Abstract
Aim: The aim of the study was the estimation of the structural and metabolic changes in cerebellum Purkinje cells of rats during the complete outside bile abduction.
Methods: Experiments were performed on male Wistar rats weighing 200 ± 25 g. In 60 rats the fistula of the common bile duct was made and all the bile secreted by the liver was collected through a plastic tube into the outside glass bile container. 60 animals of the control group underwent sham operation (no removal of bile). On the 1th, 3th, and 5th days after the operation the animals of the control and experimental groups were decapitated and cerebellum cortex samples were collected for histology, histochemistry and electron microscopy.
Results: The loss of bile induced the gradual increase in structural and metabolic abnormalities of Purkinje cells resulting in severe, irreversible disturbances and even death of some of them. The gradual decrease in Purkinje cells size, the loss of their sphericity and elongation, as well as deep changes in their nuclei and organelles (mitochondria, endoplasmic reticulum, Golgi complex and lysosomes), followed by inhibition of succinate-, NADH-, glucose-6-phosphate-, NADPhH dehydrogenases and activation of lactate dehydrogenase and the marker enzyme of lysosomes acid phosphatase were documented. All the changes reflect the ratio of the processes of damage and adaptation in the affected neurons in the setting of bile absence in the body.
Conclusion: Total loss of bile (on the 1st, 3rd, and 5th days) induces the gradual increase in structural and metabolic disturbances in cerebellum Purkinje cells and death of some of them.
Key words
cerebellum, Purkinje cells, structure, cytochemistry, loss of bile.
Introduction
Bile is an important biological fluid, secreted by the liver and flows through the common bile duct into the duodenum. Bile contains bile acids (BAs), phospholipids, cholesterol, bilirubin, proteins, water and salts. The primary BAs are synthetized from cholesterol in the liver, conjugated to glycine or taurine to increase their solubility, secreted into bile, concentrated in the gallbladder during fasting, and expelled in the intestine in response to dietary fat, as well as bio-transformed in the colon to the secondary BAs by the gut microbiota, reabsorbed in the ileum and colon back to the liver. BAs in the intestine not only regulate the digestion and absorption of cholesterol, triglycerides, and fat-soluble vitamins, but also play a key role as signaling molecules in modulating epithelial cell proliferation, gene expression, and lipid and glucose metabolism by activating farnesoid X and G-protein-coupled bile acid receptor-1 in the liver, intestine, muscle and brown adipose tissue [1]. BAs receptors expressed in endothelial cells and may have important effects on both systemic and portal circulation [2]. BAs, for decades considered only to have fat-emulsifying functions in the gut lumen, have recently emerged as novel cardio-metabolic modulators. They have real endocrine effects, acting via multiple intracellular receptors in various organs and tissues. BA affect energy homeostasis through the modulation of glucose and lipid metabolism, predominantly by activating the nuclear farnesoid X receptor (FXR), as well as the cytoplasmic membrane G protein-coupled BA receptor TGR5 in a variety of tissues. The roles of BA in the pathogenesis of diabetes, obesity, metabolic syndrome, and cardiovascular diseases are seriously being considered, and BA and their derivatives seem to represent novel potential therapeutics to treat these diseases of civilization [3].
Obstructive (mechanical) jaundice induced by bile stones or malignancies preventing the normal outflow of bile and induces inexorably progressing hyperbilirubinemia with its consequent deleterious effects [4]. It requires a surgical treatment followed by external biliary drainage. The early and adequate decompression of bile ducts decreases postoperative complications, but lead to the external loss of bile [5]. The long-term loss of bile induces the disturbances in the digestion of lipids, in blood coagulation and other symptoms of vitamin A, B, D, E, and K deficiencies, in calcium metabolism disorder followed by bone osteomalation, disturbances in acid-base balance of blood and functions of the liver, kidneys and nervous system [6-8]. In our previous studies we found that 3-5 days of external biliary drainage (with full loss of bile) in rats induces the severe structural and metabolic disturbances in brain histaminergic neurons [9] as well as in neurons of parietal brain cortex [10]. The aim of the present paper was the estimation of histological changes in the cerebellum, in particular in its Purkinje cells, in rats in the similar setting of bile loss.
Materials and methods
Animals, experimental design and chemicals
Experiments were performed on 120 male Wistar rats weighing 200±25 g. Rats were housed in vivarium with free access to standard laboratory food and kept under controlled environmental conditions. All experimental procedures complied with European Community Council Directive (86/609/EEC) for care and use of laboratory animals. This study was approved by the Biomedical Ethics Committee of the Grodno State Medical University.
In 60 rats the proximal part of common bile duct (3-5 mm below the confluence of the lobular hepatic ducts) was cut, ligated and all the bile secreted by the liver was collected through a polyethylene catheter into the outside glass bile container (Figure 1). The container was fixed to the skin on the right side of the rat body. 60 animals of the control group underwent sham operation (no removal of bile). On the 1th, 3th, and 5th days after the operation the animals of the control and experimental groups were anesthetized with ethylic ether and decapitated. The samples of their cerebellum were collected and fixed in the mixture of alcohol, chloroform and acetic acid in the ratio of 6:3:1, then treated with alcohol and xylen and embedded in paraffin. Other pieces of cerebellar cortex were frozen and stored in liquid nitrogen for further histochemistry; other small pieces of cerebellar cortex on the 5th days after the operation were quickly taken, fixed in 1% OsO4, dehydrated and embedded in epoxy resin for electron microscopy.
All the chemicals were obtained from Sigma-Aldrich (USA).
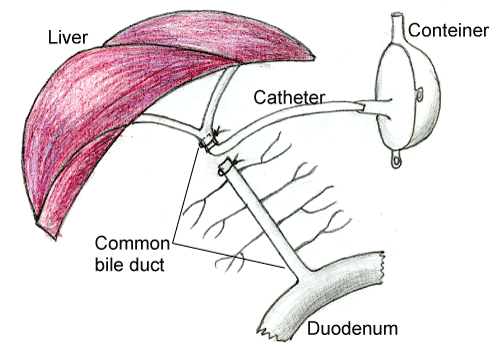
Figure 1. Scheme of operation on making a common bile duct fistula.
Histology
7 μm paraffin sagittal sections of the cerebellum cortex were stained with hematoxylin and eosin, 0.1% solution of thionine (the Nissl method) to assess general cytology of neurons and for the identification of dying neurons [11]. The examination of histological preparations, their microphotography and morphometry was carried out using microscope Axioskop 2 plus) equipped with digital camera Axiocam MRc5 (Carl Zeiss, Germany). In the preparations stained by the Nissl method the total amount of cerebellum Purkinje cells in the 1 µm interval of cortex gyrus was estimated, as well as the amount of normal and pathological types of neurons according to the intensity of their cytoplasm chromatophilia and the shape of cells bodies: normochromic (normal, medium staining), hyperchromic (intense staining), hyperchromic shrunken, hypochromic (pale staining) and shadow cells (very pale remnants of dead neurons).
Morphometry and cytophotometry of Purkinje cells in paraffin sagittal sections stained by the Nissl method were carried out using computer image analysis software Image Warp (Bit Flow, USA). To estimate the size and shape of neuronal bodies the images of up to 30 neurons bodies on the computer monitor were outlined by mouse cursor. Maximal and minimal diameter (D), perimeter (P), square (S), as well as form-factor (4πS/P2 – parameter of sphericity and folding) and the factor of elongation (maximal D/minimal D – parameter of sphericity) were calculated.
Histochemistry
10 µm frozen sagittal sections of the cerebellum were prepared using cryostat (Leica CM 1840, Germany). The activity of the oxidizing enzymes, such as succinate dehydrogenase (SDH, EC 1.3.99.1), lactate dehydrogenase (LDH, EC 1.1.1.27), glucose-6-phosphate dehydrogenase (G-6-PDH, EC 1.1.1.49), NADH dehydrogenase (NADHDH, EC, 1.1.1.49) and NADPhH dehydrogenase (NADPhDH, EC, 1.6.1.1), as well as the activity of marker enzyme of lysosomes acid phosphatase (AP, EC 1.4.3.4) were examined [12]. For the enzyme histochemistry the cryostat sections were placed into the corresponding incubation medium, including the buffer, substrate, co-factor, if necessary, and chromogen, for 30 min – 5 hours to visualize the location of enzymatic activity, then washed and embedded in the suitable plastic medium. The content of RNA in Purkinje cells cytoplasm was examined in paraffin sections by the Einarsson method [12]. The enzyme activities or products of histochemical reactions were determined in the cytoplasm of neurons on the optic density of chromogen obtained in the course of histochemical reactions. In each experimental group 150-200 neurons were estimated.
Electron microscopy
The MT-7000 ultramicrotome (RMC, USA) was used to prepare sections for electron microscopy. They were contrasted with uranyl acetate and lead citrate and examined using an electron microscope (JEM 100CX II, Japan), in the Center of Electron Microscopy of the Institute of Physiology, National Academy of Sciences of Belarus.
Statistics
The mean values obtained for every animal were processed with nonparametric statistics (because of the small number of animals in the groups) using software STATISTICA 10 (StatSoft, Inc., USA). In descriptive statistics, the values of median (Me) and interquartile range (IQR) were determined. The differences were considered significant at p<0.05 (Mann-Whitney U-test) because it was not a normal distribution.
Results
Histology
At the light microscopic level following 1 day of bile loss no structural changes in cerebellum Purkinje cells were found. Following 3 days of bile loss the shape of Purkinje cells became various: elongated, oval or triangle, but not pear-shaped only. The hyperchromic shrinking and dead neurons appeared. Following 5 days of bile loss the destructive changes in Purkinje cells increased. Highly elongated cells with extended apical dendrites and lyses of chromatophylic substance, or swelling of nucleus and cytoplasm, deformation and division of nuclei appeared (Figures 2, 3). The single shadow cells were also observed. But there remained the cerebellum cortex regions where neurons were still unchanged.
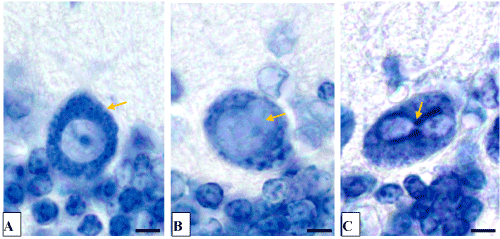
Figure 2. The cerebellum Purkinje cells.
A-control (5 days after the sham operation);
B, C-5 days of bile loss (B-swelling of nucleus, C-division of nucleus). Nissl staining. Digital microphotography. Magnification × 1000. Scale bars-5 µm
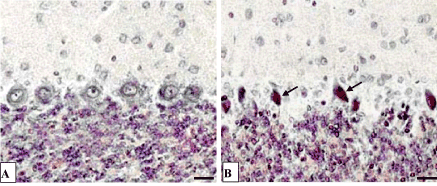
Figure 3. The cerebellum Purkinje cells. A-control (5 days after the sham operation);
B-5 days of bile loss (hyperchromic shrunken Purkinje cells, purple staining). Victorov et al. staining. Digital microphotography. Magnification × 200. Scale bars-10 µm.
The calculation of Purkinje cells have revealed that the amount of hyperchromic neurons on the 5th day of bile loss increased 3.8-fold (p=0.009), hyperchromic shrunken neurons increased 34.4-fold (p=0,009), а shadow cells – 7.5-fold (p=0.037). The amount of dead neurons estimated by the Victorov method increased 28-fold (p=0.018). In dynamics of bile loss the gradual decrease in Purkinje cells size, their elongation and loss of sphericity took place (Figure 4).
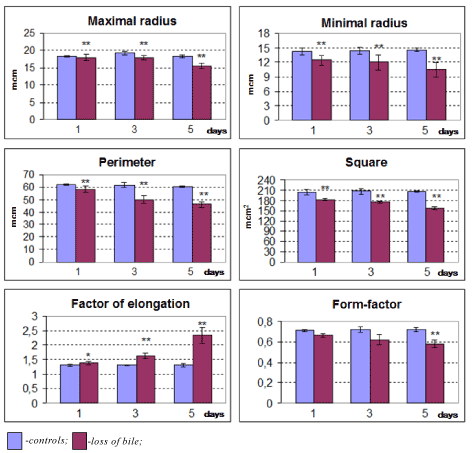
Figure 4. Changes in size and shape of cerebellum Purkinje cells in dynamics of bile loss. Me ± IQR; *-p<0.05, **-p<0.01 as compared to controls.
Histochemistry
The loss of bile induces the gradual decrease in Purkinje cells cytoplasm, the activity of the mitochondria marker enzyme succinate- and NADH-dehydrogenase, as well as glucose-6-phosphate- and NADH dehydrogenase (NADHDH, EC, 1.1.1.49) and NADPhH dehydrogenases and the activation of lactate dehydrogenase and the marker enzyme of lysosomes acid phosphatase (Figures 5-9).
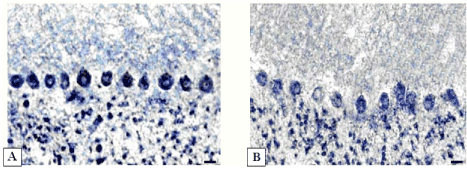
Figure 5. Activity of succinate dehydrogenase in cerebellum Purkinje cells.
А-5 days after sham operation;
B-5 days of bile loss (lower staining in cytoplasm). Staining according to Nachlas et al. Digital microphotography. Magnification × 200. Scale bars-10 µm.
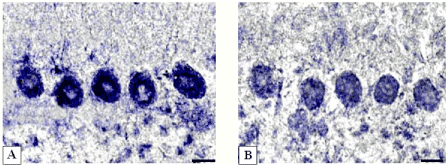
Figure 6. Activity of succinate dehydrogenase in cerebellum Purkinje cells.
А-controls (5 days after sham operation); B-5 days of bile loss (lower staining in cytoplasm). According to Nachlas et al. Digital microphotography. Magnification ×400. Scale bars-10 µm.
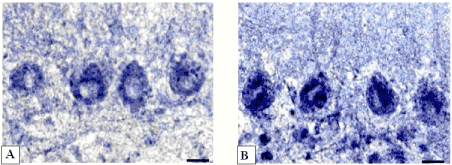
Figure 7.-Activity of lactate dehydrogenase in cerebellum Purkinje cells.
А-controls (5 days after sham operation);
B-5 days of bile loss (higher staining in cytoplasm). According to Hess, Scarpelli and Pearse. Digital microphotography. Magnification × 400. Scale bars-10 µm.
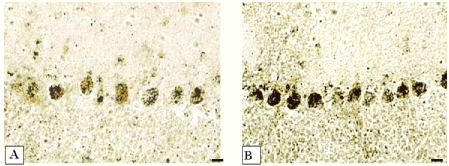
Figure 8.-Activity of acid phosphatase in cerebellum Purkinje cells.
А-controls (5 days after sham operation);
B-5 days of bile loss (higher staining in cytoplasm).
According to Gomori. Digital microphotography. Magnification × 200. Scale bars-10 µm.
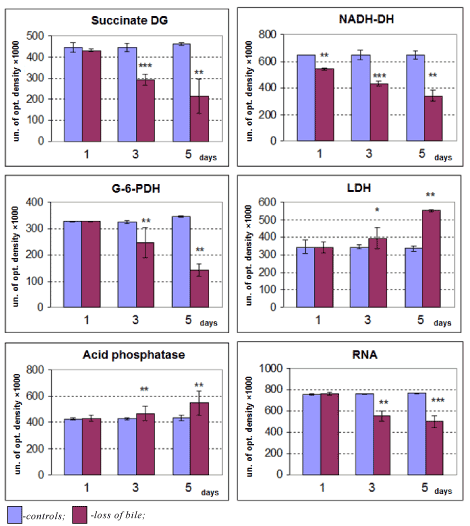
Figure 9. Changes of enzyme activities and RNA contents of cerebellum Purkinje cells cytoplasm in dynamics of bile loss. Me ± IQR; *-p<0.05, **-p<0.01 as compared to controls.
Electron microscopy
On the 5th day of bile loss we found a lot of ultrastructural changes in Purkinje cells. The nuclear envelope in those neurons became indistinct, showed deep folds; the nucleolus was enlarged and located at the periphery of the nucleus; the perinuclear space extended and the extensive output of ribonucleoprotein granules (subunits of ribosomes) was observed. In the cytoplasm of shrunken neurons the amount of organelles reduced and they were destructively changed. The width of rough endoplasmic reticulum (RER) cisterns was significantly increased (for 31%), but their surface reduced by 38% and the amount of ribosomes reduced 3.2-fold. The total number of ribosomes decreased by 43% only, because the amount of free ribosomes slightly increased. The area of smooth endoplasmic reticulum reduced by 33% (Figure 10, Table 1).
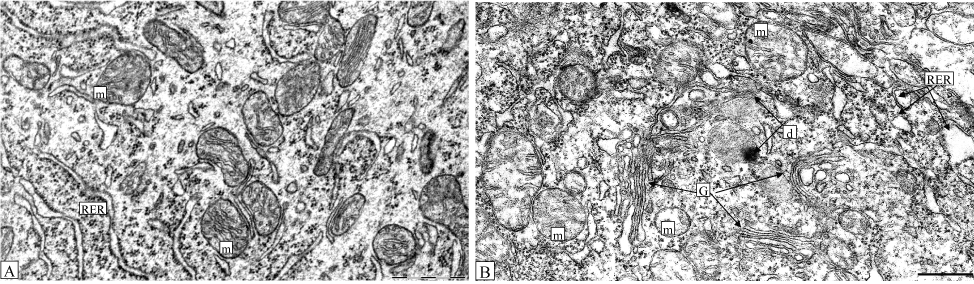
Figure 10. Cerebellum Purkinje cell cytoplasm following 5 days after sham operation (control, A) or bile loss (B): m-swelling mitochondria with destructed crista; G-hypertrophic Golgi complex with widen cisterns; d-place of cytoplasm degeneration with autophagosome; RER-extended cisterns of RER. Electron microphotogram. Scale bars-2 µm.
Table 1. Ultramicroscopic parameters of cerebellum Purkinje cells after 5 days of bile loss
Parameter |
Control |
Loss of Bile |
RER |
Area of membranes, µm2 |
4.194 ± 0,342 |
2.591 ± 0,938 ** ↓ |
Degree of cisterns extension, cu |
0.764 ± 0,067 |
1.0 ± 0,333 ** ↑ |
Ribosomes |
Total amount, in 1 µм2 |
96.50 ± 6,50 |
55.0 ± 3,0 ** ↓ |
Amount of bind, in 1 µм2 |
64.50 ± 4,50 |
20.0 ± 5,0 ** ↓ |
Amount of free, in 1 µм2 |
32.50 ± 12,50 |
39.0 ± 5,0 |
SER |
Area of membranes surface, в 1 µм2 |
2.710 ± 0,397 |
1.807 ± 0,678 ** ↓ |
Degree of extension, in cu. |
1.183 ± 0,20 |
1.750 ± 1,0 |
Mitochondria |
Amount, в 1 µм2 |
0.071 ± 0,013 |
0.049 ± 0,011 ** ↓ |
Average area, µм2 |
6.0 ± 0,934 |
3.403 ± 0,743 ** ↓ |
Degree of swelling, in cu. |
1.050 ± 0,233 |
1.40 ± 0,40 * ↑ |
Area of cristae surface, in cu. |
1.027 ± 0,236 |
0.714 ± 0,333 * ↓ |
Crista fragmentation coefficient |
0.750 ± 0,142 |
1.0 ± 0,15 * ↑ |
Golgi
complex |
Area of membranes surface, в 1 µм2 |
1.50 ± 0,293 |
3.165 ± 1,421 ** ↑ |
Degree of cisterns extension, cu |
1.0 ± 0,125 |
1.20 ± 0,550 * ↑ |
Lysosomes
|
Amount, in 1 µм2 |
4.0 ± 0,50 |
9.0 ± 1,0 ** ↑ |
Average area, in 1 µм2 |
1.227 ± 0,701 |
5.696 ± 0,956 ** ↑ |
Notes: Every data point is Me ± IQR; *-p < 0.05; **-p < 0.01 as compared to controls; cu-conditional units.
Most mitochondria swelled with distracted crista and pale matrix. In some of them the outer membrane was destroyed (Figure 9). The amount of mitochondria decreased by 31%, the degree of their swelling elevated by 33%. The area of their crista decreased by 43% and the coefficient of their fragmentation increased by 33% (Table 1).
Golgi Complex increased in size, the specific accumulations of their cisterns took place. The amount of lysosomes increased 2.3-fold and their average area increased 4.6-fold (Table 1). Quite often the local regions of cytoplasm degeneration (formless formations including small granules, irregular shaped electron dense masses and fogolysosomes) could be visible (Figure 10B).
Discussion
In our previous paper following bile loss in rats we found similar dramatic structural and metabolic disturbances in brain histaminergic and parietal cortex neurons [8, 9]. The removal of bile from the body in the present study induces the increase in the number of hyperchromic shrunken Purkinje cells and shadow cells, which seems to be too severe for neurons to survive [12]. The decrease in size, loss of sphericity and elongation of neurons took place probably as a result of rise of osmolarity of intercellular fluid. The shrinkage of neurons occurs through the activation of Na+-K+-2Cl– transport systems or coupled Na+/H+ и Cl–/HCO3– pathways of metabolism [13,14]. The shape of neurons can be disturbed also by the damage of cytoskeleton.
In the present study, on the ultrastructural level, cerebellum Purkinje cells of rats with loss of bile displayed both destructive and compensatory adaptive changes. The mitochondria were swelling, and showing the decrease in the density of cristae, as well as the reduction of their area. It corresponds to the decrease in the activity of their marker enzymes, succinate and NADH-dehydrogenases in the cytoplasm of those neurons at the light microscopic level. The increase in the nuclear envelope folding, size of nucleoli and shift of them to the nuclear envelope, the increase in the number and size of nuclear pores and the extensive output of subunits of ribosomes into the cytoplasm, the hyperplasia of free ribosomes and hypertrophy of Golgi complex may reflect the activation of synthetic processes in the affected neurons for their own needs to compensate the damaged or lost structures and to survive following the loss of bile. The reduction of bind ribosome number and RER canals indicate a decreased protein biosynthesis for export to nerve terminals. This can disturb the functions of the neurons. The significant increase in the amount and size of lysosomes in the neurons under study accompanied by the activation of the lysosomal marker enzyme acid phosphatase (at light microscopic level) may reflect the increased autophagy for the removal of damaged microstructures.
One of the possible reasons of cell membranes damage and structural disturbances of neurons can be the loss of cholesterol, which is included into the composition of biological membranes and supplied by other organs to the liver for the synthesis of biologically important substances – bile acids, which are lost during the removal of bile. In addition, in the loss of bile and absence of its entering the duodenum, a long-term afferent impulsion from duodenum results in the process of central inhibition.
Purkinje cells are very sensitive to the loss of bile, but at the same time they demonstrate high plasticity. It looks like the reparation in neurons occurs parallel to the destruction processes. If the latter dominate, the cells die.
Conclusion
The loss of bile induces the gradual increase in structural and metabolic abnormalities in Purkinje cells resulting in severe, irreversible disturbances (hyperchromic shrunken neurons) and the death of some of them. A gradual decrease in Purkinje cells size, the loss of sphericity and elongation of neurons, deep changes in their nuclei and organelles (mitochondria, endoplasmic reticulum, Golgi complex and lysosomes), followed by the activation of succinate-, NADH-, glucose-6-phosphate-, NADPhH dehydrogenases and activation of lactate dehydrogenase and the marker enzyme of lysosomes acid phosphatase take place. All these changes reflect the ratio of the processes of damage and adaptation in the neurons in the settings of bile absence in the body.
Acknowledgements
The authors are grateful to Olga A. Karnyushko for the technical assistance and the English translator Yanina Razvodovskaya for the correction of the manuscript.
2021 Copyright OAT. All rights reserv
Funding
Grodno State Medical University grant for reagents and animals.
Conflict of interest statement
None declared.
References
- Di Ciaula A, Garruti G, Baccetto RL, Molina-Molina E, Bonfrate L, et al. (2017) Bile Acid Physiology. Ann Hepatol 16: 4-14. [Crossref]
- Arab JP, Barrera F, Arrese M (2017) Bile Acids and Portal Hypertension. Ann Hepatol 16: 77-80. [Crossref]
- Vítek L (2017) Bile Acids in the Treatment of Cardiometabolic Diseases. Ann Hepatol 16: 37-46. [Crossref]
- Chandrashekhara SH, Gamanagatti S, Singh A, Bhatnagar S (2016) Current Status of Percutaneous Transhepatic Biliary Drainage in Palliation of Malignant Obstructive Jaundice: A Review. Indian J Palliat Care 22: 378-387. [Crossref]
- Chandrashekhara SH, Gamanagatti S, Singh A, Bhatnagar S (2016) Current Status of Percutaneous Transhepatic Biliary Drainage in Palliation of Malignant Obstructive Jaundice. A Review Indian. J Palliat Care 22: 378–387. [Crossref]
- Mirzoian S, Bahdasarian M, Shahbazian S (2007) Early compensation for the loss of bile during external drainage. Georgian Med News: 7-10. [Crossref]
- Bregadze IL, Ivanov PF (1965) External bile fistula. M: Medicine, pp: 144.
- Enderlin FE, Honohan T (1977) Long term bile collection in the rat. Lab Anim Sci 27: 490-493. [Crossref]
- Zimatkin SM, Baraban OV, Emel'yanchik SV (2008) Structural-metabolic changes in histaminergic neurons of the rat hypothalamus in conditions of loss of bile. Neurosci Behav Physiol 38: 907-911. [Crossref]
- Emelyanchik SV, Zimatkin SM (2013) Structural and Histochemical Changes in Rat Parietal Cortex Neurons during Biliary Drainage. Neurosci Behav Physiol 43: 329-335.
- Victorov IV., Prass K, Dirnagl U (2000) Improved selective, simple, and contrast staining of acidophilic neurons with vanadium acid fuchsin. Brain Research Protocols. 5: 135–139. [Crossref]
- Everson Pearse AG (1960) Histochemistry theoretical and applied. London, JA Churchill, United Kingdom. pp: 997.
- Lang F, Buch GI, Volke H (1998) The diversity of volume regulatory mechanisms. Cell Physiol Biochem 8: 1-45. [Crossref]
- Strange K, Emma F, Jachson PS (1996) Cellular and molecular physiology of volume-sensitive anion channel. Am J Physiol Cell Physiol 270: 711-730. [Crossref]










