Background: Limited data are available about the difference in propofol plasma levels estimated by target controlled infusion models (TCI) and measured plasma levels during prolonged operations. We hypothesized the difference to be greater at the end of surgery than at the beginning.
Methods: For this prospective observational study, adult patients were enrolled (elective surgery, estimated operating time of ≥ 3 hours, eligible to receive propofol using TCI (Schnider model)). Blood was sampled in steady state after anesthesia induction (propofol dosed to achieve BIS values of 40-60; T1) and at the end of the procedure (T2). Fluid balance was recorded. Bias and limits of agreement of actual plasma levels (Cblood) to TCI values (CTCI) were calculated, hemodynamics assessed, and performance error (PE) measured (PE(%) = ((Cblood–CTCI)/CTCI)*100) and assessed for correlations.
Results: Thirty patients were included. Mean duration of procedure was 250 min (± 136.7), fluid balance 2066 ml (± 1017), and all patients were hemodynamically stable. Mean CTCI was 2.0 µ/ml (± 0.3) and 1.8 µ/ml (± 0.6) at T1 and T2, respectively. Corresponding Cblood was 1.9 µ/ml (T1; ± 0.7; p=0.423), and 1.4 µ/ml (T2; ± 0.5; p=0.0001). PE was -4.2% (T1; IQR -31.3-10, range -64.3-115) and -27.8% (T2; IQR -44.4--9.1, range -61.1-82.4) (p=0.01513). There was a moderate correlation between PE at T2 and fluid balance (r= -0.41 [p=0.0269]).
Conclusion: TCI with additional monitoring (hemodynamic, EEG-based) allows for clinically unproblematic anesthetics. However, TCI propofol values calculated with the Schnider model may not be precise enough for scientific purposes, depending on the research question of the investigation.
propofol, target controlled infusion, general anesthesia
Propofol is one of the most widely used drugs for the administration of intravenous general anesthetics. If propofol is dosed at a constant rate based on the patient’s body weight (mg/kg/h), it has the tendency to accumulate in the blood and tissues over time [1].
Before the development of appropriate software and modern syringe pumps, the primary means of counteracting these increasing levels was to use standardized reduction protocols for long utilization periods and/or prolonged operations [2]. Later it became possible to replace the manual protocols with the automated and target controlled infusion (TCI) approach [2]. Using TCI, the operator can set the desired propofol plasma level to be reached and maintained at a constant level via a computer-controlled infusion pump. The system accounts for the duration of the infusion and patient characteristics such as age, sex, height and weight. The program deploys a multi-compartment pharmacokinetic model to make temporal adjustments of the propofol infusion for the duration of the anesthetic.
In pharmacokinetic studies and under laboratory conditions, the set TCI targets for propofol plasma levels correspond well with the measured blood plasma levels [3]. However, cases with potentially larger discrepancies between the TCI-calculated and the actual propofol plasma levels are conceivable. For example, if the TCI pharmacokinetic models was not derived from data including either obese patients or young children [4-6].
Furthermore, differences between the estimated TCI propofol plasma level and the actual measured concentration at the end of a prolonged surgery are to be estimated. The hypothesis of this study was that the difference between the TCI setting (Schnider model) and measured plasma levels was greater at the end of a long operation than at the beginning.
This prospective observational study was carried out after receiving approval by the local ethics committee (Ethikkomission Thurgau, KEKTGOV2015/09; 7th May 2015), registration with the German Register for Clinical Trials (www.DRKS.de, DRKS00009312), and obtaining the patients’ written informed consent.
Selection of subjects
We enrolled adult patients (18–75 years) who were scheduled to undergo elective surgery with general anesthesia for an estimated operating time of three hours or more but estimated blood loss of less than 1000 ml at the Cantonal Hospital in Frauenfeld (Kantonsspital Frauenfeld), Switzerland). In addition, these patients were eligible to receive a propofol-based intravenous anesthetic and an arterial line, as indicated per the local clinical guidelines. Patients with a body mass index (BMI, kg/m2) outside the pre-programmed range of the TCI model (approximately less than 16 or greater than 35) were not included in the study. Other exclusion criteria were hemodynamic instability or emergency surgery, allergy to propofol or any of its components, pregnancy, known or suspected hepatic disease, and intake of anti-epileptic drugs.
Study process
Patients received clinical care according to our internal guidelines and in accordance with the attending anesthesiologist’s clinical judgement. Throughout the procedure, normovolemia and normothermia were targeted. The volume status was individually gauged by integrating clinical and laboratory values such as blood pressure, heart rate, blood gas analysis, hemoglobin levels, diuresis, blood loss, and fluid administered. The transfusion threshold for packed red blood cells (PRBC) was at 70g/l, but was elevated to 80 or 90g/l for special clinical circumstances (i.e., patients with severe COPD, coronary heart disease, unstable circulatory situation, active bleeding).
Patients were given an oral dose of 7.5 mg midazolam before being transferred to the operating room. They received standard monitoring (non-invasive blood pressure, ECG, and pulse oximetry), at least two peripheral venous and an arterial cannula, as well as an EEG-based Bispectral Index (BIS) anesthetic monitor.
The anesthetic was conducted as a propofol-based, total intravenous anesthesia (TIVA), using a TCI syringe pump based on the Schnider pharmacokinetic model (Alaris PK Syringe Pump, CareFusion, Rolle, Switzerland) that was programmed with the patient’s age, weight, height, and gender. The standard setting for the anesthesia induction was a target effect site concentration (Cet) of 6 µ/ml. For the induction of anesthesia and endotracheal intubation, 1-3 µ/kg fentanyl and 0.5 mg/kg atracurium were also administered intravenously. After the placement of the endotracheal tube and for the maintenance portion of the anesthetic, the propofol Cet was reduced to result in a BIS value of 40-60.
Further management of the anesthetic, including opioid administration (fentanyl or remifentanil), paralysis with atracurium, admixture of volatile anesthetic (sevoflurane), and the fluid and transfusion management, was at the discretion of the attending anesthesiologist and in accordance with hospital standards.
Data Collection
Blood collection for study purposes was done after plasma concentrations of propofol reached a steady state. This was defined as CTCI - a match of the set target propofol effect site concentration (Cet), the calculated propofol plasma concentration (Cp) and the calculated propofol effect-site concentration (Ce) for at least 10 minutes.
An arterial blood gas analysis and arterial blood sample for the determination of the actual propofol plasma level was taken (T1) in the steady state at least 10 minutes after anesthesia induction and the reduction of the propofol plasma levels to a maintenance setting.
A second blood draw was performed in the identical manner towards the end of the procedure before initiating the patient to regain consciousness, with stable hemodynamic conditions, no bleeding, and the above mentioned propofol levels in steady state (T2).
The blood samples for the propofol plasma levels were immediately placed in an opaque tube, centrifuged and the plasma frozen at -70°C at the hospital’s central laboratory. The propofol plasma level measurements were carried out at the end of the study in an external laboratory (Institute for Forensic Medicine, University of Zurich, Zurich, Switzerland) by gas chromatography-mass spectrometry in single ion monitoring mode after liquid-liquid extraction (butyl-acetate). Propofol was quantified by comparison of its peak area ratio to calibration curves (propofol-D17 as internal standard; accuracy better than 15%).
In addition to the results and the time points of blood sampling, the following parameters were also recorded: patient demographic data (age, height, weight, and gender), infusion fluids and their volumes (crystalloids, colloids, blood products) that were given in between T1 and T2, urine output, estimated blood loss, duration of surgery, and body temperature. The hemodynamic stability was assessed by comparing heart rate, mean arterial pressure and lactate at times T1 and T2. Total fluid balances were calculated for each patient taking into account urinary output and the input volume of all liquids given in milliliters for the total balance.
Statistics
At both T1 and T2, measured propofol plasma levels were compared to predicted TCI levels (CTCI = Ce = Cet = Cp). The performance error (PE) was calculated, graphically displayed and assessed for relation to fluid balance by Pearson correlation. Hemodynamic stability (comparisons of heart rate, mean arterial pressure, and lactate values) was assessed by paired t-test or Wilcoxon signed rank test, as appropriate. The performance error (PE) and absolute performance error (APE) at both time points were determined using the following formula: PE (%) = ((Cblood – CTCI) / CTCI) * 100, and APE (%) = |(Cblood - CTCI)/CTCI * 100|, respectively. Divergence is the slope of a linear regression of APE against time (percentage change in APE per hour) for each individual and was calculated as follows: (APE2 - APE1)/((T2 – T1)/60). The exact Wilcoxon signed rank test was used to compare PE und APE at the two time points. A p-value of < 0.05 was considered statistically significant. All analyses were performed in the R programming language (version 3.3.3). The package “tableone” was used to compute descriptive statistics. The package “ggplot2” was used to plot the figures. The package “exactRankTests” was used to compute the exact Wilcoxon signed rank test.
Thirty ASA physical status II or III patients were included. The patients’ demographic characteristics and descriptive data about the surgical procedures are shown in Table 1. The mean administration of propofol was 1383 mg (± 548), the mean fentanyl was 0.5 mg (± 0.2), and the mean remifentanil was 2.4 g (± 1.5). The estimated mean blood loss was 230 ml (± 162). The total fluid balance at the end of surgery was + 2066 ml (± 1,017). All patients were clinically evaluated to be hemodynamically stable at the end of the procedure (Table 2). The mean body temperature at the end of surgery was 36°C (± 0.8).
Table 1. Demographic and descriptive data related to the procedures
Characteristic and study parameter |
Unit |
n = 30 |
Female sex |
n (%) |
23 (77 %) |
Age |
years |
55 (± 14) |
Height |
cm |
166 (± 6) |
Weight |
kg |
69 (± 13) |
Body mass index (BMI) |
kg / m2 |
25 (± 3.8) |
Procedure time |
min |
213 (IQR 175-261) |
Time between T1 and T2 |
min |
222 (IQR 176-270) |
Amount of infusion (crystalloids) |
ml |
2450 (IQR 2000-3100) |
Amount of infusion (colloids) |
ml |
0 (IQR 0-0) |
Transfusion quantity (PRBC and FFP) |
ml |
0 |
Transfusion quantity (Cell-Saver) |
ml |
0 |
Urine output |
ml |
400 (IQR 200-600) |
Data are presented as mean (± standard deviation) or median (IQR = interquartile range); T1 = blood collection after anesthesia induction, T2 = blood collection at the end of surgery; PRBC = packed red blood cells; FFP = fresh frozen plasma.
Table 2. Parameters for assessing hemodynamic stability at time points of blood collection
Parameter |
Unit |
T1 |
T2 |
p-Value |
Heart Rate |
1 / min |
68 (± 11) |
63 (± 11) |
*0.056 |
MAP |
mmHg |
70 (± 9.2) |
75 (± 11.5) |
*0.165 |
Hemoglobin |
g / l |
118 (IQR 110-128) |
112 (IQR 104-120) |
**0.0027 |
Lactate |
mmol / l |
0.7 (IQR 0.65-0.9) |
1 (IQR 1-1) |
**0.0229 |
Data are presented as mean (± standard deviation) or median (IQR = interquartile range); *paired t-test; **Wilcoxon signed-rank test; T1 = blood collection after anesthesia induction; T2 = blood collection at the end of surgery; MAP = mean arterial pressure.
The mean TCI level at the first time point was 2.0 µ/ml (± 0.3) and the blood measurement was 1.9 µ/ml (± 0.7) (mean difference: 0.12, 95% CI -0.18 – 0.42; p = 0.423). At the second time point, the mean measurements were as follows: TCI 1.8 µ/ml (± 0.6) and blood 1.4 µ/ml (± 0.5) (mean difference: 0.47, 95% CI 0.25 – 0.69; p = 0.0001). The median performance error (PE) at the first time point was -4.2% (IQR -31.3 - 10) and at the second time point -27.8% (IQR -44.4 - -9.1) (median difference: 17.22, 95% CI 2.75 – 30.61; Figure 1). The median absolute performance error (APE) at the first time point was 20.0% (IQR 6.5 - 33.3) and at the second time point 33.3% (IQR 15.0 - 44.4) (median difference: -8.61, 95% CI -19.84 – 5.00; Figure 2). There was a moderate correlation between PE at T2 and fluid balance (r= -0.41 [95% CI -0.68 - -0.05)]; Figure 3). Median divergence was 3.61 (IQR -1.59 - 5.31).
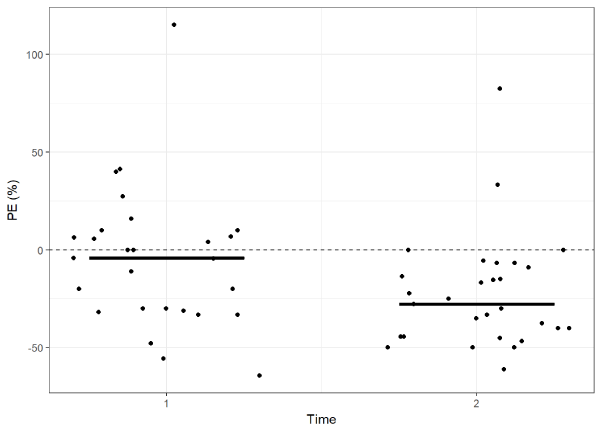
Figure 1.Distribution of performance errors of the CTCI and Cblood at T1 and T2 during prolonged surgeries (median difference: 17.22, 95% CI 2.75 – 30.61). Solid line represents median
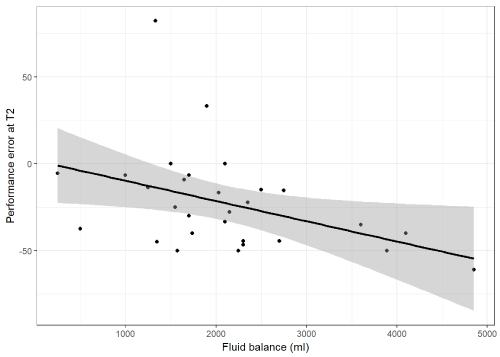
Figure 2. Absolute performance error (APE) for all patients at T1 and T2. Solid line represents median
Figure 3. Correlation between the performance error of the CTCI and Cblood and fluid balance at the end of prolonged surgery (r= -0.41, 95% CI -0.68 - -0.05 [p=0.0269])
In this study, propofol plasma levels calculated by a commercially available propofol target-controlled infusion system (TCI; Schnider model) were compared with laboratory-determined plasma levels. The blood samples for these measurements were collected under clinical conditions during prolonged surgical procedures, once after anesthesia induction and again at the end of surgery. The main result was that at the end of long-lasting surgery the performance error of propofol plasma levels predicted by the TCI model versus the laboratory levels was larger than in the beginning. The TCI model frequently overestimated the propofol levels at the end of these long procedures.
The dosing of propofol when used as the primary anesthetic should allow for adequate controllability in the clinical setting, while also guaranteeing sufficient anesthesia depth. These were the main reasons for the introduction of the target-controlled infusion method and systems [2]. TCI models have proven to provide drug administration with less or equal variability than manual infusion [7]. Propofol-based general anesthesia with TCI has been the standard primary anesthetic used in many institutions for years. However, TCI models are expected to over-predict plasma levels in one patient and to under-predict it in others because of the inter-individual variability of the patients [8]. Therefore median performance errors of up to 20% are usually considered as clinically acceptable [9].
Attempts to detect propofol and its metabolites in the expired air, similar to monitoring techniques used for inhaled anesthetics, have been introduced (https://www.bbraun.com/en/products/b1/edmon.htm; assessed 17th February 2019); but are not widely used yet [10]. Similarly, other methods of quantitative point of care detection of propofol [11] have not found frequent usage in clinical practice. Consequently, relying on propofol TCI remains the mainstay of administering propofol anesthetics. So, in order to prevent underdosing of propofol, clinical observation and usage of additional monitoring of patients with an EEG-based anesthesia monitor is prudent. This is even more crucial when the accuracy of the TCI model in use is questionable, such as pediatric [6] severely overweight [4,12] or underweight [13] patients, or in anesthetics with considerable volume shifts, such as in this study. Propofol displays a large volume of distribution [14] which together with our cases of low blood loss, makes it unlikely that a loss of propofol was the reason for our findings. Theoretically, there could have been an increase in metabolism of propofol in the patients studied, which could not be predicted by the TCI system used. Reasons for that could be the administration of inotropes intraoperatively or the previous intake of enzyme inductors such as certain anti-epileptics [15]. However, both were not the case in our patients.
As a result, we believe the reason for the larger bias between TCI prediction and laboratory measurement at the end of long-lasting surgery was the administration of rather large volumes of infusion and the resulting effect on the concentration of propofol. This is supported by the statistically significant correlation of performance error at the end of surgery with the calculated fluid balance. In a similar set-up with surgery involving major blood loss, we were unable to demonstrate an association, which was most likely due to the difficulty of calculating a reliable fluid balance in these cases [16].
There were limitations of our investigation. The relatively limited number of study patients weakened the statistical robustness to identify probable reasons for the observed deviations. Furthermore, with the restriction of only two measurements per patient, it is not absolutely appropriate to calculate the classic parameters described by Varvel et al. for the development of TCI models [17]. However, for the comparison of the bias at the two time points investigated the calculation of the percentage performance error (PE) and absolute performance error (APE) can be considered sufficient. The findings of our study are valid only for the TCI model used and its incorporation in the syringe pump used. There are newer TCI pharmacokinetic models available providing data derived from other patient groups and taking into account additional variables [18].
In conclusion, the dosing of propofol by means of target-controlled infusion assisted by additional monitoring parameters such as hemodynamic values and EEG-based anesthetic depth assessment, allowed for clinically successful and unproblematic anesthesia management for major surgical procedures. However, when carrying out scientific investigations, laboratory analyses to determine the actual propofol levels should be considered, depending on the research question.
Luis Neumann, Thomas Mohler, Marc P. Steurer, and Alexander Dullenkopf contributed substantially to all aspects of this manuscript, including conception and design; acquisition, analysis, and interpretation of data; and drafting the article. Max Züger and Thomas Kraemer contributed substantially to the conception of this manuscript; and acquisition and analysis of data. All authors approved the final version of this article.
The authors would like to thank Martina Steurer, JoEllen Welter, and Nicole Graf for their assistance with data analysis and graphic presentation.
This research did not receive any specific grant funding, neither from public agencies, commercial, nor from not-for-profit entities.
There are no conflicts of interest to declare.
- Bushuven S, Heise D (2013) Propofol up2date. Part 1: history and pharmacological characteristics. Anasthesiol Intensivmed Notfallmed Schmerzther 48: 378-385.
- Struys MM, De Smet T, Glen JI, Vereecke HE, Absalom AR, et al. (2016) The History of Target-Controlled Infusion. Anesth Analg 122: 56-69. [Crossref]
- Schnider TW, Minto CF, Struys MM, Absalom AR (2016) The Safety of Target-Controlled Infusions. Anesth Analg 122: 79-85.
- Cortinez L, De La Fuente N, Eleveld DJ, Oliveros A, Crovari F, et al. (2014) Performance of propofol target-controlled infusion models in the obese: pharmacokinetic and pharmacodynamic analysis. Anesth Analg 119: 302-310.
- Cortinez LI, Sepulveda P, Rolle A, Cottin P, Guerrini A, et al. (2018) Effect-Site Target-Controlled Infusion in the Obese: Model Derivation and Performance Assessment. Anesth Analg 127: 865-872.
- Sepulveda P, Cortinez LI, Saez C, Penna A, Solari S, et al. (2011) Performance evaluation of paediatric propofol pharmacokinetic models in healthy young children. Br J Anaesth 107: 593-600.
- Hu C, Horstman DJ, Shafer SL (2005) Variability of target-controlled infusion is less than the variability after bolus injection. Anesthesiology 102: 639-645.
- Glen JB, White M (2014) A comparison of the predictive performance of three pharmacokinetic models for propofol using measured values obtained during target-controlled infusion. Anaesthesia 69: 550-557.
- Eleveld DJ, Proost JH, Cortinez LI, Absalom AR, Struys MM (2014) A general purpose pharmacokinetic model for propofol. Anesth Analg 118: 1221-1237.
- Hornuss C, Praun S, Villinger J, Dornauer A, Moehnle P, et al. (2007) Real-time monitoring of propofol in expired air in humans undergoing total intravenous anesthesia. Anesthesiology 106: 665-674.
- Liu B, Pettigrew DM, Bates S, Laitenberger PG, Troughton G (2012) Performance evaluation of a whole blood propofol analyser. J Clin Monit Comput 26: 29-36.
- La Colla L, Albertin A, La Colla G, Ceriani V, Lodi T, et al. (2009) No adjustment vs. adjustment formula as input weight for propofol target-controlled infusion in morbidly obese patients. Eur J Anaesthesiol 26: 362-369.
- Lee YH, Choi GH, Jung KW, Choi BH, Bang JY, et al. (2017) Predictive performance of the modified Marsh and Schnider models for propofol in underweight patients undergoing general anaesthesia using target-controlled infusion. Br J Anaesth 118: 883-891.
- Bolkenius D, Dumps C, Halbeck E (2018) Drugs for intravenous induction of anesthesia: propofol. Anaesthesist 67: 147-162.
- Cortegiani A, Pavan A, Azzeri F, Accurso G, Vitale F, et al. (2018) Precision and Bias of Target-Controlled Prolonged Propofol Infusion for General Anesthesia and Sedation in Neurosurgical Patients. J Clin Pharmacol 58: 606-612
- Mohler T, Welter J, Steurer M, Neumann L, Zueger M, et al. (2019) Measuring the accuracy of propofol target-controlled infusion (TCI) before and after surgery with major blood loss. J Clin Monit Comput.
- Varvel JR, Donoho DL, Shafer SL (1992) Measuring the predictive performance of computer-controlled infusion pumps. J Pharmacokinet Biopharm 20: 63-94.
- Eleveld DJ, Colin P, Absalom AR, Struys M (2018) Pharmacokinetic-pharmacodynamic model for propofol for broad application in anaesthesia and sedation. Br J Anaesth 120: 942-959.



