and bifurcating here. D, highly reflective hypertrophic nerves with bifurcations in midstroma, about 80μm in diameter. E, thin nerve fibers present in posterior stroma. F, normal endothelium.
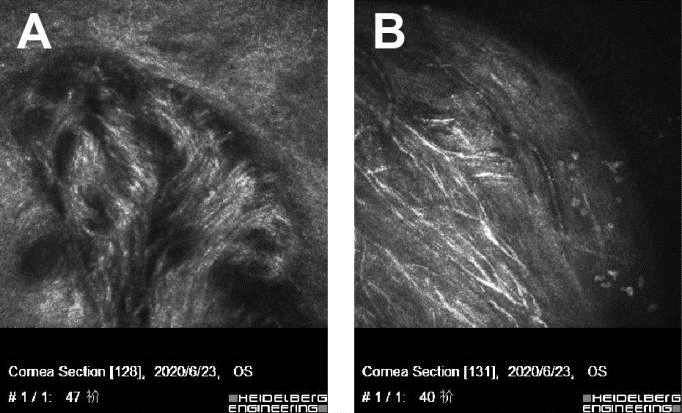
Figure 4. Conjunctival and paralimbal neuromas OS-1,2, Slit-lamp images. A and B, IVCM showing disorganized bundle of nerves (arrows) composed of elementary hyperreflective nerves with bifurcations, loops, and dilations
The patient was referred to the endocrinology department for further screening for associated neoplasia. There was no known family history of endocrine malignancy. Physical examination showed a healthy man without enlarged thyroid glands or neck masses. However, the bilateral cervical lymph nodes were slightly enlarged. Hematologic examination showed that calcitonin was 15.14 pg/ml (normal value< 9.52pg/ml). Ultrasound images showed uneven density/echoes in the thyroid. Cranial CT and MRI revealed no abnormal findings. Nevertheless, multiple endocrine neoplasia (MEN) type 2B was preliminarily ruled out by an endocrinologist.
Discussion
The cornea is one of the most sensitive tissues in the human body. The cornea nerves and sensations originate from the nasociliary branch of the first ophthalmic division of the trigeminal nerves (cranial nerve V), forming a plexus at the corneoscleral limbus [1]. From the limbal plexus, unmyelinated nerve endings maintaining transparency terminate in the corneal epithelium [2,3]. In the superficial cornea, the nerve plexus penetrates into the Bowman’s membrane and is distributed beneath the basal epithelial layer. Thickened corneal nerves are visible due to the myelination of corneal nerves [4]. Prominent corneal nerves are associated with systemic disorders such as neurofibromatosis, multiple endocrine neoplasia (MEN) type 2B, MEN-2A syndrome, Leprosy (Hansen’s Disease), primary amyloidosis, pheochromocytoma, Refsum’s disease, Marfan syndrome, congenital ichthyosis, and ectodermal dysplasia. Meanwhile, corneal nerves may be apparently thickened and more visible in some ocular diseases, such as Fuchs’ endothelial dystrophy, acanthamoeba keratitis, keratoconus, congenital glaucoma, herpes simplex, herpes zoster, posterior polymorphous dystrophy, and corneal transplantation failure [5-7].
Neurofibromatosis must be considered in any patient presenting with thickened and visible corneal nerves together with conjunctival neuromas. Therefore, in this report, we will focus on the discussion of neurofibromatosis and its ocular findings. Neurofibromatosis, a rare autosomal dominant disorder, encompasses at least three distinct disorders, referred to as NF1, NF2, and schwannomatosis. NF1, known as Von Recklinghausen’s disease, is the most common type of neurofibromatosis, with an estimated incidence of approximately 1 in 3,000 individuals to 1 in 3,500 individuals worldwide [8-10]. The clinical manifestations of NF1 are extremely varied in expressivity. Two or more features are required to establish a diagnosis of NF1: six or more café-au-lait macules (> 5 mm in diameter in prepubertal individuals and > 15 mm after puberty), two or more neurofibromas or one plexiform neurofibroma, skin-fold freckling, optic gliomas, two or more Lisch nodules, characteristic skeletal dysplasia (long bone or sphenoid wing), and a first-degree relative affected [11]. Of note, Lisch nodules, optic gliomas, and plexiform neurofibromas are hallmarks and considered diagnostic for NF1. In addition, In vivo confocal microscopy analysis showed morphological changes of the corneal structures; significant increases in the corneal endothelial cell density and in corneal nerve branching were observed, whereas corneal nerve density and the number of corneal nerve main trunks were not significantly different [12]. This patient, however, only displayed signs of three neurofibromas, meeting one of the clinical diagnostic criteria. Moreover, The IVCM shows a prominent hyperreflective, thickened nerve plexus and a normal endothelium in our case, which differ from the increased corneal endothelial cell density and corneal nerve branching in an IVCM of NF1. The diagnosis of NF1 was excluded preliminarily.
Multiple endocrine neoplasia (MEN) syndrome is an autosomal dominant disorder with three clinical subtypes: MEN1, MEN2 (MEN2A and MEN2B), and MEN4. MEN2B, also called Gorlin syndrome, is caused by germline mutations in the RET proto-oncogene and is characterized by medullary thyroid carcinoma and multiple mucosal neuromas and pheochromocytomas, with variable expressivity of intestinal ganglioneuromas, ophthalmic abnormalities, mucosal neuromas, and maxillofacial and orthopedic changes. Varying ophthalmic manifestations include enlarged prominent corneal nerves due to axon and Schwann cell abundance [13,14], eyelid and subconjunctival neuromas, dry eye disease, lid margin eversion or thickening, ptosis, and prominent perilimbal blood vessels [15-17]. An IVCM of the cornea indicated an increased density and diameter of the subbasal nerve plexus and a normal-appearing endothelium [18].
Pure mucosal neuroma syndrome is a rare distinct subgroup of MEN2B, which presents with typical features of MEN2B but no evidence of mutation in the RET proto-oncogene or associated endocrinopathies (medullary thyroid carcinoma, pheochromocytoma). Prominent corneal nerves combined with conjunctival neuromas may be an early sign of MEN2B [16,19]. We report a 41- year-old man who presented with prominent corneal nerves, conjunctival neuromas, blocked gland orifices in the upper and lower eyelids, and light conjunctival hyperaemia, but without a RET-gene mutation, medullary thyroid carcinoma, or pheochromocytoma. Our patient had MEN2B-like ocular findings, suggesting that he may represent a distinct subgroup termed “pure mucosal neuroma syndrome” [20]. A British investigation identified a genetic cause of pure MNS in which SOS1 frameshift mutations were related to isolated mucosal neuromas and gingival hypertrophy [21]. Nevertheless, a larger series of pure MNS patients is needed to confirm a detailed phenotype of this disorder.
In summary, in this report, the clinical and microstructural features of ocular involvement in NF1 and MEN2B are described. A detailed ocular examination and IVCM were performed in this patient. Ocular findings included blocked gland orifices in the upper and lower eyelids, light conjunctival hyperaemia, conjunctival neuromas, and prominent corneal nerves. Pathological results could not be obtained because the patient refused to accept the surgical resection. However, in vivo confocal microscopy (IVCM) is a non-invasive approach that provides a micro ultrastructure observation of living tissues. We detected a prominent hyperreflective and thickened nerve plexus and a normal endothelium in the IVCM for our case. Our case illustrates the importance of recognizing the ocular features of MNS, a rare presentation of MEN2B, in order to provide a narrow window of opportunity for prophylactic thyroidectomy to prevent medullary thyroid carcinoma; however, prophylactic thyroidectomy is not mandatory in these patients [21].
Competing Interests and Funding
The authors have neither competing interests nor specific funding for this manuscript.
References
- Shaheen BS, Bakir M, Jain S (2014) Corneal nerves in health and disease. Survey of Ophthalmology. 59: 263-285. [Crossref]
- Marfurt CF, Cox J, Deek S, Dvorscak L (2010) Anatomy of the human corneal innervation. Exp Eye Res 90: 478-492. [Crossref]
- Müller LJ, Marfurt CF, Kruse F, Tervo TMT (2003) Corneal nerves: structure, contents and function. Exp Eye Res 76: 521-542. [Crossref]
- Oliveira-Soto L, Efron N (2001) Morphology of corneal nerves using confocal microscopy. Cornea 20: 374. [Crossref]
- Gorlin RJ, Sedano HO, Vickers RA, Cervenka J (1968) Multiple mucosal neuromas, pheochromocytoma and medullary carcinoma of the thyroid--a syndrome. Cancer 22: 293-299. [Crossref]
- Baum and Jules L (1972) Pheochromocytoma, medullary thyroid carcinoma, multiple mucosal neuroma. A variant of the syndrome. Arch Ophthalmol 87: 574. [Crossref]
- Kane LA, Tsai MS, Gharib H, Khosla S, Robertson DM, et al. (1995) Familial medullary thyroid cancer and prominent corneal nerves: clinical and genetic analysis. J Clin Endocrinol Metab 80: 289-293. [Crossref]
- Paine RS (1956) A clinical, pathological and genetic study of multiple neurofibromatosis. Am J Hum Genet 24: 149-149. [Crossref]
- Samuelsson B, Axelsson R (1981) Neurofibromatosis. A clinical and genetic study of 96 cases in Gothenburg Sweden. Acta Derm Venereol Suppl 95: 67-71. [Crossref]
- Huson SM (1989) A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genetics 26: 704-711. [Crossref]
- Kinori M, Hodgson N, Zeid JL (2018) Ophthalmic manifestations in neurofibromatosis type 1. Survey of Ophthalmol 63: 518-533. [Crossref]
- Moramarco A, Sacchetti M, Franzone F, Segatto M, Cecchetti D, et al. (2020) Ocular surface involvement in patients with neurofibromatosis type 1 syndrome. Graefes Arch Clin Exp Ophthalmol. 258: 1757-1762. [Crossref]
- Javadi MA (2012) Confocal scan imaging and impression cytology of the cornea in a case of multiple endocrine neoplasia type-2b. J Ophthalmic Vis Res 7: 176-179. [Crossref]
- Takai S (1992) Prominent corneal nerves in patients with multiple endocrine neoplasia type 2A: diagnostic implications. World J Surg 16: 620-623. [Crossref]
- Kim SK, Dohlman CH (2001) Causes of enlarged corneal nerves. Int Ophthalmol Clin 41: 13-23. [Crossref]
- Eter N, Klingmüller D, Höppner W, Spitznas M (2001) Typical ocular findings in a patient with multiple endocrine neoplasia type 2b syndrome. Graefes Arch Clin Exp Ophthalmol 239: 391-404. [Crossref]
- Malhotra C (2016) In Vivo Confocal Microscopic Architecture of Corneal Nerves in a Case of Multiple Endocrine Neoplasia Type 2b. Middle East Afr J Ophthalmol 23: 326-328. [Crossref]
- Lam D (2019) In Vivo Confocal Microscopy of Prominent Conjunctival and Corneal Nerves in Multiple Endocrine Neoplasia Type 2B. Cornea 38: 1453-1455. [Crossref]
- Jacobs JM, Hawes MJ (2001) From eyelid bumps to thyroid lumps: report of a MEN type IIb family and review of the literature. Ophthalmic Plast Reconstr Surg 17: 195-201. [Crossref]
- Spyer G (2006) Phenotypic multiple endocrine neoplasia type 2B, without endocrinopathy or RET gene mutation: implications for management. Thyroid 16: 605-608. [Crossref]
- Owens M, Kivuva E, Quinn A, Brennan P, Caswell R, et al. (2016) SOS1 frameshift mutations cause pure mucosal neuroma syndrome, a clinical phenotype distinct from multiple endocrine neoplasia type 2B. Clin Endocrinol (Oxf) 84: 715-719. [Crossref]




