Background: Pulmonary hypertension (PHT) is common in patients with end stage renal disease (ESRD). Moderate to severe PHT is a strong independent predictor of mortality in hemodialysis (HD) patients, and in those undergoing noncardiac surgery. The studies which have evaluated the association of PHT with renal transplant outcomes have shown conflicting results. We performed a systematic review and meta-analysis of the current available evidence examining the effect of existing PHT on relevant clinical outcomes following renal transplantation.
Materials and methods: Major databases (Pubmed, Embase, Cochrane, Web of Science, and Scopus) were searched for studies of patients undergoing renal transplantation that reported pulmonary pressures and transplantation outcomes. Data were extracted from the original publications.
Results: Out of 259 publications, only 3 (with a total of 502 patients) were eligible for inclusion in the current analysis. Our meta-analysis of these three studies suggests a three-fold increase in mortality after renal transplantation in patients with PHT compared to those without PHT (OR 3.15, 95% confidence interval 1.42-6.97; p = 0.005). A qualitative review indicates that PHT is associated with both early graft dysfunction and worse renal function at 12 months post-transplant.
Conclusions: There is paucity of clinical trial data examining the effect of pulmonary hypertension and its management on renal transplant outcomes. In this meta-analysis we found that there is an increased risk of mortality in patients with pulmonary hypertension who undergo renal transplantation
Pulmonary hypertension, renal transplantation, end stage renal disease
Pulmonary hypertension (PHT) is common in patients with end stage renal disease (ESRD). The reported prevalence of PHT is 40-50% in the general ESRD population [1,2] and 17-32% in patients being evaluated for a renal transplant [3,4]. Moderate to severe PHT is a strong independent predictor of mortality in hemodialysis (HD) patients [5], and in those undergoing noncardiac surgery [6]. Severe PHT is a contraindication to liver transplantation due to increased post-transplant mortality and morbidity [7].
Despite the high prevalence there is a paucity of literature regarding the effect of pulmonary hypertension on outcomes after renal transplantation. Moreover, the studies which have evaluated the association of PHT with renal transplant outcomes have shown conflicting results. Several indicate that PHT is an independent predictor of early and delayed graft dysfunction [8,9] as well as mortality [4,10]. In the report showing an increased risk of mortality, there was no relationship between PHT and graft survival, a finding that contradicts others [4]. In other publications, the prevalence of PHT was not different in patients with and without major adverse cardiovascular events (MACE) [11]. Renal transplantation may indeed improve PHT in this population [12]. It has therefore been suggested that the presence of significant PHT may be another criterion for expeditious transplantation rather than a contraindication [13].
This inconsistency is reflected in the current guidelines for patients undergoing renal transplantation [14]. The 2012 ACC/AHA guidelines have made only suggestions rather than strong recommendations for echocardiographic screening and evaluation for PHT [14].
In view of this we performed a systematic review and meta-analysis of the current available evidence examining the effect of existing PHT on relevant clinical outcomes following renal transplantation.
Data source and search strategy
The systematic review was carried out in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [15]. The search strategies were developed in PubMed, and translated to match the subject headings and keywords for Embase, Cochrane Central Register of Controlled Trials, ISI Web of Science, and Scopus from database inception through January 23rd, 2015. The following MeSH, Emtree and keyword search terms were used in combination: pulmonary hypertension, pulmonary artery systolic pressure, pulmonary artery pressure, right ventricular systolic pressure, right sided pressures, echocardiographic parameters, renal transplant outcomes, kidney transplant outcomes, major adverse cardiac events, major adverse cardiac outcomes, MACE, mortality, graft survival, graft dysfunction, post renal transplant. The search accounted for plurals and variations in spelling with the use of appropriate wildcards. To identify further articles, we manually searched references and related citations. All results were downloaded into Endnote (Thompson Reuters) and duplicate citations were identified and removed.
Study selection
Two authors (AM, ARK) independently assessed the eligibility of the identified studies. Unfortunately, there were no RCTs looking at our outcomes of interest in this population. Those chosen were retrospective studies that focused on the effect of pre-transplant pulmonary hypertension on mortality and graft outcomes in patients undergoing renal transplantation. We included studies which used echocardiography to identify patients with pulmonary hypertension prior to renal transplantation and then studied mortality rates and graft outcomes in patients with and without pulmonary hypertension. The methodological quality of selected studies was assessed using the Newcastle scale [16].
Data extraction
Two authors (AM, ARK) independently extracted data on the year of publication, cohort size, patient characteristics, presence or absence of pulmonary hypertension and relevant outcomes. The main outcome was mortality during the follow-up period. The articles differed slightly in the pre-specified definition of pulmonary hypertension by echocardiography and were included as defined in the individual experiences. Two of the studies utilized RVSP ≥ 35 mm Hg (or TR jet > 3m/s) as their cut off to study effects of pulmonary hypertension on renal transplants while a third one used a cut off of RVSP ≥ 50 mm Hg. Findings were considered suggestive of PH in one study if the tricuspid regurgitant jet velocity (TRV) was greater than 2.8 m/s, pulmonary artery acceleration time (PAAT) of 120 ms or right ventricular dilatation (diameter greater than 2.6 cm) The publications were extremely heterogeneous in their definition of graft function; therefore, the data on graft outcomes were not pooled for statistical analysis.
Data synthesis and statistical analysis
The inverse variance method was used in a fixed-effects model to pool mortality as an outcome and corresponding forest plots were constructed. Some reported their results as dichotomous outcomes and odds ratios were calculated before pooling them in the analysis. Cochran's Q test was used to assess heterogeneity, and was complemented by the I2 statistic [17]. Publication bias was not assessed because of the small number of reports. Sensitivity analysis was performed in a random effects model to assess the robustness of the results. Analyses were performed using Review Manager (RevMan) version 5.2.
Quality assessment
Two authors(SK, RP) independently assessed the methodological quality of selected articles using the New-Castle Ottawa Quality Assessment scale for cohort studies [16]. This scale is used to explore selection, comparability and outcome assessment between groups [16]. Those with a score of 7 or more are considered to be of good methodological quality.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework, which has been adopted by the Cochrane collaboration to evaluate evidence reported in systematic reviews, was used to interpret our findings [18]. For purposes of systematic reviews, the GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. Quality of a body of evidence involves consideration of within-study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias [18].
The search identified 259 publications, out of which 3 [4,9,10] were eligible for inclusion in the analysis (Figure 1). Others analyzed echocardiographic parameters in transplant recipients but did not report PHT as one of their echo parameters and were not included [19,20]. One review of major adverse cardiovascular events (MACE) in renal transplant recipients with and without PHT was evaluated [11]. The statistical analysis included absolute levels of pulmonary pressures in patients who did or did not have MACE after transplantation and did not show a significant difference. We wrote to the authors to retrieve mortality data in patients with and without PHT but did not receive a response. Thus, this was not included in our statistical analysis.
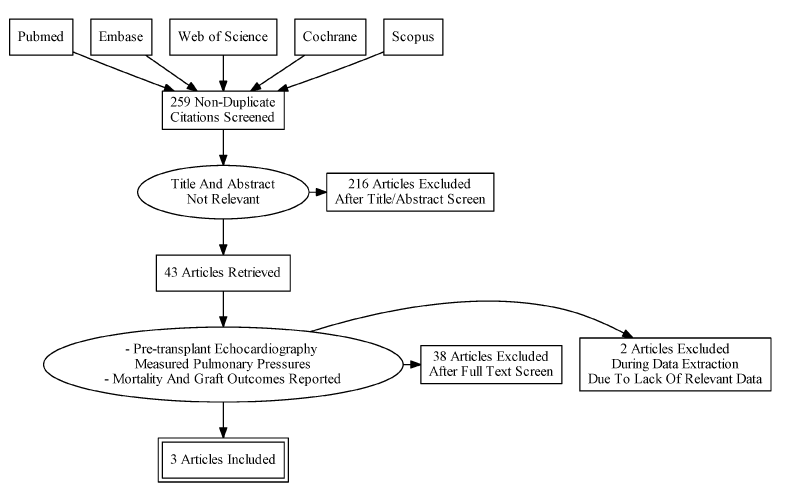
Figure 1. Flow diagram of citations reviewed.
There was excellent agreement between the reviewers regarding the inclusion of the analyzed publications, data abstraction and quality assessment. Table 1 summarizes the characteristics of the included reports. All had moderate methodological quality with New-Castle Ottawa Quality Assessment scale.
Table 1. Characteristics of Included Studies and Patients.
Authors |
Year |
Country |
Inclusion (I) and Exclusion (E) Criteria |
Patient, Total (n) |
Age (yr) |
PHT, n (%) |
Male (%) |
DM (%) |
RRT |
Follow-up (m) |
Mortality n (%) |
HD (%) |
PD (%) |
None (%) |
Issa
et al |
2008 |
US |
(I) Pre transplant echo with RVSP
(E)No RVSP or no echo |
215 |
55 ± 11 |
32 |
61 |
46 |
47.4 |
4.60 |
48 |
23 ± 12 |
14 (6.5) |
Gazzana et al |
2013 |
Brazil |
(I) Pre transplant echo with RVSP
(E)No RVSP or no echo |
81 |
49 ± 12 |
12 |
56 |
NR |
NR |
NR |
NR |
28 ± 12 |
8 (9.8) |
Mehra
et al |
2013 |
US |
(I) Pre transplant echo with RVSP
(E)No RVSP or no echo |
206 |
50 |
19 |
55 |
43 |
77 |
17 |
6 |
12 |
11 (5.3) |
Abbreviations: DM: Diabetes Mellitus, RVSP: Right ventricular systolic pressure; RRT: Renal Replacement Therapy; HD: Hemodialysis; PD: Peritoneal Dialysis; NR: Not Reported
There were a total of 502 patients in these 3 cohorts who underwent renal transplantation and 118 (23.5%) of those had PHT by echocardiography prior to transplant. Two were from United States [4,9] and one was from Brazil [10]. The prevalence of PHT ranged from 12% to 32%. Most of the patients were relatively young with mean age ranging from 49 - 55 years. The percentage of patients not yet on dialysis was 48% in one, 6% in another and not reported in the third. Males comprised 55-60% of the patients while the mean length of follow up ranged from 12 to 28 months.
Mortality: All-cause mortality was reported to be 6.5% [4], 5.3 % [9] and 9.8% [10] respectively. Two [4], [10] of the studies concluded that the presence of PHT is associated with a statistically significant increase in mortality after renal transplantation while one [9] concluded that there was no statistically significant increase in mortality associated with presence of PHT.
Our meta-analysis of these three studies suggests a three-fold increase in mortality after renal transplantation in patients with PHT compared to those without PHT (OR 3.15, 95% confidence interval 1.42-6.97; p = 0.005) (Figure 2). There was no heterogeneity between the studies (I2 = 0%; p = 0.49). A random effects model revealed a similar risk.
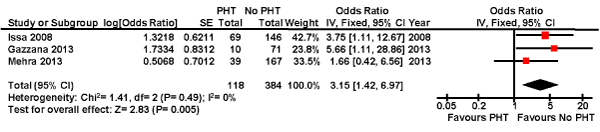
Figure 2. Forest plot comparing the mortality after renal transplantation in patients with and without pulmonary hypertension.
Graft function: As mentioned above the definition of outcome of graft dysfunction was heterogeneous in the included studies, hence the outcomes were not pooled in a quantitative manner. However, a qualitative review indicates that PHT is associated with both early graft dysfunction and worse renal function at 12 months post-transplant [8,9]. This effect of early graft dysfunction is most pronounced in deceased donor renal transplants and was not seen in living donor transplants in one report [8]. Delayed graft function (defined as the need for dialysis within a week of renal transplantation or serum creatinine >3 mg/dL) seen in this group was associated with PHT even after adjusting for important donor, graft and recipient characteristics such as age, cold ischemia time and donor graft criteria among others. Some other studies on graft function did not evaluate the effect of donor, recipient and graft characteristics on the final outcomes and hence their results should be taken with a grain of salt [9]. Other observational data from a large study indicate that PHT did not affect graft survival [4]. The type of immunosuppression used and baseline characteristics affecting graft outcomes are also not clearly defined in some of these retrospective studies making it difficult to identify PHT an independent predictor of worse graft outcome [4,9].
In this systematic review and meta-analysis we found an increased risk of mortality in patients with pulmonary hypertension who underwent renal transplantation. Our analysis suggests a threefold increase in risk of death after renal transplantation in the presence of PHT.
The pathogenesis of PHT in this population remains poorly understood with reported associations including AV fistulae, cardiac dysfunction, fluid overload, bone mineral disorders, non-biocompatible dialysis membranes, uremia-induced endothelial dysfunction [21,22].
While it is well known that cardiovascular diseases including coronary artery disease, are the most frequent causes of death in patients post renal transplantation, the data on patients with PHT who have undergone renal transplantation is limited. At the same time PHT is not an all or none phenomenon with a wide variety of clinical and prognostic implications depending on not only the severity but also the underlying cause and reversibility or lack thereof of the elevated pulmonary pressures. There is also lack of data on the severity of PHT and its association with risk of mortality and there are no studies that show how the different types of PHT and different degrees of reversibility are associated with renal transplant outcomes. Current guidelines for liver transplantation mandate a screening echocardiography to search for the presence of PHT [23]. The goal identifies and treat those patients at the highest risk of cardiovascular adverse events during and after transplant. Failure to reduce mean pulmonary artery pressure to <35 mm of Hg is considered by most liver transplant centers to be a relative contraindication to transplant [24]. The relatively high prevalence of PHT in renal transplant recipients makes it imperative to better define scientific guidelines in this arena.
While our analysis found increased odds of mortality after renal transplantation in the presence of PHT, the strength of the available evidence is affected by the observational design of the studies. The evidence was largely based on a pooled analysis of observational studies that are inherently limited by the study design and lack of randomization. Moreover, the data in the individual studies was not adjusted for possible potential confounders. The patient population may have differed in the type of immunosuppression, donor or graft characteristics, age, baseline comorbidities, severity of pulmonary hypertension and duration of follow-up; which may have led to different risk profiles and possible differences in observed outcomes. However, the effect of these patient variables can only be evaluated in individual patient data meta-analysis. Thus, this association constitutes low level of evidence as per the GRADE framework. Factors that negatively affect the evidence are observational design, lack of adjustment for confounders in the individual studies and the variability in the results. Additionally, the evidence is derived from single centers with small sample size and a fewer number of events. The large effect size may have upgraded the evidence in favor of the association but the above mentioned factors prevented us from upgrading the level of evidence.
The current systematic review indicates that PHT portends increased mortality after renal transplantation. At the same time there are numerous observations of improvement in PHT along with other echocardiographic parameters after renal transplantation [1,12,25,26]. This has led many to propose that PHT can be used as an indication for expeditious renal transplantation [12,25,26]. However, data also suggest that resolution of echocardiographic parameters may not necessarily confer survival benefit [27]. Most of the data comes from these retrospective, small sized studies, which are plagued by discrepancies inherent to such a study design. In addition, the studies did not account for whether pulmonary hypertension is reversible or not (either by achieving an optimal volume state or by pulmonary vasodilator therapy).
According to a recent census by the Organ Procurement Transplant Network (OPTN) database, there are 101,569 patients on the waiting list for a kidney transplant. Among these 16,485 kidney transplants were performed in U.S. in 2012. For patients with ESRD, transplant offers a second chance at life. Therefore, being classified as a suboptimal candidate due to PHT can be devastating news. However, it is important to determine the impact of a patient’s PHT severity on transplant outcome to ensure a favorable risk/benefit ratio taking into account the transplant program and the appropriate use of organs. All the current studies included defined PHT based on echocardiography. Invasive confirmation and further definition of the type of PHT based on right heart catheterization (RHC) was not done frequently. Because the PHT seen in ESRD patients has unique properties with respect to pathogenesis and hemodynamics therefore we feel it is imperative in this population to exactly define these mechanisms by a right heart catheterization. It has been shown that pulmonary hypertensive disease of HD patients is a unique form of PHT in which elevated cardiac output (CO) and uremic-induced endothelial dysfunction co-exist [21]. Given the unique characteristics of PHT in ESRD population a RHC to accurately define the severity and type of PHT should be mandatory, as it will help delineate treatment goals and strategy. Some authors have even suggested that measures of right ventricular (RV) function and the integration of RV function with RV afterload (i.e., Pulmonary Vascular Resistance obtained by RHC) are likely better indicators of prognosis than the degree or presence of PHT itself in patients being considered for renal transplantation [28].
At this time, the propensity of data seems to indicate that patients with mild to moderate PHT (RVSP<50 mm of Hg) may be considered for expedited transplantation to prevent the PHT from worsening and potentially improve it. Those with severely elevated pressures should potentially undergo targeted treatment depending on RHC findings to reduce the pulmonary pressures before transplantation to improve patient and graft outcomes. There is some evidence in liver transplant candidates that patient with moderate or severe PHT who are able to reduce their mPAP to <35 mm Hg with vasodilation therapy have excellent survival following liver transplantation [24]. The ultimate goal should be to define the degree and type of PHT and accurately classify patients into those where expedited transplantation is the treatment option as compared to others where initial management to reduce pulmonary pressures and subsequent transplantation will be of utmost clinical benefit.
2021 Copyright OAT. All rights reserv
Strengths and limitations of our analysis
To our knowledge this is the first systematic review and meta-analysis on the effect of PHT on patient survival after renal transplantation. Our analysis involves a comprehensive literature search with inclusion of all relevant studies adding substantially to the cumulative evidence. We have also examined qualitatively the association of PHT with graft dysfunction after renal transplantation. The results of our analysis are weakened by the limitations inherent to meta-analysis and those of included studies, which were observational in design and retrospective in nature. The small number of patients in these studies and the comparatively low incidence of mortality further limit the strength of the analysis. The fact that one study used RVSP > 50 mm Hg to divide patients into PHTN group and the other two used RVSP> 35 mm Hg is another limitation of our analysis. All these factors may have had an influence on the pooled estimate, which may be considered as a weakness in our analysis.
Implications
Data regarding the evaluation of PHT and its associated risk to the patient prior to renal transplantation are limited. There is evidence of an associated increased mortality during the first year post transplant as compared to dialysis [29,30]. Our analysis also suggests decreased survival in patients with renal transplant who have PHT. A clear cutoff of severity of pulmonary artery pressures beyond which mortality risk increases is not well defined. It has been suggested that patients with mild to moderate PHT may be considered for expedited transplantation to prevent the PHT from worsening and potentially improve it [1,12,25,26] and severe PHT may be treated before transplantation to improve patient and graft outcomes.
The results of our analysis emphasize the need for screening for PHT as a part of the workup for renal transplantation. Further studies would be needed to confirm our findings and evaluate the outcomes of the suggested approach to elevated pulmonary pressure prior to transplantation. We propose that all renal transplantation candidates should undergo screening for PHT using transthoracic echocardiography. Patients with estimated right ventricular systolic pressures higher than 50 mmHg should undergo right heart catheterization for confirmation and elucidation of the underlying etiology (pre-or post capillary). Decisions regarding transplant candidacy or deferring transplant until the pulmonary pressures are better controlled; can be made with a better understanding after a RHC indicates not only the severity but also the type and reversibility of PHT. A proposed algorithm to guide such management is shown in Figure 3. Long-term effects on mortality and graft function depending on the type of PHT have not been studied. Randomized clinical trials utilizing right heart catheterization guided targeted management compared to conventional treatment before transplant with data on long term mortality and graft function are needed to better define management guidelines in this field.
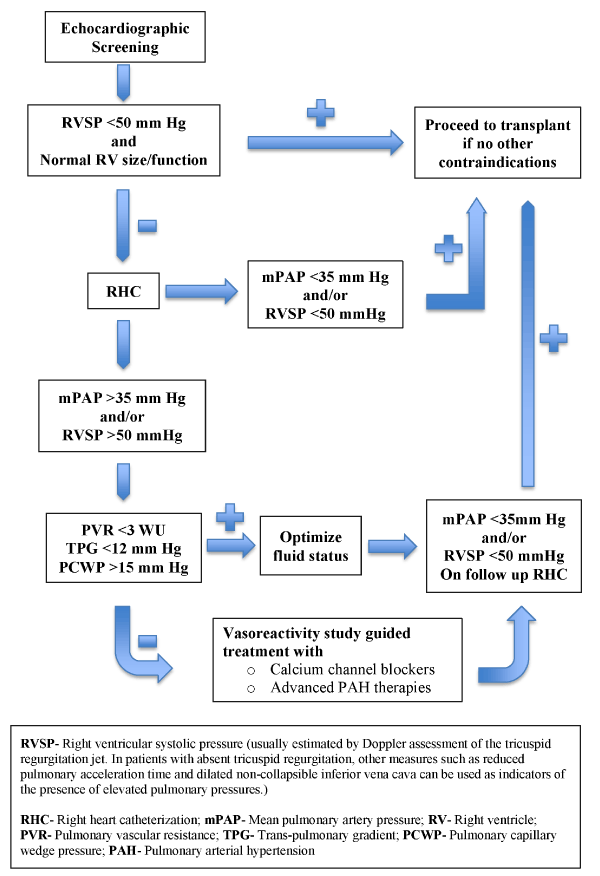
Figure 3. Proposed algorithm to guide management of renal transplant candidates with pulmonary hypertension.
There is paucity of clinical trial data examining the effect of pulmonary hypertension and its management on renal transplant outcomes. In this meta-analysis, we found that there is an increased risk of mortality in patients with pulmonary hypertension who undergo renal transplantation. However, the results constitute low quality evidence as per the GRADE framework. Based on the above findings, the diagnosis and management of pulmonary hypertension in the renal transplantation candidates may help improve outcomes post transplantation. Further studies are needed not only to confirm our findings but also to help in the clinical decision making in this patient population.
Compliance with ethical standards
Potential conflicts of interest: None relevant to the study
Informed consent: This is a review of literature and did not involve direct contact with patients
Funding sources: none relevant to the study
Disclosures: All the authors have declared no competing interest.
This article does not contain any studies with human participants or animals performed by any of the authors.
- Yigla M, Nakhoul F, Sabag A, Tov N, Gorevich B, et al. (2003) Pulmonary hypertension in patients with end-stage renal disease. Chest 123: 1577-1582. [Crossref]
- Havlucu Y, Kursat S, Ekmekci C, Celik P, Serter S, et al. (2007) Pulmonary hypertension in patients with chronic renal failure. Respiration 74: 503-510. [Crossref]
- Bozbas SS, Akcay S, Altin C, Bozbas H, Karacaglar E, et al. (2009) Pulmonary hypertension in patients with end-stage renal disease undergoing renal transplantation. Transplant Proc 41: 2753-2756. [Crossref]
- Issa N, Krowka MJ, Griffin MD, Hickson LJ, Stegall MD, et al. (2008) Pulmonary hypertension is associated with reduced patient survival after kidney transplantation. Transplantation 86: 1384-1388. [Crossref]
- Yigla M, Fruchter O, Aharonson D, Yanay N, Reisner SA, et al. (2009) Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int 75: 969-975. [Crossref]
- Ramakrishna G, Sprung J, Ravi BS, Chandrasekaran K, McGoon MD (2005) Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol 45: 1691-9. [Crossref]
- Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB; ERS Task Force Pulmonary-Hepatic Vascular Disorders (PHD) Scientific Committee (2004) Pulmonary-Hepatic Vascular Disorders (PHD). Eur Respir J 24: 861-80. [Crossref]
- Zlotnick DM, et al. (2010) Non-invasive detection of pulmonary hypertension prior to renal transplantation is a predictor of increased risk for early graft dysfunction. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 25: 3090-3096.
- Mehra Sanjay EJ, Cynthia C, Sunil S (2013) Pulmonary hypertension in patients undergoing kidney transplant- A single center experience. International Journal of Cardiovascular Research 2.
- Azzana MB (2013) Pulmonary Hypertension Identified by Color Doppler Echocardiography in Renal Transplant Candidates: Prevalence and Effect on Survival. American Journal of Transplantation 13: 381-381.
- Gu H, Akhtar M, Shah A, Mallick A, Ostermann M, et al. (2014) Echocardiography predicts major adverse cardiovascular events after renal transplantation. Nephron Clin Pract 126: 75-80. [Crossref]
- Casas-Aparicio G, Castillo-Martínez L, Orea-Tejeda A, Abasta-Jiménez M, Keirns-Davies C, et al. (2010) The effect of successful kidney transplantation on ventricular dysfunction and pulmonary hypertension. Transplantation proceedings. 42: 3524-3528. [Crossref]
- Reddy YN, et al. (2013) Progressive pulmonary hypertension: another criterion for expeditious renal transplantation. Saudi journal of kidney diseases and transplantation: an official publication of the Saudi Center for Organ Transplantation 24: 925-929.
- Lentine KL, Costa SP, Weir MR, Robb JF, Fleisher LA, et al. (2012) Cardiac disease evaluation and management among kidney and liver transplantation candidates: A scientific statement from the american heart association and the American college of cardiology foundation. Circulation 126: 617-663. [Crossref]
- Liberati A (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62: e1-34.
- Wells GSB, O’Connell D, J Peterson, V Welch, M Losos, et al. (2015) The Newcastle- Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. Ottawa, Ontario: The Ottawa Health Research Institute. Available: http://www.ohri.ca/programs/clinicalepidemiology. Last accessed january 25th 2015. 2015.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557-560. [Crossref]
- Schünemann HJ, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. (2011) Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
- McGregor E (1998) Pre-operative echocardiographic abnormalities and adverse outcome following renal transplantation. Nephrol Dial Transplant 13: 1499-1505.
- Sharma R (2007) Echocardiography-based score to predict outcome after renal transplantation. Heart 93: 464-469.
- Nakhoul (2005) The pathogenesis of pulmonary hypertension in haemodialysis patients via arterio-venous access. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association 20: 1686-1692.
- Kawar B (2013) Pulmonary hypertension in renal disease: epidemiology, potential mechanisms and implications. Am J Nephrol 37: 281-290.
- Murray KF, Carithers RL (2005) AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology 41: 1407-1432.
- Ashfaq M (2007) The impact of treatment of portopulmonary hypertension on survival following liver transplantation. Am J Transplant 7: 1258-1264.
- Stallworthy EJ (2013) Do echocardiographic parameters predict mortality in patients with end-stage renal disease? Transplantation. 95: 1225-1232.
- Bozbas, S.S., et al., (2011) Renal transplant improves pulmonary hypertension in patients with end stage renal disease. Multidisciplinary Respiratory Medicine 6: 155-160.
- McGregor E (200) Early echocardiographic changes and survival following renal transplantation. Nephrol Dial Transplant 15: 93-98.
- Tedford RJ, Forfia PR (2013) Hemodynamic Evaluation of Pulmonary Hypertension in Chronic Kidney Disease. Advances in Pulmonary Hypertension 12: 82.
- Gill JS, Rose C, Pereira BJ, Tonelli M (2007) The importance of transitions between dialysis and transplantation in the care of end-stage renal disease patients. Kidney Int 71: 442-447. [Crossref]
- Wolfe RA (1999) Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725-1730.



