Background and Objective: During the action of sitting, up to 35% of the patients push to the affected side after an acute hemispheric stroke. We analyze the deviation of the upper trunk to the affected side (DUTAS) during walking in patients having recovered from a hemispheric stroke and demonstrate the negative impact of DUTAS on gait performance.
Methods: In 99 patients having recovered after a hemispheric stroke (mean age: 58yrs) with more than 10-meter walking ability, kinematics (during walking at the preferred gait speed) were recorded using an array of 8 VICON cameras. Reflective markers were attached to the body, especially at the sacrum and C7 cervical vertebra. The deviation angle of the vertical from the line through the sacrum and C7-marker was measured. Walking abnormalities, including the abnormal posture of the upper trunk, were rated by five rehabilitation experts.
Results: Relevant DUTAS was observed in 35.6% of the right-side, and 37% of the left-side affected patients. There was a highly significant (p<.0001) positive correlation between rating of gait abnormalities and DUTAS and a significant (p<.01) negative correlation between DUTAS to the right or left side and gait speed. Additionally, the post-stroke period (shorter than four months or longer than 4 up to 120 months) had no influence on DUTAS.
Conclusions: The lateral deviation of the upper trunk to the affected side during walking is frequently observed in post-stroke patients. Because of the associated negative impact on gait performance, correction of trunk lateral bending is essential in post-stroke gait rehabilitation.
Hemispheric stroke, pushing, lateral deviation of the upper trunk, reduction of gait speed
Stroke affects at least 150 to 200 out of 100 000 people in industrial countries per year [1]. A large proportion of stroke survivors suffer from a severe long-term gait disorder limiting their ability to ambulate in the community [2]. Reduced ability to adapt to environmental constraints [3], poor endurance [4] and slow gait speed [5] characterize walking after stroke. Weakness in specific muscle groups, development of spasticity, reduced afferent sensory feedback and sensory perception [6], as well as disturbed cognition [7], contribute to the complex movement disorder of upper motor neuron syndrome (UMNS).
Pushing after stroke has first been described in 1985 by Davies [8], and is a disorder where a patient “pushes strongly towards the hemiplegic side in all positions and resists any attempt at passive correction of posture” [8,9]. The incidence of pushing after stroke is dependent on the sensitivity of the standardized measurement used [10] and lies between 8% [11] and 60% [12]. In most of the patients, a significant improvement of “pushing” has been reported from week 1 to week 12 after stroke [13]. Prolonged pushing behavior is a strong predictor for poor outcome after stroke [14] and compromised rehabilitation [9]. Initially, “pushers” were classified using criteria set out by Davies in 1990 [14]. Later on, criteria for “pushers” were refined using the Scale for Contraversive Pushing (SCP) [10]. Today three established tools can be used for scoring pushing behavior [12]. However, only the Burke scale analyses aspects of pushing during walking [15].
Pushing is usually analyzed during sitting. In the sitting position, it could be confirmed that one of the major reasons for pushing is an alteration of the subjective vertical (SV) [16] and an adaptation of the postural vertical (PV) to the altered SV [16]. After a right-hemisphere stroke, SV is tilted counterclockwise, after the left-hemisphere stroke clockwise [17,18].
In the upright standing position, this adaptation of the postural vertical to the subjective vertical and the tendency of the patients to bend the trunk to the affected side is a serious problem. This has to be compensated, or otherwise, the patient will fall over to the affected side, which may lead to severe injuries. Broad-based stance and shift of the lower body to the less affected side [19] are apparent compensatory mechanisms [20] which allow bending of the upper body to the impaired side without falling over.
During walking these compensations have to be increased since the movement of the tilted mass of the upper body causes further difficulties. Quantification of this “pushing during walking” or tilt of the upper trunk affords the measurement of the movements of the upper body in relation to the lower body. In a small sample of severely affected, free walking, adult post-stroke patients, ten out of eleven patients tilted the upper body to the affected side during walking [20].
It has been postulated that pushing behavior may reflect the severe end of a continuum of right-hemisphere extinction syndromes [9]. Because of the frequent finding of “pushing during walking” or tilt of the upper body to the affected side during walking we extend this postulation and hypothesize that “pushing during walking” may indicate the mild end of this continuum of right-hemisphere extinction syndromes [9].
There is convergent evidence that the posterior insular and parietal cortex of the right hemisphere is heavily involved in the determination of SV and the etiology of pushing [21]. However, “pushing during walking” has not been compared in the left-side and right-side affected patients. Besides, no study is available on “pushing during walking” comparing patients with a short and a long duration since stroke to analyze the possible influence of the development of spasticity after a stroke on the deviation of the upper trunk to the affected side (DUTAS).
Therefore, the following study was performed on the tilt of the upper body in the frontal plane during walking in 99 patients with a wide range of duration since stroke (0.3 to 349 months) being affected either on the right (n=45) or the left side of the body (n=54).
This study was performed according to the guidelines of good clinical practice (GCP) and in line with the declaration of Helsinki. The authors obtained institutional review board approval for instrumental gait analysis and clinical examination of stroke patients from the ethics committee of the University Duisburg-Essen (application number 18.7988).
Patients
Patients were consecutively recruited from in-patients who were transferred to the Clinic of Neurorehabilitation Essen/Rhein/Ruhr (Germany). Patients were informed on the purpose, design, and data security of the study and were included after they had given written informed consent.
Inclusion criteria for the present study were: (i) age >= 18yrs; (ii) hemispheric stroke according to a recent CT- or MRI-scan; (iii) no hint in clinical examination for the presence of orthopedic or neurological deficits interfering with walking other than the stroke; (iv) ability to walk a distance of 10 m at least 6 times with intermittent pauses without external support. Excluded were patients under care. 124 patients were screened and included (ITT-group). In 25 patients, technical problems (as difficulties to fix the markers) led to incomplete data. Five patients were cane users. Their data was thoroughly analyzed and marked by triangles. Final gait and statistical analysis were based exclusively on 94 free walking patients and 5 cane users with a complete data set.
Patients were subdivided into a subgroup of individuals who were affected on the right side of the body (RS-group; n=45) and a further subgroup of patients who were affected on the left side (LS-group; n=54). Patients were also subdivided into patients with a short duration (<4 months) since stroke (SD-group; n=79) and patients with a longer duration (>=4 months) since stroke (LD-group; n=20). The cut-off value of 4 months was chosen, because a considerable improvement of pushing during sitting during the first 3 to 4 months after stroke has been reported [13]. Demographical data, scoring, and measurement data of all patients as well as of all four subgroups are presented in (Table 1).
Table 1. Demographic, clinical and gait analysis data of all patients and all 4 patient subgroups. SD-group = subgroup of patients with a duration <4 months after stroke; LD = subgroup of patients with a duration >= 4 months after stroke; RS-group = subgroup of right-side affected patients; LS-group = subgroup of left-side affected patients; f = female; m = male; abs (DUTAS) = absolute value of the deviation angle of the upper trunk from the vertical in the frontal plane.
|
all patients
n=124 |
SD-group
n=94 |
LD-group
n=30 |
RS-group
n=45 |
LS-group
n=54 |
age
(yrs) |
55.1/11.2
26.0-82.0 |
56.8/10.4
30.8-80.3 |
49.8/12.5
26.0-82.0 |
54.5/10.8
27.3-80.3 |
55.6/12.1
26.0-82.0 |
sex (f/m)
percentage of females (%) |
25/99
25.3% |
15/79
19.0% |
10/20
50% |
11/34
32.4% |
10/44
22.7% |
duration since
stroke (months) |
12.1/40.4
0 - 349 |
1.57/0.75
0 - 3 |
45.1/73.6
4 - 349 |
8.0/32.6
0 - 220 |
14.4/48.7
0 - 349 |
% outside
+/-2 degrees range |
36 / 99
36.4% |
26 / 76
34.2% |
10 / 23
43.5% |
16 / 45
35.6% |
20 / 54
37.0% |
abs (DUTAS)
(degrees) |
1.77/1.52
0.11-7.69 |
1.78/1.60
0.20-7.69 |
1.74/1.24
0.11-3.88 |
1.77/1.21
0.11-2.22 |
1.77/1.75
0.18-7.69 |
gait speed
(m/s) |
0.84/0.26
0.19-1.36 |
0.85/0.27
0.19-1.36 |
0.80/0.21
0.44-1.17 |
0.85/0.26
0.33-1.36 |
0.83/0.26
0.19-1.29 |
subscore (iii):
upper trunk posture
n=61 |
1.29/0.55
0.4 – 2.6
n=61 |
1.35/0.58
0.4-2.6
n=45 |
1.14/0.42
0.4-2.0
n=16 |
1.25/0.50
0.4-2.6
n=27 |
1.35/0.57
0.4-2.6
n=34 |
total ReHabX-score
n=61 |
1.10/0.60
0.38-2.70
n=61 |
1.14/0.64
0.10-2.67
n=45 |
0.99/0.46
0.38-2.03
n=16 |
1.06/0.57
0.10-2.22
n=27 |
1.14-0.57
0.4-2.6
n=34 |
Data collection
At first, patients underwent a detailed clinical examination; after that, the gait analysis was performed. Before data collection, an experienced lab technician attached 15 small (2.5 cm diameter), light-weight, reflective markers (Oxford Metrics, Ltd., Oxford, England) to the skin with double-sided adhesive tape according to a standardized protocol [22]. The movement measurement system consisted of eight 50 Hz infrared cameras, two integrated digital video cameras (JVC, Victor, Japan), and the VICON 512 gait system (Oxford Metrics, Ltd. Oxford, England) using Vicon Polygon v. 2.2 software for data collection and reduction. The system was calibrated daily. Foot, lower and upper leg, pelvis, sacrum, Th10 vertebra, C7 vertebra, shoulder, and arm movements were collected bilaterally in the sagittal, frontal, and horizontal plane during at least six complete trials of walking across a 10-meter walkway. Patients walked at their preferred walking speed without any restrictions and without any attempts to hit a particular place on the floor.
Data extraction for “pushing during walking” and measurement of gait speed
Data analysis of the deviation of the upper trunk to the affected side at a time point T of the gait cycle (DUTAS(T)) has been described in detail elsewhere [20]. In short, the vector from the sacrum marker to the C7 marker (rC7) was projected into the frontal (=coronal) plane (frC7) in parallel to the floor. The angle between frC7 and the vertical direction was used to measure the trunk lateral bending in degrees at this time point T (DUTAS(T); see (Figure 1)). The angle was positive when it deviated to the right side and negative if it deviated to the left side. Since neither DUTAS(T) nor gait speed (GS(T)) were equally distributed over a complete gait cycle the mean values of DUTAS(T) and mean values of gait speed (GS(T)) were determined by averaging DUTAS(T) and GS(T) over six complete gait cycles. These mean values were called DUTAS and GS in the following.
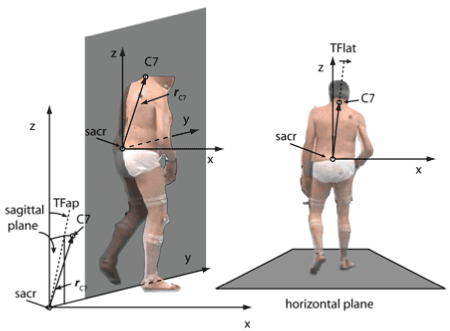
Figure 1. A. A coordinate system (x=direction to the right; y=direction of walking; z=vertical) with the sacrum marker as origin was defined and the vector (rc7) from the sacrum marker (sacr) to the C7-marker (C7) was determined. B. The deviation angle from the vertical at a time T of a gait cycle (TFlat=DUTAS(T)) was the angle between the projection of rc7 into the frontal (x,z)-plane at the time T. It was positive when it deviated to the right side and was negative when it deviated to the left side
Values of DUTAS within the +/- 2 degrees range were classified as normal, values outside this range were classified as abnormal. The +/- 2 degrees range was taken from control experiments on the determination of the subjective veritical (SV) during sitting [16]. For the present study, no control population was analyzed.
Rating of gait abnormalities
Gait abnormalities were scored using the ReHabX-score, which has been described elsewhere [23] and used in a previous study [20]. The ReHabX-score can easily be determined either immediately by inspection of patient´s walking during the gait analysis or later on by inspection of a pair of videos (one from the lateral view, the other from the a/p-view) being recorded during gait analysis. A rater has to score 6 different aspects of spastic gait: (i) abnormalities of the posture of the affected arm, (ii) abnormalities of the movements of the affected leg, (iii) abnormalities of upper trunk posture, (iv) reduction of gait speed, (v) fluency of gait, (vi) suspected risk for falls. It has to be scored whether these abnormalities are absent (score=0), mildly present (score=1), moderately present (score=2) or severely present (score=3). The 6 individual subscores range from 0-3 and are summed up to yield the total ReHabX-score (= sum of the 6 subscores divided by 6; range: 0-3) [23].
In 61 patients, the ReHabX-score was determined. Five experienced raters who were blind to the results of the gait analysis scored 2x61 videos of the patients to determine the ReHabX-score. For each patient, each rater determined all 6 subscores and the total ReHabX-score. For further analysis, the mean subscore (iii) (abnormalities of upper trunk posture) and the mean total ReHabX-score were determined across all five raters.
Statistics
An ANOVA was calculated to study the influence of age, sex, duration since the stroke and side affected on DUTAS and gait speed (GS). Due to a large number of patients, the Pearson product-moment correlation was used. All statistical procedures were part of the commercially available SPSS statistics package (version 25; IBM, Armonk; USA).
Demographical, Scoring and Measurement Data of All Patients and All Four Subgroups
Age distribution (comp. Table 1) of our patient cohort did not differ from other stroke patient cohorts (compare, e.g. Perennou et al. [16]). Only one-fourth of the patients were females. Gait speed covered a wide range (mean: 0.84 m/s; SD: 0.26 m/s; range: 0.19 to 1.36 m/s) as did DUTAS during walking (mean: 1.77 degs; SD: 1.52 degs; range: 0.11 to 7.69 degs) and duration since stroke (mean: 12.1 months; SD: 40.4 months; range: 0.3 to 349 months; see Table 1).
An ANOVA did not show any influence of age, gender, duration since the stroke, and side of the body affected neither on gait speed (GS) nor on DUTAS (see Table 1). The distribution of duration since stroke was not different for the right-side and left-side affected patients (Table 1).
The percentage of patients with DUTAS outside the +/- 2 degrees range was 36.4% in the entire cohort. DUTAS did not differ between the right-side (35.6%) and left-side affected patients (37.0%). There was a tendency to more affected patients in the longer duration (LD-)group compared to the shorter duration (SD-)group (see Table 1), but the ANOVA did not show an influence of duration after stroke on DUTAS.
DUTAS Measured by the VICON-System and scored by means of the ReHabX-score
Scoring of DUTAS during walking appears to be difficult, especially when DUTAS is small (Figure 2A). It has to be kept in mind that the upper trunk is continuously moving during walking and sways back and forth and from right to left. Nevertheless, there was a highly significant (r=.547; p<.0001) correlation between the clinical scoring of abnormalities of upper trunk posture (subscore (iii)) and measurement of the absolute value of DUTAS (abs(DUTAS); Figure 2A). This correlation appears to be higher when the tilt of the upper body exceeds 2 degrees (Figure 2A).
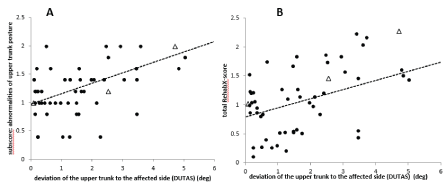
Figure 2. A. There is a highly significant (p<.0001) positive correlation between the deviation of the upper trunk to the affected side (DUTAS) and the subscore “abnormalities of upper trunk position”. Triangles indicate cane users. B. There is a highly significant (p < .001) positive correlation between DUTAS and the clinical scoring of patient´s gait utilizing the mean total RehabX-score. Triangles indicate cane users
The mean total ReHabX-score (averaged over 5 rating experts) also showed a highly significant (r=.453; p<.001) correlation with abs(DUTAS) (Figure 2B), indicating a worse walking ability with an increase of DUTAS. This is in agreement with the fact that persisting pushing during sitting is a predictor for poor outcome after stroke.
Correlation between DUTAS and gait speed
When the upper trunk is bent to the side, gait speed (GS) is reduced. There is a highly significant negative correlation between gait speed and scoring of abnormalities of upper trunk posture (subscore (iii): r=-.471; p<.0001), total ReHabX-score (r=-.684; p<.0001) and abs(DUTAS) (r=-.260; p<.01; (Figure 3A)). The slope of the regression line is steeper when the analysis is restricted to abs(DUTAS)>2 degrees. The +/- 2 degrees range is indicated by two vertical bars in Figure 3A and Figure 3B.
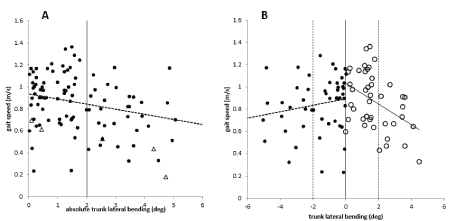
Figure 3. A. There is a significant (p<.01) negative correlation between abs (DUTAS) (x-axis), and gait speed (y-axis) of the entire group of patients. Triangles indicate cane users. B. There is a significant (p<.01) negative correlation between abs (DUTAS), and gait speed in right-side affected patients (open circles) with abs (DUTAS) plotted to the right. There is also a significant (p<.01) negative correlation between abs (DUTAS) and gait speed in left-side affected patients (full circles) with abs (DUTAS) plotted to the left. Triangles indicate cane users. The ± 2 degrees is indicated by 2 vertical bars in Figure 3A and Figure 3B. This was found to be the normal range of the subjective vertical in experiments on pushing during sitting [16]
The correlation between gait speed and abs(DUTAS) appears to be steeper in the right-side affected patients (open circles in (Figure 3B)) than in the left-side affected patients (full circles in Figure 3B), but the difference was not statistically different.
Correlation between DUTAS and gait speed in patients with different durations of the post-stroke period
Duration since stroke did not show a normal distribution, most of the patients (75.8%) had a short duration since stroke (<4 months; open circles in (Figure 4A)). Though the ANOVA did not yield a significant influence of the duration of the post-stroke period on DUTAS, plotting of DUTAS against duration since stroke revealed a tendency to an increase of DUTAS with increasing duration since a stroke in the LD-group (full circles in Figure 4A). We also looked for a difference in the relation between DUTAS and gait speed normalized for leg length (NGS) in the SD- and the LD-groups. The correlation between abs(DUTAS) and NGS tended to be stepper for SD-patients (open circles in (Figure 4B)) compared to LD-patients (full circles in Figure 4B), but there was no significant difference. The negative impact of DUTAS on walking appears to decrease with duration since the stroke.
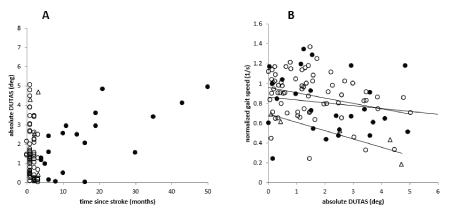
Figure 4. A. The open circles indicate patients with a duration after the stroke of less than four months (SD-group), the full cycles indicate patients with a duration after the stroke of more than four months (LD-group). Some patients suffer from trunk lateral bending already immediately after stroke, whereas in other patients’ trunk lateral bending seems to develop with duration of the post-stroke period. B. There is a significant negative correlation between gait speed and abs(DUTAS) , which is more pronounced in patients with a short duration since stroke (<4 months; open circles) compared to the patients with a longer duration (>=4 months; full circles) since stroke. Triangles indicate cane users
Relevant “pushing during walking.”
Relevant “pushing during walking” (with a mean deviation of the upper trunk to the affected side of more than 2 degrees) is a frequent finding in both left-side affected (37%) and the right-side affected adult post-stroke patients (35.6%). This is in full agreement with the observation that the incidence of pushing during sitting after stroke lies between 8% [11] and 60% [12] depending on the sensitivity of the measurement tools used [10].
The criterion that abs(DUTAS) >2degs is relevant is based on experiments by Perennau et al. [16] demonstrating that in a sitting position the deviation of the subjective vertical (SV) from the vertical varies between -2 and +2 degrees in healthy controls [16]. Similar experiments during walking are not available. Patients adjust their postural vertical (PV) to their subjective vertical (SV). Our observation that during walking right-side affected patients tilt their upper body predominantly to the right side and left-side affected patients to the left side is consistent with the well-known fact that after right-hemisphere stroke SV is tilted counterclockwise, after left-hemisphere stroke clockwise [17,18]. In some patients, the upper trunk was bent to the less affected side. These exceptional cases suffered from a severe paresis of the hip flexors and foot extensors.
A mean tilt of the upper trunk to the affected side (DUTAS) of 2 to 5 degrees (as presented in Figures 2,3, and 4) appears to be small. However, DUTAS(T) considerably varies over the time T of a gait cycle and from trial to trial. Patients with a mean DUTAS around 3 degrees may have a range of DUTAS(T) during a complete gait cycle between -5 to +8 degrees or even more.
A tilt of the upper body to the side of more than 5 degrees may cause considerable balance problems and has to be compensated by a broad-based gait and shift of the lower body to the less affected side. The previously reported highly significant correlation between the shift of the lower body to the less affected side and gait speed [19] nicely matches the correlation between gait speed and tilt of the upper to the affected side in the present study. We, therefore, think that the “adaptive changes of the thorax alignment during walking” do not compensate reduced mobility or faulty muscle control at the hip, knee or ankle” [24], but reflect more general difficulties in keeping the postural vertical within normal limits. Pushing during sitting is probably the severe end and lateral deviation of the upper trunk to the affected side during walking the mild end of a continuum of deficits resulting from a right-hemisphere motor extinction syndrome [9].
Reduction of gait speed with DUTAS
A tilt of the upper trunk to the affected side shortens the ipsilateral hip flexors and reduces their force production, which results in the inability of the patient to support themselves. With the increase of DUTAS, the difficulty to transport the affected leg forward increases and gait speed declines. In this case, the ability of the patient to walk for long distances, and their speed is reduced significantly. This correlation between gait speed and DUTAS (Figure 3A) was observed in left-side affected (full circles in Figure 3B) and right-side affected patients (open circles in Figure 3B) and in patients with a short duration (open circles in Figure 4B) and a longer duration after stroke (full circles in Figure 4B). Since the CNS-centers used for the determination of subjective verticals are probably located in the right posterior insular and parietal cortex [21], one might have expected more disturbances in the left-side affected patients. However, we did not succeed to demonstrate a significant difference in the DUTAS/GS-relation between the RS- and LS-group (Figure 3B).
Improvement of pushing during walking
Pushing during sitting spontaneously improves during the first three to four months after an acute stroke [13]. For “pushing during walking,” the situation appears to be less precisely analyzed. DUTAS tended to increase with the duration of the post-stroke period (Figure 4A). Whether this is due to the development of spasticity in trunk or extremity muscles, no analysis has been carried out so far to find out the exact causes. Neither gait speed, nor DUTAS nor the DUTAS/GS-relation showed a significant difference between the SD- and the LD-group.
An easy way to compensate deviation of the upper trunk to the affected side (DUTAS) during walking is the use of a cane [25]. Utilizing a cane in the less affected hand the patient can quickly and reliably push his upper body back to the affected side so that there is no risk to lean the upper body more to the non-affected side. The cane gives the patients extra support, which helps them to balance their bodies. The use of a cane allows the patient to keep his upper body closer to the midline [20]. Similarly, patients keep the upper trunk closer to the midline after injections of botulinum toxin (BTX) into the affected arm [20]. Whether this is a simple biomechanical compensation mechanism or due to the BTX-induced modulation of muscle spindle feedback to the parietal cortex [26] leading to an improved determination of the SV has not been clarified so far. However, this finding may be essential to improve trunk rehabilitation.
“Pushing during walking” appears to be a multifactorial problem. The present cross-sectional study on adult post-stroke in-patients in a rehabilitation clinic documents that “pushing during walking” is present in at least 1/3 of these patients and has a negative impact on walking ability. However, the temporal course of “pushing during walking” and the influence of the development of spasticity and trunk muscle weakness have not been addressed in various ways. Therefore, prospective studies are recommended to analyze the natural history of “pushing during walking” after acute right- and left-hemisphere lesions, to measure the subjective vertical during walking and to record EMG-activity of trunk muscles during walking in parallel.
The authors are grateful to all the members of the ReHaBoard-team for help during the measurement of the patients and fruitful discussions.
This work has been supported by the European Union and the government of Nordrhine-Westphalia: Grant number: Z1104me31c // 005-1111-0056
All Authors have no conflict of interests
- Bakhai A (2004) The burden of coronary, cerebrovascular and peripheral arterial disease. Pharmacoeconomics 4: 11-18. [Crossref]
- Lamontagne A, Stephenson JL, Fung J (2007) Physiological evaluation of gait disturbances post stroke. Clinical Neurophysiology 118: 717-729.
- Said CM, Goldie PA, Patla AE, Sparrow WA, Martin KE (1999) Obstacle crossing in subjects with stroke. Arch Phys Med Rehabil 80: 1054-1059. [Crossref]
- Dean CM, Richards CL, Malouin F (2001) Walking speed over 10 metres overestimates locomotor capacity after stroke. Clin Rehabil 15: 415-421. [Crossref]
- von Schroeder HP, Coutts RD, Lyden PD, Billings E, Jr., Nickel VL (1995) Gait parameters following stroke: a practical assessment. J Rehabil Res and Develop 32: 25-31.
- Lamontagne A, Fung J, McFadyen B, Faubert J, Paquette C (2010) Stroke affects locomotor steering responses to changing optic flow directions. Neurorehabil Neural Repair 24: 457-468. [Crossref]
- Haggard P, Cockburn J, Cock J, Fordham C, Wade D (2000) Interference between gait and cognitive tasks in a rehabilitating neurological population. J Neurol Neurosurg Psychiatry 69: 479-486. [Crossref]
- Davies PM (2000) Out of Line (the Pusher Syndrome). In: Steps to Follow. Springer, Berlin, Heidelberg.
- Punt TD, Riddoch MJ (2002) Towards a theoretical understanding of pushing behaviour in stroke patients. Neuropsychol Rehabil 12: 455-472.
- Verheyden G, Nieuwboer A, Van de Winckel A, De Weerdt W (2007) Clinical tools to measure trunk performance after stroke: a systematic review of the literature. Clin Rehabil 21: 387-394. [Crossref]
- Bohannon RW (1986) The listing phenomenon of hemiplegic patients. Neurology Report 10:43-44.
- Babyar SR, Peterson MG, Bohannon R, Perennou D, Reding M (2009) Clinical examination tools for lateropulsion or pusher syndrome following stroke: a systematic review of the literature. Clin Rehabil 23: 639-650. [Crossref]
- Danells CJ, Black SE, Gladstone DJ, McIlroy WE (2004) Poststroke "pushing": natural history and relationship to motor and functional recovery. Stroke 35: 2873-2878. [Crossref]
- Davies PM (1990) Right in the Middle (Selective Trunk Activity in the Treatment of Adult Hemiplegia). 1 ed: Springer-Verlag Berlin Heidelberg.
- D'Aquila MA, Smith T, Organ D, Lichtman S, Reding M (2004) Validation of a lateropulsion scale for patients recovering from stroke. Clin Rehabil 18: 102-109. [Crossref]
- Perennou DA, Mazibrada G, Chauvineau V, Greenwood R, Rothwell J, et al. (2008) Lateropulsion, pushing and verticality perception in hemisphere stroke: a causal relationship? Brain 131: 2401-2413.
- Brandt T, Dieterich M, Danek A (1994) Vestibular cortex lesions affect the perception of verticality. Ann Neurol 35: 403-412. [Crossref]
- Yelnik AP, Lebreton FO, Bonan IV, C Colle FM, Meurin FA, et al. (2002) Perception of Verticality After Recent Cerebral Hemispheric Stroke. Stroke 33: 2247-2253. [Crossref]
- Tyson SF (1999) Trunk kinematics in hemiplegic gait and the effect of walking aids. Clin Rehabil 13: 295-300. [Crossref]
- Hefter H, Rosenthal D (2017) Improvement of upper trunk posture during walking in hemiplegic patients after injections of botulinum toxin into the arm. Clin Biomech (Bristol, Avon) 43: 15-22. [Crossref]
- Rousseaux M, Braem B, Honore J, Saj A (2015) An anatomical and psychophysical comparison of subjective verticals in patients with right brain damage. Cortex 69: 60-67. [Crossref]
- Westhoff B, Hirsch MA, Hefter H, Wild A, Krauspe R (2004) Wie reliabel sind Informationen aus der 3D-Ganganalyse? Sportverletz Sportschaden 18: 76-79.
- Ferreira P, Raab D, Rosenthal D, Sielbler M, Hefter H, et al. (2015) Numerical evaluation of stroke patients gait disorders based on instrumental motion analysis. Paper presented at: Biomechanics Portuguese.
- Perry J, Burnfield JM (2010) Gait Analysis: Normal and Pathological Function. J Sports Sci Med 9: 353-353. [Crossref]
- Kuan TS, Tsou JY, Su FC (1999) Hemiplegic gait of stroke patients: the effect of using a cane. Arch Phys Med Rehabil 80: 777-784. [Crossref]
- Ceballos-Baumann AO, Passingham RE, Warner T, Playford ED, Marsden CD, et al. (1995) Overactive prefrontal and underactive motor cortical areas in idiopathic dystonia. Ann Neurol 37: 363-372. [Crossref]




