A 38-year-old female with history of nephropathy C1Q in peritoneal dialysis, who developed papilledema secondary to a pseudotumor cerebri, supported by increased intracranial pressure (38 cm H2O) in lumbar puncture, without evidence of lesions in the central nervous system. Who received management with intravenous steroids in the acute phase and oral topiramate for a long time, with good response at 6 six months’ follow-up.
pseudotumor cerebri; papilledema; kidney diseases; intracranial hypertension
The Pseudotumor Cerebri (PTC) was described for first time by Quincke under the name “Meningitis Serosa” in 1893, but it has been known by various names: Idiopathic Intracranial Hypertension (IIH), Benign intracranial Hypertension and others [1]. The variation of the nomenclature reflects the unclear etiology of this pathology.
This is an idiopathic condition, characterized by signs and symptoms of increased intracranial pressure (ICP) above 25 cm of water in adults, in the absence of causes that can explain it [2,3]. The pathogenesis has not been fully elucidated, but is strongly associated with female gender, obesity, renal failure, anemia, endocrine disorders and some drugs like tetracycline, vitamin A and the derivatives of retinoids among others [3,4].
The diagnostic criteria have been described by Dandy in 1937 and modified over time, (it has been modified due to the current interest in diagnosing as a primary event and not as a diagnosis of exclusion), and the last update was performed by Friedman, et al. in 2013 [2], including the papilledema, normal imagining studies and high opening pressure in the lumbar puncture.
The C1q nephropathy is rare disorder with a prevalence of 0.2 to 2.5% in biopsies, corresponding to a glomerular disease that courses in patients without evidence of systemic lupus erythematosus or membranoproliferative glomerulonephritis and it is characterized by the presence of mesangial C1q deposition leading to kidney failure and bad response to corticosteroid treatment [5].
There are some reports about the association of PTC and kidney disease; however, there are not reports in cases with C1q nephropathy. We present a case of a patient with diagnosis of renal failure secondary to C1q Nephropathy who developed PTC with bilateral papilledema that improved with Corticosteroid and Topiramate therapy with follow-up of 6 months.
A 38-year-old female patient, with a history of arterial hypertension and renal failure secondary to C1q nephropathy, confirmed by blood and histopathological studies, in renal replacement therapy with peritoneal dialysis and treated with oral anti-hypertensive and mineral supplements and waiting for performing kidney transplantation.
She came to ophthalmology department for bilateral fluctuating decrease vision, headache and tinnitus. On physical examination the best corrected visual acuity (BCVA) was 20/20 in both eyes; the Slit-lamp examination, intraocular pressure and the pupillary reflexes were normal; at fundus examination in both eyes showed an optic disc swelling, blurring of the margins disc, with peridiscal hemorrhages and axoplasmic deposits, absence of excavation and spontaneous venous pulse (Figure 1); the macula and retinal periphery were normal. Imaging studies were performed, cerebral nuclear magnetic resonance (MRI) and venography were normal. A lumbar puncture (LP) was performed finding an opening pressure of 38 cm H2O with normal cytochemical study. The high-definition spectral domain optical coherence tomography (HD SD OCT) of retinal nerve fiber layer (RNFL) showed an increased thickness in the right and left eye of 273 and 313 um respectively with diffuse loss of ganglion cell in both eyes (Figure 2). A neurological visual field testing showed isolated defects without compromise of macula (Figure 3). It was concluded that this is an PTC secondary to C1q nephropathy, starting with dietary and nutritional measures, endovenous steroids for three days and oral topiramate (standard doses) for 6 months with good response, getting reduction of bilateral RNFL thickness to 192 µm and 220 µm respectively.
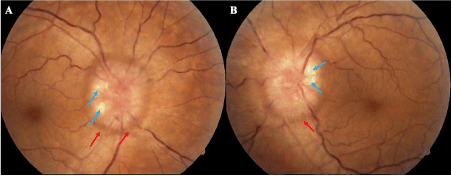
Figure 1. Optic disc swelling, look the absence of the excavation, the hemorrhages (red arrow) and the axoplasmic deposits (blue arrow) (a) right eye (b) left eye
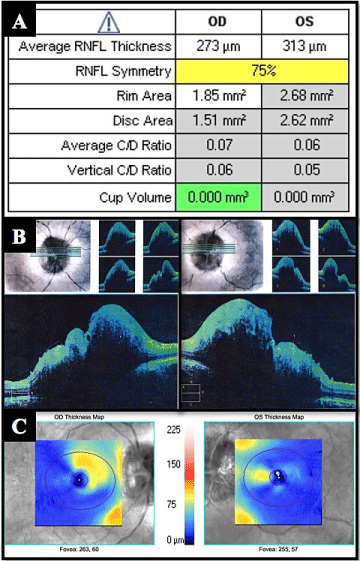
Figure 2. (A) OCT values in the ONH and RNFL OU analysis. (B) Section of the optic nerve in OCT HD 5 lines raster images on right and left eye. (C) Ganglion cell OU analysis images on right and left eye
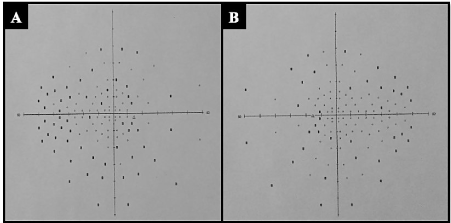
Figure 3. Neurological visual field of 120 points (a) right eye (b) left eye
There are several mechanisms tried to explicate the dysregulation of ICP in PTC, one of this mechanism is the is the increased cerebral blood volume and excessive cerebrum spinal fluid (CSF) production, as in renal failure, that induce an unbalance of hydro electrolyte system and the Natriuretic peptides, leading to water retention and subsequently making an increase levels of ICP, which would have justified the high frequency in patients with nephropathy [2,5-8]. In recent years, it has been shown the contribution of some inflammatory factors like leptin, interleukins [5,9], macrophage chemotactic protein-1 (MCP-1/ CCL2) and plasminogen activator inhibitor-1 (PAI- 1) [3]; and these two factors are present in patients with nephropathy C1q, a typical immune complex-mediated glomerular disease characterized by the presence of mesangial C1q deposition with a pro inflammatory state [5]. Other mechanism is the obstruction of venous outflow and compromised CSF resorption, related with the raise of abdominal pressure make an elevation of the ICP explained the high prevalence in obese patients [2,4].
The papilledema is a common finding in patients with IIH [1-12], for this reason is imperative for an adequate approach, that doctors suspect in any type of intracranial lesion and request extension studies, although the presence of papilledema is not mandatory, and in his absence implies a unilateral or bilateral abducens nerve palsy for suspect PTC; even if these two findings are absent, other clinical and imaging criteria must be present [10,11].
The diagnosis of PCT requires a lumbar puncture test and the exclusion of any cause of intracranial hypertension using imaging studies [2,12]. These criteria have been described by Dandy in 1937 and modified over time, the last update was performed by Friedman, et al. (Table 1) in 2013 [2,10], due to the current interest in diagnosing as a primary event and not a diagnosis of exclusion [11], and not fall into the over diagnosis of this condition [13].
Table 1. Diagnostic criteria for idiopathic intracranial hypertension
Actually, there are monitoring techniques such as OCT, been the thickness of the RNFL the most important parameter [12], that as in the case of our patient, shows a decrease with treatment at 6 months’ follow-up. Although other studies that can help us in monitoring as clinical manifestations and neurological visual field [14].
On treatment, it has been found that Acetazolamide is safe and effective option [2,3,10], given the first line of treatment; however, due to kidney damage present in our patient, it could not be administered at the discretion of the nephrology department, therefore a second-line management was initiated with intravenous steroids only in the acute phase in conjunction with standard dose topiramate for 6 months. Some studies such as Celebisoy, et al., indicate similar efficacy in the treatment of PTC between acetazolamide and topiramate, giving an additional effect in the case of topiramate with a greater weight loss compared to acetazolamide [15]. Showed that in the case of our patient improve clinically and in the thickness of RNFL of the optic nerve.
We must recognize that it is often diagnosed by Ophthalmologist due the findings in the optic nerve (papilledema), and although in most cases the visual acuity may be preserved as our case, but in chronic or atrophic stages could compromise irreversibly the vision, whereby is important to know that kidney disorders like this, correspond to risk factors.
The combination of steroids and topiramate shown a good response in the treatment of PTC with 6 months’ follow-up. In the best of our knowledge, this is the first report in the literature of PTC secondary to C1q nephropathy.
- Ruggieri M, Salprietro V, Johanson CE (2015) The history of pseudotumor cerebri syndrome among “courses” and “recourses”. J Pediatr Neurol 13: 3–7.
- Markey KA, Mollan SP, Jensen RH, Sinclair AJ (2016) Understanding idiopathic intracranial hypertension: Mechanisms, management, and future directions. Lancet Neurol 15: 78–91. [Crossref]
- Baykan B, Ekizoğlu E, Uzun GA (2015) An update on the pathophysiology of idiopathic intracranial hypertension alias pseudotumor cerebri. Agri 27: 63-72. [Crossref]
- Sheldon CA, Kwon YJ, Liu GT, McCormack SE (2015) An integrated mechanism of pediatric pseudotumor cerebri syndrome: Evidence of bioenergetic and hormonal regulation of cerebrospinal fluid dynamics. Pediatr Res 77: 282-289. [Crossref]
- Vizjak A, Ferluga D, Rozic M, Hvala A, Lindic J, et al. (2008) Pathology, clinical presentations, and outcomes of C1q nephropathy. J Am Soc Nephrol 19: 2237-2244. [Crossref]
- Shaw D, Priestman W, McIntyre CW (2002) Benign intracranial hypertension in a patient with chronic renal failure, precipitated by hemodialysis. Clin Nephrol 58: 458-460. [Crossref]
- Ghosh S, Sarkar K, Mukhopadhyay S, Bhaduri G (2008) Idiopathic intracranial hypertension in a child after hemodialysis. Pediatr Neurol 39: 272-275. [Crossref]
- Ahmed US, Bacaj P, Iqbal HI, Onder S (2016) IgA Nephropathy in a Patient Presenting with Pseudotumor Cerebri. Case Rep Nephrol 2016: 5273207. [Crossref]
- Sinclair AJ, Ball AK, Burdon MA, Clarke CE, Stewart PM, et al. (2008) Exploring the pathogenesis of IIH: an inflammatory perspective. J Neuroimmunol 201-202: 212-220. [Crossref]
- Friedman DI, Liu GT, Digre KB (2013) Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 81: 1159-1165. [Crossref]
- Bidot S, Saindane AM, Peragallo JH, Bruce BB, Newman NJ, et al. (2015) Brain imaging in idiopathic intracranial hypertension. J Neuroophthalmol 35: 400-411. [Crossref]
- Optical Coherence Tomography Substudy Committee, NORDIC Idiopathic Intracranial Hypertension Study Group (2015) Papilledema outcomes from the optical coherence tomography substudy of the idiopathic intracranial hypertension treatment trial. Ophthalmology 122: 1939-1945.e2. [Crossref]
- Fisayo A, Bruce BB, Newman NJ, Biousse V (2016) Overdiagnosis of idiopathic intracranial hypertension. Neurology 86: 341-350. [Crossref]
- The NORDIC idiopathic intracranial hypertension study group writing committee (2016) Visual field outcomes for the idiopathic intracranial hypertension treatment trial (IIHTT). Invest Ophthalmol Vis Sci 57: 805-812. [Crossref]
- Celebisoy N, Gökçay F, Sirin H, Akyürekli O (2007) Treatment of idiopathic intracranial hypertension: Topiramate vs acetazolamide, an open-label study. Acta Neurol Scand 116: 322-327. [Crossref]



