Abstract
Eucommia ulmoides is a traditional herbal medicine known to have various systemic effects. In recent years, the bone pharmacological properties of Eucommia ulmoides leaf have been reported. However, studies on the effect of Eucommia ulmoides leaf on bone-related cells are insufficient. Therefore, this study aimed to investigate the efficacy of Eucommia leaf extract (ELE) for osteoblast proliferation and maturation by using primary cultured osteoblasts. Primary osteoblast cultures received constant applications of 10 mg/ml ELE up to 28 days in vitro (DIV). Alkaline phosphatase activity and Ca2+ accumulation were significantly increased after 28 DIV. In addition, 10 mg/ml ELE application significantly increased the intracellular Ca2+ concentration after 14 DIV and significantly increased the expression of RANKL and Col1a2 mRNA after 7 DIV on RT-qPCR. However, ELE application did not affect 3-(4, 5-dimethylthiazol-2-yl) 2, 5-diphenyl tetrazolium bromide (MTT) reduction in a MTT assay. These results suggest that ELE application has no effect on proliferation of osteoblasts but has a regulatory effect on osteoblast ossification. From the standpoint of prophylactic pharmacology, drinking ELE tea as a self-medication potentially could prevent metabolic bone disorders.
Keywords
Eucommia leaf extract (ELE), osteoblast, osteogenesis, prophylactic pharmacology, self-medication
Introduction
Eucommia ulmoides is a deciduous tree native to China. The Eucommia ulmoides bark is used as a traditional herbal medicine, which is described in the Shennong Bencaojing that was compiled >1600 years ago in China. It is said that Eucommia ulmoides bark has no side effects, and it has been used to treat kidney dysfunction and as an analeptic. The Eucommia ulmoides leaves are edible, and a decoction of the leaves is drunk as a tea in China, Korea, and Japan for health promotion. Eucommia ulmoides is known to have various systemic effects. It is known that various ingredients are contained in Eucommia ulmoides [1]. Although the component concentrations are different, the ingredients contained in the Eucommia ulmoides bark as a medicine are also contained in Eucommia ulmoides leaves as a food. This means that it is possible to easily purchase “natural” active ingredients with no side effects as foods in the market. In addition, it is possible that ingestion of foods considered to be effective for health can be a form of preventive self-medication against possible future diseases.
In recent aging society, it is expected that bone metabolic diseases, such as osteoporosis, will occur more frequently in elderly people. However, bone metabolic diseases were already known in Chinese medicine from much earlier times. Eucommia ulmoides bark also is a traditional Chinese medicine that is used to treat bone metabolic diseases. Indeed, applications of the lignan component extracted from Eucommia ulmoides bark to postmenopausal osteoporosis model rats by ovariectomy have been reported to prevent osteoporotic lesions [2]. Additionally, application of Eucommia leaf extract (ELE), which is a food product, to postmenopausal osteoporosis model rats by ovariectomy has been found to have a preventive effect on osteoporotic lesions [3]. Therefore, it is possible that ingestion of ELE as a food could prevent bone metabolic diseases. However, the effect of ELE on ossification has not been fully clarified at present. This study aimed to investigate the efficacy of ELE on osteoblast proliferation and maturation by using primary cultured osteoblasts.
Material and Methods
Preparation of ELE
In this study, we used Eucommia leaves collected in the Sichuan District of China. To prepare the ELE, fresh Eucommia leaves were steamed at 100–110°C and then dried and roasted. Two tons of roasted Eucommia leaves were steeped in 10 tons of hot water at 90°C for 1 h, and the extract was then filtered and concentrated. The concentrate was allowed to stand for 1 day. The concentrate was then filtered and concentrated, vacuum-dried, and powdered (yield: 18%) as previously described [4].
Preparation of primary cultured osteoblasts
Appropriate animal experiments that considered animal welfare were conducted according to the “Suzuka University of Medical Science Animal Experiment Guide.” All procedures were also performed according to the National Institutes of Health guidelines regarding the principles of animal care (1996). All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques.
Under pentobarbital (Kyoritsu Seiyaku Corporation. Tokyo, Japan) anesthesia conditions, osteoblasts were isolated from the calvaria of 1-to-2-day-old Wistar neonatal rats. In brief, rat calvaria were gently incubated at 37°C for 10 min with 0.2% (w/v) collagenase (Worthington Biochemical Corporation. NJ, USA) in a-modified minimum essential medium (a-MEM) (Wako Pure Chemical Industries, Ltd., Osaka, Japan), followed by collection of cells in supernatants thus obtained. This incubation was consecutively repeated 5 times. Then, the last 3 digestion supernatants were collected together in a-MEM containing 10% fetal bovine serum (FBS) and 10-units/ml penicillin–10 mg/ml streptomycin (Wako Pure Chemical Industries), followed by centrifugation at 1,500 rpm for 5 min. The pellets were suspended in a-MEM containing 10% FBS. Cells were plated at a density of 1 × 104 cells/ml in appropriate dishes, and then cultured at 37°C for different periods under 5% CO2 with medium change every 3 days. Throughout the experiments, a-MEM containing 10% FBS, 50 mg/ml ascorbic acid (Sigma-Aldrich Co. LLC., MO, USA), 5 mM sodium b-glycerophosphate (Sigma-Aldrich), and 40 mM NaHCO3 (Sigma-Aldrich) were used [5].
ELE treatment protocol
The adjusted primary osteoblast cultures were divided into the control group and the ELE 10 μg/mL application group. Then, the groups were cultured for ≤28 days, and the medium was changed every 3 days in vitro (DIV). Osteoblast cultures of 7 DIV, 14 DIV, and 28 DIV periods were used in each experiment.
MTT reduction assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide, yellow tetrazole (MTT) (DOJINDO LABORATORIES, Kumamoto, Japan) assay is a method for examination of the viability and growth rate of cultured cells. The MTT is reduced to violet-colored insoluble formazan in living cells. Then, the obtained formazan is quantified by absorptiometry. In this study, MTT assays using cultured osteoblasts of the control group and ELE application group for 7, 14, and 28 DIV periods were performed. The cultured osteoblasts for different periods were washed in phosphate-buffered saline (PBS). Subsequently, the MTT substrate in PBS (500 µg/mL) was added to osteoblast cells, and the cells were maintained for 1 hour in a CO2 incubator at 37°C. After the incubation, isopropanol/0.04 N HCl was added to MTT-treated cells, and the cells were agitated to promote elution of formazan. Then, the supernatants were quantified by measuring the absorbance at 550 nm in a SpectraMax M5e microplate reader (Molecular Devices, Minneapolis, MN, USA) [6].
Determination of ALP activity
Osteoblasts cultured for each period were washed with PBS and then sonicated in 0.1 M Tris–HCl buffer (pH 7.5) containing 0.1% Triton X-100. The assay buffer composed of 0.05 M 2-amino-2-methylpropanol, 2 mM MgCl2, and 10 mM p-nitrophenylphosphoric acid was added at a volume of 200 ml into 10 ml of cell suspensions, followed by a reaction for 30 min at 37°C and subsequent immediate determination of absorbance of p-nitrophenol at 405 nm in a SpectraMax M5e microplate reader (Molecular Devices, Minneapolis, MN, USA). Simultaneously, the protein concentrations of cell suspension were determined by using the Bradford method, and the obtained alkaline phosphatase (ALP) activities were standardized by the protein concentration and the incubation time of the ALP assay [5].
Determination of Ca2+ concentration
Osteoblasts cultured for each period were washed with PBS and then sonicated in 0.1 M Tris–HCl buffer (pH 7.5) containing 0.1% Triton X-100. HCl at 6 M was added to these cell lysates to make a final concentration of 2 M HCl for calcium release. Then, the cell suspensions were incubated for 16 to 24 hours at room temperature. Finally, the cell suspensions were centrifuged at 15,000 rpm for 5 min, and the Ca2+ content was determined in the supernatant by using a calcium E-TEST kit (Wako Pure Chemical Industries, Ltd. Osaka, Japan) [5].
Determination of Ca2+ accumulation by Von Kossa staining
Osteoblasts cultured for each period were washed with PBS and fixed with 4% paraformaldehyde for 10 minutes. The cells were then washed twice with ddH2O, followed by the addition of 5% silver nitrate (KANTO CHEMICAL CO., INC., Tokyo, Japan) to the cells and exposure to ultraviolet light for 1 hour. Next, the silver nitrate was removed, and the cells were washed twice with ddH2O followed by addition of 5% sodium thiosulfate (Sigma-Aldrich) to the cells for 5 min to stop the reaction. Thereafter, the cells were washed with ddH2O and 30% glycerol was then added to the cells. The stained cells were observed on an inverted microscope and photographed to capture the area of mineralization, which was indicated by black staining. Ten images per culture dish well were randomly acquired, and then the mineralized area was quantified by using Image J software [6].
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from osteoblasts cultured for each period by using the standard TriPure Isolation Reagent procedure (Roche Holding AG. Basel, Swaziland). After total RNA extraction, the RNA was subjected to synthesis of the first-strand cDNA by using a ReverTraAce qPCR RT kit (Toyobo CO., LTD., Osaka, Japan). The cDNA (50 ng/μl) were analyzed by RT-PCR using a FastStart Universal SYBER Green Master mix (Roche Holding AG, Basel, Swaziland) and an ABI PRISM 7300 system (Applied Biosystems). Individual cDNAs were ampli?ed in a reaction mixture containing a cDNA aliquot, 2 × master mix of FastStart Universal SYBR Green Master (Rox) (Roche Holding AG) and the relevant sense and antisense primers. The amplification program included an initial denaturation step at 95°C for 10 min, 40 cycles of denaturation at 95°C for 15 s, and annealing/extension at 59°C–60°C for 1 min. The oligonucleotide primer sequences are shown in Table 1. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels were used as an internal control. Each gene was normalized to GAPDH by subtracting the cycle threshold (Ct) value of GAPDH from the Ct value of the target gene (CT [target]). The relative expression of the target gene was calculated by using sodium dodecyl sulfate v1.2 with RQ software (Applied Biosystems), with ΔCt[target] compared with the ΔCt values of the reference; i.e., ΔΔCt = ΔCt[target] − ΔCt[reference]. The degree of difference (expressed in fold difference) between the target and reference was calculated as 2−ΔΔCt [4].
Table 1. Primers sequence
Gene Name |
Accession No |
Forward primer (5’- 3’) |
Reverse primer (5’ – 3’) |
ALP |
J03572.1 |
gatggtatgggcgtctccac |
atctccagccgtgtctcctc |
RANKL |
NM 057149.1 |
ggccaagatctctaacatga |
ccatcagctgaagatagtcc |
Runx2 |
NM 001278483.1 |
ttcgtcagcgtcctatcagttc |
cttccatcagcgtcaacacc |
Col1a1 |
NM 053304.1 |
tgcaacatggagacaggtcag |
cttcttctccttggggtttgg |
Col1a2 |
NM 053356.1 |
cattctgcagggctccaac |
gcaggcgagatggcttattc |
BMP-2 |
NM 017178.1 |
catgtgaggattagcaggtctttg |
gcttccgctgtttgtgtttg |
Osteocalcin |
NM 013414.1 |
ctgcattctgcctctctgacc |
ccttactgccctcctgcttg |
Osteopontin |
M 99252.1 |
ggtgaaagtggctgagtttgg |
ctgcttctgagatgggtcagg |
Osterix |
AY 177399.1 |
atggcgtcctctctgcttg |
gcttctttgtgcctccttttc |
ALP; alkaline phosphatase, RANKL; receptor activator of NF-kappaB ligand, Runx2; Runt-related transcription factor 2, Col1a1; Collagen, type I, alpha 1, Col1a2; Collagen, type I, alpha 2, BMP-2; bone morphogenetic protein 2,; |
Statistical analysis
Data analysis results were expressed as the mean ± S.E., and statistical significance was determined by one-way analysis of variance (ANOVA) with Tukey’s test and/or Dunnett’s test.
Results
Efficacy of ELE for osteoblast proliferation
Osteoblast differentiation and maturation were examined by using cell proliferation as determined by MTT reduction rates. Therefore, the MTT reduction was examined for each culture day (Figure 1). The osteoblasts of 7 DIV showed high MTT reduction rates, which indicated that cell proliferation was actively occurring. However, high MTT reduction rates were not observed after 14 DIV in the control group and 10 mg/ml ELE application group. The MTT reduction rates after 14 DIV and 28 DIV were significantly decreased relative to those after 7 DIV, which indicated that the proliferative function of osteoblasts was not as active in both groups (Figure 1). In addition, even if ELE at a concentration of 10 mg/ml was constantly applied, the application did not influence osteoblast proliferation on any of the culturing days (Figure 1).
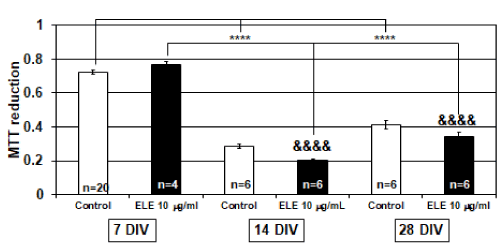
Figure 1. Efficacy of ELE for osteoblast proliferation
The graph shows the MTT reduction level in the control group and ELE 10 mg/ml application group in several culture days’ primary osteoblasts. In 7, 14, and 28 days in vitro (DIV), MTT reduction’s changes were not observed by ELE 10 mg/ml application. And in 14 and 28 DIV, the cells were significantly decreased the MTT reduction compared with 7 DIV cells. Each value represent the mean ±SEM, ****p<0.0001, &&&&p<0.0001. (Dunnett)
Efficacy of ELE for ALP activity
It is known that osteoblasts promote ossification by secreting ALP when osteoblasts are hyperactive. Therefore, we examined the effect of ELE application on osteoblast differentiation by using ALP activity as an index. We found that ALP activity increased as the number of culture days progressed, and differentiation was promoted after cell proliferation settled. In the 10 mg/ml ELE application group, significantly increased ALP activity was observed after 14 DIV and 28 DIV relative to that after 7 DIV, which indicated that the ALP activities were significantly increased even when 14 DIV and 28 DIV. Similar tendencies were observed in the control group. Additionally, significant increases in ALP activity were observed between 7 DIV and 28 DIV and between 14 DIV and 28 DIV (Figure 2). As described above, cell proliferation was actively occurring after 7 DIV (Figure 1), and ALP activities were not different between the control group and the 10 mg/ml ELE application group (Figure 2). However, although active cell proliferation was not detected after 28 DIV (Figure 1), a significant increase in ALP activity was detected by 10 mg/ml ELE application after 28 DIV (Figure 2).
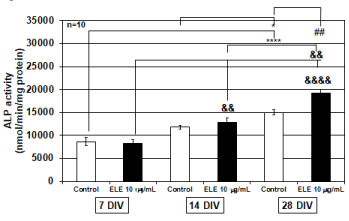
Figure 2. Efficacy of ELE for ALP activity
The graph shows the alkaline phosphatase (ALP) activity in the control group and ELE 10 mg/ml application group in several culture days’ primary osteoblasts. In the control group, the ALP activity was significantly elevated from 7 DIV to 28 DIV and from 14 DIV to 28 DIV, whereas the ALP activity significantly increased in the ELE 10 mg/ml application group in association with the increase in the number of culture days. In addition, it was observed that the ALP activity was significantly increased by ELE 10 mg/ml application as compared with the control group on 28 DIV. Each value represent the mean ±SEM, *p<0.05, ****p<0.0001, &&p<0.01, &&&&p<0.0001, ##p<0.01. (Turkey)
Efficacy of ELE for Ca2+ contents
The changes in intracellular Ca2+ concentration required for osteoblasts to cause osteogenesis were investigated by using cultured osteoblasts of each culture day. As a result, in both the control group and 10 mg/ml ELE application group, significant increases in the concentration of Ca2+ in the cultured osteoblasts were observed with increasing number of culture days. In addition, after 14 DIV, 10 mg/ml ELE application significantly increased the intracellular Ca2+ concentration in the osteoblasts. After 28 DIV, the control group’s intracellular Ca2+ concentration in the osteoblasts was the same as that in the ELE application group (Figure 3).
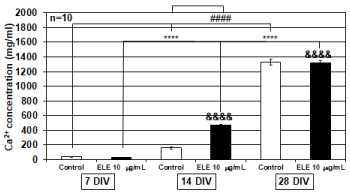
Figure 3. Efficacy of ELE for Ca2+ contents
The graph shows the Ca2+ contents levels in the control group and ELE 10 mg/ml application group in several culture days’ primary osteoblasts.
In both the control group and the ELE 10 mg/ml application group, a significant increase in the Ca2+ concentration of the cultured osteoblasts was observed with the increase in the number of culture days. In addition, on the 14 DIV, the Ca2+ concentration of cultured osteoblasts was significantly increased by ELE application compared with the control group. Each value represent the mean ±SEM, ****p<0.0001, &&&&p<0.0001, ####p<0.0001. (Tukey)
Efficacy of ELE for Ca2+ accumulation
Von Kossa staining was continuously performed to examine the efficacy of ELE application on Ca2+ accumulation. Von Kossa staining is a method that stains calcium phosphate black, which enables detection of the accumulated Ca2+. Therefore, osteoblasts cultured for 14 DIV in which the intracellular calcium concentration had increased were used for Von Kossa staining. Consequently, 14-DIV osteoblasts did not show any significant change in Ca2+ accumulation caused by ELE application. However, the 28-DIV osteoblasts showed that significant Ca2+ accumulation was induced by 10 mg/ml ELE application (Figure 4).
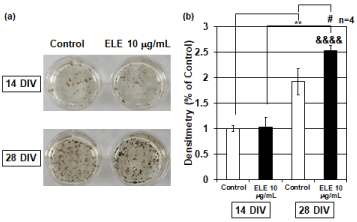
Figure 4. Efficacy of ELE for Ca2+ accumulation
(a) The photographs show the Von Kossa staining results in the control group and ELE 10 μg/ml application group. Black spots are silver phosphate precipitates, which are caused by reaction of silver ions with phosphate of calcium phosphate. (b) The graph shows the Ca2+ accumulation levels in the control group and ELE 10 mg/ml application group in several culture days’ primary osteoblasts. No change in Ca2+ accumulation due to the ELE 10 μg/ml application was observed at 14 DIV compared to the control group. However, a significant increase in Ca2+ accumulation was observed by ELE 10 μg/ml application at 28 DIV. Each value represent the mean ±SEM, **p<0.01, &&&&p<0.0001, #p<0.05. (Turkey)
Efficacy of ELE for osteoblast’s differentiation markers
In turn, osteoblast gene samples from each culture day were examined by using various differentiation marker primers of osteoblasts on RT-qPCR. The various differentiation markers changed according to the number of culture days in the early, middle, and terminal stages of differentiation and the dynamics of known differentiation markers could be reproduced. However, the 10 mg/ml ELE application groups did not show any significant changes in the following differentiation marker’s mRNA expression levels: ALP, Runx 2, Colla 1, BMP-2, osteocalcin, osteopontin, Osterix . On the other hand, the mRNA expression levels of the RANKL and Col1a2 differentiation markers were significantly increased by 10 mg/ml ELE application after 7 DIV (Figure 5).
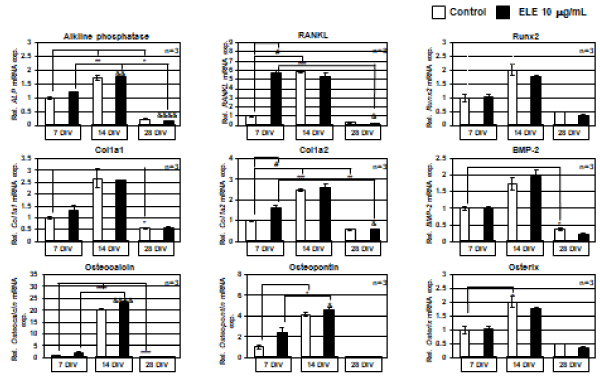
Figure 5. Efficacy of ELE for osteoblast’s differentiation markers
The graphs show the RT-qPCR results of mRNA expression level changes of osteoblast differentiation marker by ELE 10 μg/ml application. The mRNA expression level changes of ALP, Runx 2, Col1a1, BMP-2, Osteocalcin, Osteopontin, and Osterix were not observed by ELE 10 μg/ml application at every culture days. However, in RANKL and Col1a2, the mRNA expression levels were significantly increased at 7 DIV. Each value represent the mean ±SEM, **p<0.01, &&&&p<0.0001, #p<0.05. (Tukey)
Discussion
The effect of Eucommia ulmoides on bone metabolism in vivo have been investigated in ovariectomized rats [3] and granulomas of aged model rats [7] . On the other hand, the effect of Eucommia ulmoides on bone metabolism in vitro have been investigated in osteoblast-like cell lines [8] and co-cultured osteoblasts with osteoclasts [9]. In a report on the effect of Eucommia ulmoides leaf as a food on bone metabolism, the collagen content in granulomas of aged model rats was increased by administration of ELE [7] and the osteoporosis-like symptoms were prevented in ovariectomized rats by ELE administration [3]. In addition, it has been reported that ELE exerted an antioxidant effect by suppressing oxidative stress, and the effect increased with age and maintained the function of osteoblasts [8]. However, studies on the effect of ELE on bone-related cells are insufficient. According to the MTT assay results in this study, ELE did not affect osteoblast proliferation. This finding is in contrast to the finding in a previous study in which the lignan component contained in the Eucommia ulmoides bark promoted osteoblast proliferation [2]. This discrepancy might have been caused by differences between the components of the Eucommia ulmoides bark and Eucommia ulmoides leaves. Indeed, the main components of the Eucommia ulmoides leaves are not lignans [1,10]. On the other hand, the present study showed that ALP activity was significantly increased by 10 mg/ml ELE application in 28 DIV osteoblasts, and the accumulation of calcium phosphate was also significantly increased by 10 mg/ml ELE application. In addition, the increase in intracellular Ca2+ concentration caused by 10 mg/ml ELE application was already significantly increased in 14 DIV osteoblasts, and it is inferred that the preparation for calcium accumulation is significantly advanced. These results suggest that ELE has an accelerating effect on the osteogenesis of osteoblasts. The RT-qPCR results suggested that the differentiation of osteoblasts by ELE application might have an accelerating effect at an early stage of osteoblasts. Osteoblasts differentiate from mesenchymal stem cells and progressively differentiate into osteoprogenitor cells, pre-osteoblasts, osteoblasts, mature osteoblasts, and osteocytes [11]. In the present study, Col1a2 was observed during osteoblast differentiation to be significantly induced by ELE application in the osteoprogenitor cell stage. Col1a2 is a type I collagen known as a bone matrix [12]. In addition, promotion of osteoblast calcification was observed by ELE application. These findings suggest that the promoting effect of osteoblast calcification by ELE application is facilitated by expression of Col1a2 in the osteoprogenitor cell stage and increased Ca2+ accumulation in the collagen matrix in the mature osteoblasts stage. This possibility is consistent with previous findings that ELE application increased collagen content [13,14]. On the other hand, a significant increase in RANKL mRNA, which activates osteoclasts in the early stage of osteoblast differentiation, was observed in the present study. This suggests the possibility that remodeling is promoted by renewing bone metabolism.
According to recent reports, Eucommia ulmoides bark and Eucommia ulmoides leaf have shown the following various effects on health: hepatoprotective [15,16], neuroprotective and hypnotic [17-22], anti-obesity and anti-metabolic syndrome [10,23-26], antibacterial [27], antiviral [28], anti-inflammatory [29,30], inhibitory action against diabetic complications [31-33], antioxidative [8,34-37], antihypertensive [38-41], anticancer [42,43] and bone metabolism effects [2,3,7-9,13,14,44-47].
Conclusion
These various findings clearly show that Eucommia ulmoides is a traditional herbal medicine that has systemic effects. In addition, Eucommia ulmoides leaves are treated as food, not pharmaceuticals, and can be purchased as a tea at neighborhood supermarkets at low prices. The results of the present study suggest that continued ingestion of ELE from an early age promotes ossification and may lower the risk of future bone metabolic disorders. From the standpoint of prophylactic pharmacology, ingestion of ELE is considered very useful for preventive self-medication.
Acknowledgments
This study was supported by a research grant from the Japanese Society of Eucommia. The Eucommia leaf extract samples used in this study were provided by KOBAYASHI Pharmaceutical Co., Ltd. (Osaka, Japan).
Conflict of interest
There are no conflicts of interest to declare.
References
- Hussain T, Tan B, Liu G, Oladele OA, Rahu N, et al. (2016) Health-Promoting Properties of Eucommia ulmoides: A Review. Evid Based Complement Alternat Med 2016: 5202908. [CrossRef]
- Zhang R, Pan YL, Hu SJ, Kong XH, Juan W, et al. (2014) Effects of total lignans from Eucommia ulmoides barks prevent bone loss in vivo and in vitro. J Ethnopharmacol 155: 104-112. [CrossRef]
- Zhang W, Fujikawa T, Mizuno K, Ishida T, Ooi K, et al. (2012) Eucommia leaf extract (ELE) prevents OVX-induced osteoporosis and obesity in rats. Am J Chin Med 40: 735-752. [CrossRef]
- Fujikawa T, Hirata T, Hosoo S, Nakajima K, Wada A, et al. (2012) Asperuloside stimulates metabolic function in rats across several organs under high-fat diet conditions, acting like the major ingredient of Eucommia leaves with anti-obesity activety. J Nutr Sci 1: e10. [CrossRef]
- Fujimori S, Osawa M, Iemata M, Hinoi E, Yoneda Y (2006) Increased GABA transport activity in rat calvarial osteoblasts cultured under hyperglycemicconditions. Biol Pharm Bull 29: 297-301. [CrossRef]
- Sá MA, Ribeiro HJ, Valverde TM, Sousa BR, Martins-Júnior PA, et al. (2016) Single-walled carbon nanotubes functionalied with sodium hyaluronate enhance bonemineralization. Braz J Med Biol Res 49: e4888.
- Hirata T, Ikeda T, Fujikawa T, Nishibe S (2014) Chapter 8 – The Chemistry and Bioactivity of Eucommia ulmoides Oliver Leaves. Studies in Natural Products Chemistry, 41, ELSEVIER, 225-260.
- Lin J, Fan YJ, Mehi C, Zhu JJ, Chen H, et al. (2011) Eucommia ulmoides Oliv, antagonizes H2O2-induced rat osteoblastic MC3T3-E1 apoptosis by inhibiting expressions of caspases 3, 6, 7, and 9. J Zhejiang Univ Sci B 12: 47-54. [CrossRef]
- Ha H, Ho J, Shin S, Kim H, Koo S, et al. (2003) Effects of Eucommiae Cortes on osteoblast-like cell proliferation and osteoclast inhibition. Arch Pharm Res 26: 929-936.
- Fujikawa T, Hirata T, Wada A, Kawamura N, Yamaguchi Y, et al. (2010) Chronic administration of Eucommia leaf stimulates metabolic function of rats across several organs. Br J Nutr 104: 1868-1877. [CrossRef]
- Siddiqui JA, Partridge NC (2016) Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology (Bethesda) 31: 233-245. [CrossRef]
- Ricard-Blum S, Ruggiero F (2005) The collagen superfamily: from the extracellular matrix to the cell membrane. Pathol Biol 53: 430-442. [CrossRef]
- Li Y, Sato T, Metori K, Like K, Che QM, et al. (1998) The promoting effects of geniposidic acid and aucubin in Eucommia ulmoides Oliver leaves on collagen synthesis. Biol Pharm Bull 21: 1306-1310. [CrossRef]
- Li Y, Kamo S, Metori K, Koike K, Che QM, et al. (2000) The promoting effect of eucommiol from Eucommiae cortex on collagen synthesis. Biol Pharm Bull 23: 54-59. [CrossRef]
- Jin CF, Li B, Lin SM, Yadav RK, Kim HR, et al. (2013) Mechanism of the Inhibitory Effects of Eucommia ulmoides Oliv. Cortex Extracts (EUCE) in the CCl 4 -Induced Acute Liver Lipid Accumulation in Rats. Int J Endocrinol 2013: 751854.
- Lv PY, Feng H, Huang WH, Tian YY, Wang YQ, et al. (2017) Aucubin and its hydrolytic derivative attenuate activation of hepatic stellate cells via modulation of TGF-b stimulation. Environ Toxicol Pharmacol 50: 234-239. [CrossRef]
17.Zhou Y, Liang M, Li W, Li K, Li P, et al. (2009) Protective effects of Eucommia ulmoides Oliv. bark and leaf on amyloid b-induced cytotoxicity. Environ Toxicol Pharmacol 28: 342-349. [CrossRef]
- Kwon SH, Lee HK, Kim JA, Hong SI, Kim SY, et al. (2011) Neuroprotective effects of Eucommia ulmoides Oliv. Bark on amyloid beta (25-35) -induced learning and memory impairments in mice. Neurosci Lett 487: 123-127.
- Guo H, Shi F, Li M, Liu Q, Yu B, et al. (2015) Neuroprotective effects of Eucommia ulmoides Oliv. and its bioactive constituent work via ameliorating the ubiquitin-proteasome system. BMC Complement Altern Med 15: 151. [CrossRef]
- Li CP, Qiu GZ, Liu B, Chen JL, Fu HT (2016) Neuroprotective effect of lignans extracted from Eucommia ulmoides Oliv. on glaucoma-related neurodegeneration. Neurol Sci 37: 755-762. [CrossRef]
- Li X, Tang Z, Fei D, Liu Y, Zhang M, et al. (2016) Evaluation of the sedative and hypnotic effects of astragalin isolated from Eucommia ulmoides leaves in mice. Nat Prod Res 26: 1-5. [CrossRef]
- Wang J, Li Y, Huang WH, Zeng XC, Li XH, et al. (2017) The Protective Effect of Aucubin from Eucommia ulmoides Against Status Epilepticus by Inducing Autophagy and Inhibiting Necroptosis. Am J Chin Med 45: 557-578. [CrossRef]
- Choi MS, Jung UJ, Kim HJ, Do GM, Jeon SM, et al. (2008) Du-zhong (Eucommia ulmoides Oliver) leaf extract mediates hypolipidemic action in hamsters feda high-fat diet. Am J Chin Med 36: 81-93. [CrossRef]
24.Jin X, Amitani K, Zamami Y, takatori S, Hobara N, et al. (2010) Ameliorative effect of Eucommia ulmoides Oliv. leaves extract (ELE) on insulin resistance and abnormal perivascular innervation in fructose-drinking rats. J ethnopharmacol 128: 672-678. [CrossRef]
- Hirata T, Kobayashi T, Wada A, Ueda T, Fujikawa T, et al. (2011) Anti-obesity compounds in green leaves of Eucommia ulmoides. Bioorg Med Chem Lett 21: 1786-1791. [CrossRef]
- Kobayashi Y, Hiroi T, Araki M, Hirokawa T, Miyazawa M, et al. (2012) Facilitative effects of Eucommia ulmoides on fatty acid oxidation in hypertriglyceridaemic rats. J Sci Food Agric 92: 358-365. [CrossRef]
- Zhang L, Ravipati AS, Koyyalamudi SR, Jeong SC, Reddy N, et al. (2013) Anti-fungal and anti-bacterial activities of ethanol extracts of selected traditional Chinese medicinal herbs. Asian Pac J Trop Med 6: 673-681. [CrossRef]
- Nakano M, Nakashima H, Itoh Y (1997) Anti-human immunodeficiency virus activity of oligosaccharides from rooibos tea (Aspalathus linearis) extracts in vitro. Leukemia 3: 128-130. [CrossRef]
29.Kwon SH, Ma SX, Hwang JY, Ko YH, Seo JY, et al. (2016) The Anti-Inflammatory Activity of Eucommia ulmoides Oliv. Bark. Involves NF-κB Suppression and Nrf2-Dependent HO-1 Induction in BV-2 Microglial Cells. Biomol Ther (Seoul) 24: 268-282. [CrossRef]
30.Wang JY, Yuan Y, Chen XJ, Fu SG, Zhang L, et al. (2016) Extract from Eucommia ulmoides Oliv. ameliorates arthritis via regulation of inflammation, synoviocyte proliferation and osteoclastogenesis in vitro and in vivo. J Ethnopharmacol 194: 609-616. [CrossRef]
- Niu HS, Liu IM, Niu CS, Ku PM, Hsu CT, et al. (2016) Eucommia bark (Du-Zhong) improves diabetic nephropathy without altering blood glucose in type 1-like diabetic rats. Drug Des Deve Ther 10: 971-978. [CrossRef]
- Sugawa H, Ohno R, Shirakawa J, Nakajima A, Kanagawa A, et al. (2016) Eucommia ulmoides extracts prevent the formation of advanced glycation end products. Food Funct 7: 2566-2573. [CrossRef]
- Liu B, Li CP, Wang WQ, Song SG, Liu XM (2016) Lignans Extracted from Eucommia Ulmoides Oliv. Protects Against AGEs-Induced Retinal Endothelial Cell Injury. Cell Physiol Biochem 39: 2044-2054. [CrossRef]
- Liu E, Han L, Wang J, He W, Shang H, et al. (2012) Eucommia ulmoides bark protects against renal injury in cadmium-challenged rats. J Med Food 15: 307-314. [CrossRef]
35.Dragland S, Senoo H, Wake K, Holte K, Blomhoff R (2003) Several culinary and medicinal herbs are important sources of dietary antioxidants. J Nutr 133: 1286-1290. [CrossRef]
36.Akinmoladun AC, Obuotor EM, Farombi EO (2010) Evaluation of antioxidant and free radical scavenging capacities of some Nigerian indigenousmedicinal plants. J Med Food 13: 444-451. [CrossRef]
- Hendra R, Ahmad S, Oskoueian E, Sukari A, Shukor MY (2011) Antioxidant, anti-inflammatory and cytotoxicity of Phaleria macrocarpa (Boerl.) Scheff Fruit. BMC Complement Altern Med 11: 110. [CrossRef]
- Jing X, Huang WH, Tang YJ, Wang YQ, Li H, et al. (2015) Eucommia ulmoides Oliv. (Du-Zhong) Lignans Inhibit Angiotensin II-Stimulated Proliferation by Affecting P21, P27, and Bax Expression in Rat Mesangial Cells. Evid Based Complement Alternat Med 2015: 987973.
- Yan JK, Ding LQ, Shi XL, Donkor PO, Chen LX, et al. (2017) Megastigmane glycosides from leaves of Eucommia ulmoides Oliver with ACE inhibitory activity. Fitoterapia 116: 121125.
- Hosoo S, Koyama M, Watanabe A, Ishida R, Hirata T, et al. (2017) Preventive effect of Eucommia leaf extract on aortic media hypertrophy in Wistar-Kyoto rats fed a high-fat diet. Hypertens Res 40: 546-551. [CrossRef]
- Takayama F, Fujihara Y (2017) How does Eucommia leaf extract prevent smooth muscle cell proliferation induced by high-fat diets at the aortic tunica media? Hypertens Res 40: 541-543. [CrossRef]
- Fujiwara A, Nishi M, Yoshida S, Hasegawa M, Yasuma C, et al. (2016) Eucommicin A, a b-truxinate lignan from Eucommia ulmoides, is a selective inhibitor of cancer stem cells. Phytochemistry 122: 139-145. [CrossRef]
- Li Q, Zhang Y, Shi JL, Wang YL, Zhao HB, et al. (2016) Mechanism and Anticancer Activity of the Metabolites of an Endophytic Fungi from Eucommiaulmoides Oliv. Anticancer Agents Med Chem 17: 982-989. [CrossRef]
- Zhang R, Liu ZG, Li C, Hu SJ, Liu L, et al. (2009) Du-Zhong (Eucommia ulmoides Oliv.) cortex extract prevent OVX-induced osteoporosis in rats. Bone 45: 553-559. [CrossRef]
- Pan Y, Niu Y, Li C, Zhai Y, Zhang R, et al. (2014) Du-zhong (Eucommia ulmoides) prevents disuse-induced osteoporosis in hind limb suspension rats. Am J Chin Med 42: 143-155. [CrossRef]
- Kim JY, Lee JI, Song M, Lee D, Song J, et al. (2015) Effects of Eucommia ulmoides extract on longitudinal bone growth rate in adolescent female rats. Phytother Res 29: 148-153. [CrossRef]
- Xie GP, Jiang N, Wang SN, Qi RZ, Wang L, et al. (2015) Eucommia ulmoides Oliv. bark aqueous extract inhibits osteoarthritis in a rat model of osteoarthritis. J Ethnopharmacol 162: 148-154. [CrossRef]





