Objective: The occurrence of breakthrough pain (BTP) in cancer patients is associated with a poor prognosis and a negative impact on their quality of life (QoL). Their symptom profile and how adequate symptom control influences QoL is not known.
The study objective was to assess symptom control in cancer patients with BTP attending the palliative care unit (PC) and its relationship to QoL.
Methods: A four-week observational study in patients aged over 18 years with cancer, BTP and Karnofsky over 30. Symptoms were assessed using the Edmonton Symptom Assessment Scale (ESAS). At baseline and at the end of the study the EuroQoL-5D-5L was completed.
Results: The study included 80 patients, 78.8% males, with a mean age of 69.9 years. A significant improvement was seen in all symptoms at the end of the study (p=0.001). In 7 of 10 symptoms improved symptom control (ESAS≤4) between 48 hours and two weeks (p<0.01). The proportion of patients with all ESAS symptoms controlled improved from 2.5% (n=2) to 52.6% (n=40) at the end of the study. Pain reduction was the only symptom able to improve QoL independently (p=0.031). Control of the 10 ESAS symptoms improved QoL 11.5 mm (95% CI 1.3-21.8; p=0.028).
Conclusions: Pain is the symptom requiring priority treatment, since improves QoL independently. Adequate control of most symptoms was achieved in less than two weeks, after patient referred to our PC unit. The patient quality of care objective in our department will be to achieve control of the 10 ESAS symptoms in at least 52.6% of patients in one month.
breakthrough pain, cancer, palliative, symptom management
Cancer patients referred to palliative care (PC) unit usually experience multiple symptoms that must be characterized and successfully managed. Symptoms might be affected by many factors: location of cancer, histological type, extension -both locoregional and distant-, presence of comorbidities, toxicity due to the treatment itself, etc. [1].
Pain has been reported in 35-96% of cancer patients [1]. Additionally, as cancer progresses, patients may also experience acute episodes of pain, independent from background pain, which due to their characteristics have been recognized as a specific clinical entity called breakthrough pain (BTP). Portenoy et al. defined BTP as “a transient exacerbation of pain that occurs either spontaneously, or in relation to a specific predictable or unpredictable trigger, despite relatively stable and adequately controlled background pain” [2]. These episodes are severe, short-lasting, usually less than 60 minutes, and may occur on average about three to five times a day [3,4].
BTP is present in up to 66% of cancer patients, is common in patients in advanced stages of the disease and is associated with a poor prognosis [3-5]. BTP has a significant impact on patient quality of life (QoL) and is likely to demand more healthcare resources [6-8].
In addition to pain, patients have numerous symptoms, related or not to cancer or derived from their treatment, which impact their general condition and whose adequate control may improve their QoL [9]. It is not known whether cancer patients with BTP have a different profile in terms of frequency and severity of their symptoms, and the impact of their control on patient QoL is also unknown.
Symptom prevalence studies have a number of difficulties for their comparability, such as variability in symptom definition, use of different measurements and scales, inclusion of different types of cancer at different stages, the type of professional collecting the information, and the care setting [1]. For these reasons, it is important to have validated tools for symptom assessment that allow for uniform monitoring of patients attending our clinics, and therefore for adequate quality control in their care.
PC has experienced a significant advance in recent years, however, there are still areas susceptible of improvement [9]. To achieve these improvements assessment and monitoring of the quality of care provided, the healthcare services and the health programs through a systematic methodology is required and proactive attitude of the whole team. Based on this idea, the primary objective of this study was to assess the quality of symptom care based on symptom control in cancer patients with BTP referred to our PC unit. Adequate symptom control was considered a quality indicator of patient care. We also evaluated improvement of patients’ symptoms, and time required to achieve this improvement. As a secondary objective, the impact of symptom control on patient QoL during four weeks of follow-up was analyzed.
Study design and ethical standards
A four-week prospective follow-up observational study was conducted in the Palliative Care Unit of Complejo Hospitalario de Orense. Patients completed four visits: baseline, 48 hours, 2 weeks and 4 weeks.
The study was authorized by the Spanish Agency for Medicinal Products and Medical Devices and the Regional Research Ethics Committee of Galicia (2017/132).
Selection criteria
Patients had to meet the following criteria: 1) men or women over 18 years of age; 2) with a history of cancer; 3) first consultation for occurrence of BTP; 4) Karnofsky score over 30; 5) written informed consent for participation in the study. Patients with cognitive impairment that did not allow them to complete the study scales were excluded.
Davies algorithm was used to make the differential diagnosis of BTP [10]. BTP was defined as “a transient exacerbation of pain that occurs either spontaneously, or in relation to a specific predictable or unpredictable trigger, despite relatively stable and adequately controlled background pain” [2]
Participants were consecutively selected from patients attending the clinic between 3-4-2017 and 21-5-2018 who met the screening criteria. No study-specific treatment was administered.
Study variables
Information was recorded on: year of birth, sex, socioeconomic level (low: incomes less than 2/3 of average salary; middle: incomes between 2/3 and twice the average salary; or high: incomes higher than twice the average salary), weight, height, medical history, type of cancer, date of diagnosis, and presence of metastases.
Patient performance status was assessed using the Karnofsky performance scale, with scores ranging from 0 to 100 indicating lower to higher functional capacity, and survival prognosis using the Palliative Prognostic Score (PaP score) [11-13].
Baseline dyspnea was assessed using the Mahler Baseline Dyspnea Index (BDI) containing three subscales: magnitude of task, functional impairment, and magnitude of effort. Each subscale is rated in five grades (0-4), with the total sum ranging from 0 to 12 points, where the lower the score the greater the severity of dyspnea [14].
The number of daily BTP episodes, their mean duration (minutes), and their characteristics were recorded (Table 1).
Table 1. Characteristics of breakthrough pain in study patients
Description |
n |
% |
|
Location of BTP
N=65 |
Head |
1 |
1.5 |
Neck |
7 |
10.8 |
Shoulder |
5 |
7.7 |
Arm |
0 |
0 |
Chest |
23 |
35.4 |
Abdomen |
21 |
32.3 |
Hip |
7 |
10.8 |
Leg |
1 |
1.5 |
|
Form of onset
N=80 |
Gradual |
40 |
50 |
Sudden |
40 |
50 |
|
Severity
N=80 |
Mild |
0 |
0 |
Moderate |
4 |
5 |
Severe |
55 |
68.8 |
Unbearable |
21 |
26.3 |
|
Is the pain intensified by any event?
N=76 |
No, it is spontaneous |
49 |
64.5 |
Yes |
27 |
35.5 |
|
When does it predominantly occur?
N=79 |
At night |
7 |
8.9 |
During the day |
26 |
32.9 |
Unrelated |
46 |
58.2 |
|
How does the pain develop?
N=78 |
It is unpredictable |
57 |
73.1 |
It is predictable |
21 |
26.9 |
|
Type of pain
N=80 |
Somatic |
28 |
35 |
Visceral |
22 |
27.5 |
Neuropathic |
2 |
2.5 |
Mixed |
28 |
35 |
Clinical information was collected from the following scales at all visits:
1) Edmonton Symptom Assessment System (ESAS) is a validated self-report measure tool for the evaluation of severity of 10 common symptoms experienced in the last 24 hours: pain, fatigue, nausea, depression, anxiety, drowsiness, appetite, well-being, dyspnea and insomnia were rated on 10-point individual visual analog scales (VAS) [15,16]. Adequate control of symptoms was considered to be met when symptom scores were less than or equal to 4 out of 10 points, where 10 points represented the highest intensity of symptoms and 0 represented the absence of symptoms [17].
2) Borg Scale was used to assess dyspnea severity. This is a VAS ranging from 0 (none) to 10 (worst) points that also presents descriptors associated with several of the categories of breathlessness [18].
3) Bristol Stool Form Scale, to explore the occurrence or course of constipation. This is a visual table that classifies the form of stool into seven groups: Types 1 and 2 represent hard stools, slow transit (constipation); types 3 and 4 are soft stools, regular transit; types 5, 6, and 7 are mushy or watery stools, very rapid transit (diarrhea) [19].
At baseline and at 4-week visit, information was collected from:
1) Hospital Anxiety and Depression Scale (HADS): The scale consists of two subscales: the anxiety subscale and the depression subscale, each of one assessed by seven items. Each item is rated on four points with values from 0 to 3. Total score for each subscale ranges from 0 to 21 points. A score between 0 and 7 indicates normality, between 8 and 10 points suggests a borderline case, and over 11 points suggests the presence of a mood disorder [20,21].
2) EuroQoL Quality of Life (EuroQoL-5D-5L), for the assessment of health status at the time of the interview. This is a generic questionnaire describing five dimensions (mobility, self-care, daily activities, pain/discomfort, and anxiety/depression) with five possible levels (no problems, slight problems, moderate problems, severe problems and extreme problems) and a patient self-rated overall health with a VAS (from 0 -“The worst health you can imagine” to 100 mm -“The best health you can imagine”) [22,23].
Treatments administered to the patients during the study were recorded.
Statistical analysis
The primary study endpoint was to assess the differences in the proportion of patients with all ESAS symptoms under adequate control, between initiation of the study and after one-month follow-up. With a sample of 80 patients, a power of 85.8% was achieved to show differences of 15%, with a two-sided alpha error of 0.05 (Sample Power SPSS).
A descriptive analysis was performed measuring frequencies and percentages for qualitative variables and the mean, standard deviation, minimum and maximum values and 95% confidence intervals for quantitative variables. Comparisons between variables were performed using the Fisher or Chi-squared tests for qualitative variables and using Student’s t-test or Mann Whitney U test for comparisons of independent groups for quantitative variables. The analysis of variance model was applied for comparisons in quantitative variables between patient groups or for repeated measures, using Bonferroni or Games-Howell corrections for control of multiple comparisons error, depending on homogeneity of variances. An exploratory linear regression analysis was performed to analyze the relationship between changes in QoL and symptom control and course. The level of statistical significance was established at 0.05. Statistical analysis was performed using SPSS 25.0.
A total of 80 patients were included in the study. Of them, 95% (n=76) completed four weeks of follow-up. Four patients died due to disease progression: one patient between 48 hours and two weeks, and three patients between two and four weeks.
Table 1 describes the main characteristics of BTP. The mean number of BTP episodes per day was 3.7 (95% CI 3.2-4.2), ranging from 1 to 10, with a mean duration of 29.3 minutes (95% CI 23.9-34.7), ranging from 0.5 to 120 minutes. Naïve BTP patients with the first episode of BTP at study entry were 71.8% (n=56). Table 2 shows the sociodemographic and clinical data of the patients. Table 3 shows data on cancer characteristics and patient performance status and prognosis.
Table 2. Sociodemographic and clinical data of study patients
Sociodemographic data n=80 |
Sex: n (%) |
Male |
63 (78.8) |
Female |
17 (21.3) |
Age: mean (95% CI) |
|
69.9 (67.1-72.8) |
Socioeconomic level: n (%) |
Low |
23 (29.5) |
Medium |
37 (47.4) |
High |
18 (23.1) |
Unknown |
2 (2.5) |
Medical history data n=80 |
Concomitant illness on current treatment: n (%) |
No |
18 (22.5) |
Yes |
62 (77.5) |
Patients with the following associated diseases in treatment at baseline, n (%) (One or more each patient) |
Respiratory |
15 (18.8) |
Cardiovascular |
40 (50) |
Gastrointestinal |
3 (3.8) |
Genitourinary |
9 (11.3) |
Musculoskeletal |
3 (3.8) |
Neurological |
3 (3.8) |
Endocrine |
27 (33.8) |
Hematological |
1 (1.3) |
Dermatological |
1 (1.3) |
Psychiatric |
3 (3.8) |
Surgical |
2 (2.5) |
Allergy |
0 (0) |
Mahler baseline dyspnea index: mean (95% CI) |
Dimension A - Magnitude of Task (0-4) |
3.4 (3.2-3.6) |
Dimension B - Functional Impairment (0-4) |
3.3 (3.1-3.5) |
Dimension C - Magnitude of Effort (0-4) |
3.3 (3.1-3.5) |
Total score (0-12) |
9.98 (9.3-10.6) |
Body mass index classification: n (%) |
Cachexic BMI <20 kg/m2 |
6 (7.5) |
Normal BMI >=20 and <25 kg/m2 |
32 (40) |
Overweight BMI >=25 and <30 kg/m2 |
34 (42.5) |
Obese BMI >=30 kg/m2 |
8 (10) |
Table 3. Cancer characteristics and performance status at study entry
Cancer characteristics |
Cancer type: n (%), n=80 |
Breast |
3 (3.8) |
Gynecological |
4 (5) |
Prostate |
3 (3.8) |
Urological |
5 (6.3) |
Lung |
35 (43.8) |
Gastrointestinal |
19 (23.8) |
Otorhinolaryngologic |
7 (8.8) |
Hematological |
2 (2.5) |
Cutaneous |
2 (2.5) |
Time since cancer diagnosis in months: mean (95% CI), n=76 |
|
20.2 (15.2-25.2) |
Current cancer therapy: n (%), n=80 |
No |
55 (68.8) |
Yes |
25 (31.3) |
Metastasis: n (%), n=80 |
No |
10 (12.5) |
Yes |
70 (87.5) |
Karnofsky Performance Status: n (%), n=80 |
30 |
2 (2.5) |
40 |
2 (2.5) |
50 |
9 (11.3) |
60 |
15 18.8) |
70 |
19 (23.8) |
80 |
19 (23.8) |
90 |
10 (12.5) |
100 |
4 (5) |
PaP-Score (probability of surviving at 30 days): n (%), n=80 |
>70% |
60 (75) |
30-70% |
20 (25) |
Symptom assessment using the ESAS scale
Symptoms at baseline: All patients had two or more symptoms (VAS score >0) at baseline and 75% (n=60) had more than five symptoms. The mean number of symptoms present at baseline was 7.1 (95% CI 6.6-7.5), ranging from 2 to 10 symptoms. Pain was present in 97.5% (n=78) of patients; fatigue in 95% (n=75); nausea in 38.7% (n=31); depression in 73.3% (n=59); anxiety in 65% (n=52); drowsiness in 32.5% (n=26); loss of appetite in 85% (n=68); malaise in 100% (n=80); shortness of breath in 46.2% (n=37); and difficulty sleeping in 72.5% (n=58).
Mean score: Figure 1 shows the change in the scoring of the 10 ESAS symptoms from baseline to 4 weeks. Significant improvements were seen in the mean scores of all symptoms between the baseline and the last visit at 4 weeks (p=0.001), except for nausea (p=0.213), drowsiness (p=0.242), and shortness of breath (p=0.939).
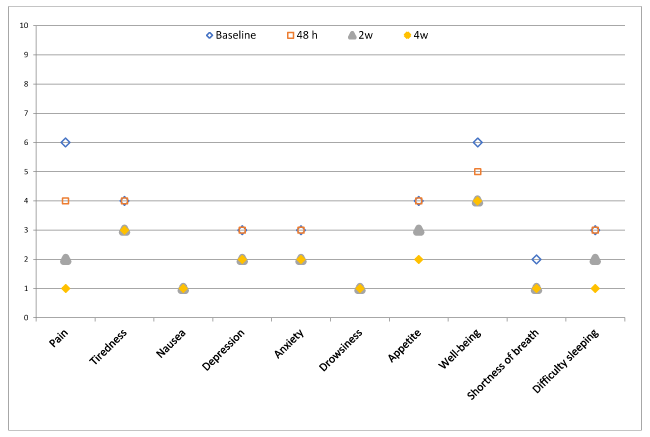
Figure 1. Mean symptom severity assessed by the ESAS scale (0-10 points) from baseline to 4 weeks of follow-up
Proportion of patients with adequate symptom control: Figure 2 shows the change in the proportion of patients in whom symptom control was achieved (VAS≤4) at each follow-up visit. Mean number of symptoms controlled at baseline was 6.2 (95% CI 5.6-6.7), ranging from 1 to 10. The proportion of patients with control of pain, nausea, and appetite had already significantly increased at 48 hours (p<0.001). The proportion of symptom control significantly improved at two weeks in depression (p=0.0001), anxiety (p=0.002), well-being (p=0.002) and difficulty sleeping (p=0.003). The proportion of patients with each symptom controlled increased significantly from baseline to the end of the study at 4 weeks (p=0.001) in pain, nausea, depression, anxiety, appetite, well-being, and difficulty sleeping. The proportion of patients with symptom control did not change in fatigue (p=0.344), drowsiness (p=1.0) or shortness of breath (p=1.0) from baseline to the end of the study at 4 weeks.
Control of all symptoms significantly changed from baseline to the end of the study (p=0.0001). Control of all symptoms was observed in 2.5% (n=2) of the patients at baseline, 15% (n=12) at 48 hours, 39.2% (n=31) at 2 weeks, and 52.6% (n=4) of the patients at 4 weeks (Figure 2).
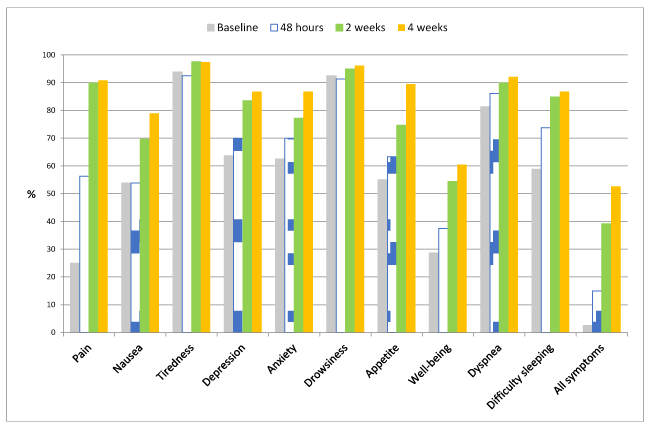
Figure 2. Proportion of patients with adequate control of each symptom (VAS score ≤4) and of all symptoms at each follow-up timepoint
Change in dyspnea
Dyspnea severity as assessed by the Borg scale did not change significantly.
Change in constipation
Of note, the worsening of constipation observed within the first 48 h of the study. Thus, the Bristol constipation scale score decreased significantly (harder stools) at 48 hours (p=0.032) and was no longer modified during follow-up with an initial score of 2.7 (95% CI 2.4-3) and 2.3 (95% CI 2-2.5) at 48 hours. This could be explained by the adjustment and increase in opioid doses required by these patients for more adequate pain control, together with the latency period inherent to the laxative drugs used, with a mean of 48-72 hours.
Change in HADS anxiety and depression scale
The anxiety score improved significantly from baseline to the end of the 4 weeks follow-up period (p=0.001), from 11.1 points (95% CI 9.6-12.6) to 8.7 points (95% CI 7.3-10.1), with a difference of 2.4 points (95% CI 1.2-3.6).
The depression score improved significantly from baseline to the end of the 4 weeks (p=0.001), from 13.2 points (95% CI 11.6-14.8) to 10.4 points (95% CI 8.9-12), with a difference of 2.7 points (95% CI 1.4-4.1).
Change in EuroQoL-5L scale
A statistically significant improvement was seen in the EuroQoL VAS score from baseline to the end of the 4 weeks (p<0.0001). Baseline score was 42.4 (95% CI 36.5-48.3) and final score was 52.7 (95% CI 46.2-59.1), improving 10.2 points (95% CI 4.4-16). Differences greater than 12 mm (95% CI 9-15) are considered clinically relevant in the 100 mm VAS. The result was therefore clinically relevant [24]. Scores on the five dimensions of the EuroQOL-5D-5L were also significantly improved: mobility (p=0.004), self-care (p=0.045), daily activities (p=0.004), pain-discomfort (p=0.001), and anxiety-depression (p=0.001).
No differences were seen in QoL changes depending on type of cancer.
The impact of symptom control in the quality of life of the patients
An exploratory multivariate analysis was performed to study factors related to the change in EuroQoL VAS QoL score and symptoms as assessed by the ESAS scale, controlled by age, sex, and Karnofsky at baseline. The increase in the QoL score from the start of the study to 4 weeks was calculated. A positive score was an improvement in QoL, and a negative score was a worsening. Table 4 shows the regression equations analyzed.
Table 4. Exploratory linear regression equation of the relationship between the increase in EuroQol-5D-5L visual analogue scale quality of life score and the control of each ESAS symptom
|
Equation A |
|
Equation B |
Variable |
B (95% CI) |
p |
Variable |
B (95% CI) |
p |
|
(Constant) |
-20.4 (-58.6-17.8) |
0.291 |
(Constant) |
-38.8 (-74.7--2.8) |
0.035 |
|
Sex (female vs male) |
11.6 (-0.4-23.6) |
0.059 |
Sex (female vs male) |
13.9 (3-24.7) |
0.013 |
|
Age |
0.1 (-0.3-0.5) |
0.495 |
Age |
0.011 (-0.3-0.4) |
0.951 |
|
Karnofsky |
0.2 (-0.2-0.5) |
0.334 |
Karnofsky |
0.001 (-0.3-0.3) |
0.994 |
|
Control of all symptoms at 4 weeks |
11.5 (1.3-21.8) |
0.028 |
No. of symptoms controlled at 4 weeks |
5 (2.8-7.2) |
<0.0001 |
|
|
|
Equation C |
|
Equation D |
Variable |
B (95% CI) |
p |
Variable |
B (95% CI) |
p |
|
(Constant) |
-43.2 (-88.2-1.8) |
0.060 |
(Constant) |
-13.7 (-52.3-24.9) |
0.480 |
|
Sex (female vs male) |
14.6 (2.9-26.4) |
0.015 |
Sex (female vs male) |
9 (-2-20) |
0.107 |
|
Age |
0.095 (-0.3-0.5) |
0.627 |
Age |
-0.02 (-0.4-0.4) |
0.933 |
|
Karnofsky |
-0.01 (-0.3-0.3) |
0.937 |
Karnofsky |
0.04 (-0.2-0.3) |
0.768 |
|
Controlled pain* |
-4.2 (-23.1-14.8) |
0.662 |
r Pain |
2.1 (0.2-4.1) |
0.031 |
|
Controlled nausea* |
-1.6 (-17-13.8) |
0.836 |
r Nausea |
2.9 (-0.3-6) |
0.076 |
|
Controlled fatigue* |
20 (-19.2-59.1) |
0.312 |
r Tiredness |
1.2 (-0.9-3.3) |
0.246 |
|
Controlled depression* |
14.6 (-5.1-34.4) |
0.144 |
r Depression |
-1 (-5-3.1) |
0.639 |
|
Controlled anxiety* |
-0.3 (-27.2-26.5) |
0.981 |
r Anxiety |
1 (-2.7-4.6) |
0.595 |
|
Controlled drowsiness* |
-16 (-43.5-11.5) |
0.249 |
r Drowsiness |
-0.2 (-3-2.6) |
0.877 |
|
Controlled appetite* |
10 (-9.7-29.7) |
0.316 |
r Appetite |
1.1 (-0.7-2.9) |
0.232 |
|
Controlled well-being* |
8 (-4.5-20.5) |
0.205 |
r Well-being |
3.2 (-0.5-6.9) |
0.085 |
|
Controlled dyspnea* |
24.1 (-1.8-49.9) |
0.067 |
r Dyspnea |
0.8 (-2-3.6) |
0.560 |
|
Controlled difficulty
sleeping* |
-5.5 (-26.9-16) |
0.612 |
r Difficulty
sleeping |
-8.9 (-3.3-1.5) |
0.470 |
|
*A symptom is considered controlled if the visual analogue scale score for the symptom is less than or equal to 4 points;r: Increase 4 weeks - baseline.
Equation A: Dependent variable: Increase in EuroQoL-5D-5L visual analog scale score 4 weeks - baseline (B); analysis of patients with all 10 symptoms controlled versus patients with less than 10 symptoms controlled at 4 weeks.
Equation B: Dependent variable: Increase in EuroQoL-5D-5L visual analog scale score 4 weeks - baseline (B); analysis of number of symptoms controlled (VAS ≤4) at 4 weeks.
Equation C: Dependent variable: Increase in EuroQoL-5D-5L visual analog scale score 4 weeks - baseline (B); analysis of control of each symptom (VAS≤4) at 4 weeks.
Equation D: Dependent variable: Increase in EuroQoL-5D-5L visual analog scale score 4 weeks - baseline (B); analysis of increase of each symptom score (VAS 0 to 10) from baseline to 4 weeks. |
|
The control of all ESAS symptoms is associated with higher improvement of quality of life: A statistically significant relationship (p=0.028) was observed between the improvement of the QoL and having all 10 ESAS symptoms controlled, regardless of patient’s age, sex, and Karnofsky at baseline (Table 4, Equation A). Patients who had all 10 symptoms controlled improved 11.5 mm more (95% CI 1.3-21.8) than patients with less than 10 symptoms controlled. The improvement of QoL observed was considered clinically significant [24].
How many ESAS symptoms should be controlled to improve quality of life? Data show that the greater the number of controlled symptoms, the higher the QoL improvement (p=0.0001), regardless of age, sex and Karnofsky status at study start (Table 4, Equation B).
For each symptom controlled, QoL improved 5 mm (95% CI 2.8-7.2), on the QoL VAS score. Therefore, for a clinically significant improvement of QoL at least two symptoms should be controlled (5 mm x 2 = 10 mm difference) [24].
Furthermore, symptom control improved QoL more in women than in men (p=0.013), regardless of the number of symptoms controlled, patient age or Karnofsky score at study start. The difference in the QoL scale between men and women was 13.9 mm (95% CI 3-24.7), which was clinically significant.
Which ESAS symptoms have the greatest impact on quality of life improvement if controlled: No individual symptom, when adequately controlled, significantly improved the QoL score, considering age, sex, and Karnofsky score at study start (Table 4, Equation C).
How much ESAS symptoms score need to increase to improve quality of life? The difference between the final score and the initial score for each ESAS symptom was calculated, and this data was included in the linear regression equation where the dependent variable was the increase in QoL. Patient’s age, sex and Karnofsky score at baseline were included as control variables (Table 4, Equation D).
Pain was the only symptom significantly affecting QoL. For each point (0-10) that the ESAS pain score improved, QoL improved 2.1 mm (95% CI 0.2-4.1) in a statistically significantly manner (p=0.031). Change in other symptoms did not result in significant improvement of the EuroQol VAS QoL score.
Treatment
Treatments were administered according to standard clinical practice depending of the symptoms present in each patient following the best support care principle and the recommendations of the Spanish strategy on palliative care document [25]. A total of 60 different products were managed. For the treatment of background pain 18 different product were administered, being fentanyl (43.7%), morphine (10.9%) and oxycodone (9.3%) the most frequently used. One patient received radiotherapy for the treatment of background pain. BTP was treated with fentanyl in 66 patients (82.5%) and with morphine sulfate in 14 patients (17.5%).
The purpose of this study was to analyze the course of symptom control in patients with cancer and breakthrough pain during the first month of follow-up in the PC unit. This evaluation would allow us to set benchmarks for the quality control process of care for our patients. The study results may serve as a reference for other centers using the same symptoms assessment (ESAS) and quality of life (EuroQoL-5D-5L) scales, already recognized and recommended internationally, whose simplicity is adapted to the needs of PC clinics [15,22,23,25].
It is important to emphasize that patients’ symptoms should be examined systematically, the patient should be asked about them, and their course should also be monitored systematically, since the symptoms evolve over time. Symptom control will improve the experience of the disease for the patient and their prognosis [26].
Although there are numerous validated methods for symptom assessment, it is important to choose the one that is simplest, most comprehensive and useful in clinical practice [27]. In the study, we selected the 10-symptom ESAS, which encompasses the main symptoms experienced by cancer patients. The analog scales used in it make it easy to identify if each symptom is controlled, with no need for any calculation. The improvement that is defined as clinically significant for the 10-point VAS is 1.2 points (95% CI 9-1.5), allowing it to be assessed whether the change from the previous state is adequate [24]. It is used in many clinical studies, so the results can be compared to those of other centers, and its use is free.
On reviewing the main results, upon admission to our department, all patients had more than two symptoms, and 75% had more than five symptoms, in this order of frequency: malaise, pain, tiredness, lack of appetite, depression, difficulty sleeping, anxiety and shortness of breath, nausea and drowsiness. These results are within the range seen in other series, which on the other hand is highly variable due, among other reasons, to the inclusion of different types of patients, different definitions for the symptoms and different management settings [1,28-31].
The mean number of symptoms at baseline was 7.1, and a mean of 6.2 symptoms were adequately controlled before admission to the unit.
The best controlled symptoms at admission to the unit were fatigue, drowsiness, and dyspnea. Control of these three symptoms improved with treatment, although not significantly, since baseline control was already adequate in 80% of patients (Figure 2).
Control of all other symptoms improved significantly, with an increase in pain control, nausea and lack of appetite that was already significant at 48 hours (p<0.001), whereas control of depression, anxiety, malaise and difficulty sleeping improved after two weeks (p<0.01). Malaise was the symptom that was worst controlled, only in 60% of patients (Figure 2).
Only 2.5% of patients had all their ESAS symptoms controlled before admission to the unit. However, in one month of follow-up, 52.6% of patients had all their symptoms controlled. Our goal in the future will be to maintain or exceed this level of symptom control to ensure adequate quality control of patient care.
The improvement in QoL according to the EuroQoL-5D-5L was clinically significant (p<0.0001). In addition, we were interested in knowing how symptom control contributed to this improvement. Thus, we found that patients in whom control of the 10 ESAS symptoms was achieved improved their QoL more than those in whom all symptoms were not controlled. This improvement was clinically significant and independent of patient age, sex and Karnofsky at study start. Given these results, we recommended to pursue and achieve control of all symptoms, in order to obtain a greater improvement of QoL, which, as is known, also reduces patient cost and improves patient prognosis [6,9,26].
When the control of all symptoms it is not possible, we recommend to pursue a higher number of controlled symptoms as possible, since it can also improve QoL, as we observed in this study, bearing in mind that control of at least two additional symptoms resulted in clinically significant improvements in QoL. Pain was the only symptom individually related to an improvement in QoL (p=0.031). Pain control should therefore be a priority objective. In this study 71.8% of patients were included in the study with their first episode of BTP, so 28.2% were on follow-up. This can be the reason why pain was still not controlled at baseline (Figure 2) and the treatment for BTP contributed to the improvement of pain control observed in the following visits.
As study limitations, we did not use a control group of patients in the study, so we do not know whether the changes in symptom control and QoL were entirely due to the performance of the PC unit. Although we found that control of all 10 symptoms improved the patient’s QoL, it is possible that patients in whom control of all symptoms was not achieved were patients with more severe disease as it was not recorded. The equations in Table 4 were only exploratory and the results in equation C and D may be affected by error type I and II or have not enough power to obtain conclusions as the number of observations per variable were less than 20 as the sample size was of 80 patients. Interactions between symptoms were not explored due to this fact. But the procedure of the analysis is correct and is shown as an example of in deep analysis. Also, the participation of a single center could have selection bias as no analysis of number and characteristics of patients screened was completed.
However, we did show that the greater the number of controlled symptoms, the better the improvement in QoL.
As a conclusion to the study, we have shown a significant improvement in control of symptoms of cancer patients with breakthrough pain attending our PC unit. We have observed that pain is the symptom requiring priority treatment, since the impact on the improvement of patient QoL is independent. We have identified the symptoms that are most difficult to manage, as are malaise and nausea, and that require a special care. The support and care of cancer patients is important, as we observed on the improvement of the control of most symptoms (7 of 10) in a short time, between 48 hours and two weeks, after referred to our PC unit. We have also set a benchmark of 52.6% of the patients with the 10 ESAS symptoms controlled in one month of follow-up, which defines the quality of care in our department. This study reinforces the usefulness of the ESAS symptom scale for the quality control of symptom management.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its subsequent amendments or comparable ethical standards.
We thank to Ibone Huerta for the revision of the document.
The study was funded by Kyowa Kirin Farmacéutica, S.L.U. The sponsor was involved in the study design, interpretation of data and in the decision to submit the article for publication.
Antonio Javier Jiménez and Ana Cabezón Álvarez belong to the Medical Department of Kyowa Kirin Farmacéutica, S.L.U.
Begoña Soler was contracted by Kyowa Kirin Farmacéutica, S.L.U. to conduct the design, monitoring, statistical analysis, and management of the publications of the study.
The remaining authors disclose that there have no conflicts of interest with the objectives and results of the study.
Data obtained in the study will be available upon reasonable request.
- Solano JP, Gomes B, Higginson IJ (2006) A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage 31: 58-69. [Crossref]
- Portenoy RK, Hagen NA (1990) Breakthrough pain: definition, prevalence and characteristics. Pain 41: 273-281. [Crossref]
- Escobar Álvarez Y, Biete i Solà A, Camba Rodríguez M, Gálvez Mateos R, Mañas Rueda A, et al. (2013) Diagnóstico y tratamiento del dolor irruptivo oncológico: Recomendaciones de consenso. Rev Soc Esp Dolor 20: 61-68.
- Mercadante S, Marchetti P, Cuomo A, Caraceni A, Mediati RD, et al. (2018) Factors influencing the clinical presentation of breaktrough pain in cancer patients. Cancer 10: E175. [Crossref]
- Deandrea S, Corli O, Consonni D, Villani W, Greco MT, et al. (2014) Prevalence of breakthrough cancer pain: a systematic review and a pooled analysis of published literature. J Pain Symptom Manage 47: 57-76. [Crossref]
- Fortner BV, Demarco G, Irving G, Ashley J, Keppler G, et al. (2003) Description and predictors of direct and indirect costs of pain reported by cancer patients. J Pain Symptom Manage 25: 9-18. [Crossref]
- Velucci R, Mediati RD, Gasperoni S, Piergallini LM, Valentino MC, et al. (2015) Pharmacoeconomic considerations about breakthrough cancer pain. Value in Health 18: A665-A666.
- Pérez-Hernández C, Jiménez-López AJ, Sanz-Yagüe A, Mar-Medina J, Larrañaga I, et al. (2018) Observational study evaluating the economic impact of breakthrough pain in cancer patients in clinical practice in Spain: The IMDI study. Pain Ther 7: 227-240. [Crossref]
- Lorenz KA, Lynn J, Dy SM, Shugarman LR, Wilkinson A, et al. (2008) Evidence for improving palliative care at the end of life: a systematic review. Ann Intern Med 148: 147-159. [Crossref]
- Davies AN, Dickman A, Reid C, Stevens AM, Zeppetella G, et al. (2009) The management of cancer-related breakthrough pain: recommendations of a task group of the science committee of the association for palliative medicine of Great Britain and Ireland. Eur J Pain 13: 331-338. [Crossref]
- Karnofsky D, Burchenal J (1949) The clinical evaluation of chemotherapeutic agents in cancer. In: MacLeod C, ed. Evaluation of chemotherapeutic agents. New York, NY: Columbia university press, pp: 191-205.
- Maltoni M, Nanni O, Pirovano M, Scarpi E, Indelli M, et al. (1999) Successful validation of the palliative prognostic score in terminally ill cancer patients. J Pain Symptom Manage 17: 240-247. [Crossref]
- Pirovano M, Maltoni M, Nanni O, Marinari M, Indelli M, et al. (1999) A new palliative prognostic score: a first step for the staging of terminally ill cancer patients. Italian multicenter and study group on palliative care. J Pain Symptom Manage 17: 231-239. [Crossref]
- Mahler DA, Weinberg DH, Wells CK, Feinstein AR (1984) The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 85: 751-758. [Crossref]
- Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K (1991) The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care 7: 6-9. [Crossref]
- Carvajal A, Hribernik N, Duarte E, Sanz-Rubiales A, Centeno C (2013) The Spanish version of the Edmonton Symptom Assessment System-revised (ESAS-r): First psychometric analysis involving patients with advanced cancer. J Pain Symptom Manage 45: 129-136. [Crossref]
- Oldenmenger WH, de Raaf PJ, de Klerk C, van der Rijt CC (2013) Cut points on 0-10 numeric rating scales for symptoms included in the Edmonton Symptom Assessment Scale in cancer patients: A systematic review. J Pain Symptom Manage 45: 1083-1093. [Crossref]
- Noble BJ, Borg GA, Jacobs I, Ceci R, Kaiser P (1983) A category-ratio perceived exertion scale: relationship to blood and muscle lactates and heart rate. Med Sci Sports Exerc 15: 523-528. [Crossref]
- Lewis SJ, Heaton KW (1997) Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 32: 920-924. [Crossref]
- Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67: 361-370. [Crossref]
- Herrero MJ, Blanc J, Peri JM, De Pablo J, Pintor L, et al. (2003) A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen Hosp Psychiatry 25: 277-283. [Crossref]
- EuroQol Group (1990) EuroQol-a new facility for the measurement of health-related quality of life. Health Policy 16: 199-208. [Crossref]
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, et al. (2011) Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 20: 1727-1736. [Crossref]
- Kelly AM (2001) Setting the benchmark for research in the management of acute pain in emergency departments. Emerg Med (Fremantle) 13: 57-60. [Crossref]
- Estrategia en Cuidados Paliativos (2014) Sistema Nacional de Salud. Madrid: Ministerio de Sanidad y Consumo; 2014. Accessed March 20, 2019. http://www.mscbs.gob.es/organizacion/sns/planCalidadSNS/docs/paliativos/cuidadospaliativos.pdf.
- Ambroggi M, Biasini C, Toscani I, Orlandi E, Berte R, et al. (2018) Can early palliative care with anticancer treatment improve overall survival and patient-related outcomes in advanced lung cancer patients? A review of the literature. Support Care Cancer 26: 2945-2953. [Crossref]
- Kirkova J, Davis MP, Walsh D, Tiernan E, O'Leary N, et al. (2006) Cancer symptom assessment instruments: a systematic review. J Clin Oncol 24: 1459-1473. [Crossref]
- Gómez-Batiste X, Porta-Sales J, Espinosa-Rojas J, Pascual-López A, Tuca A, et al. (2010) Effectiveness of palliative care services in symptom control of patients with advanced terminal cancer: a Spanish, multicenter, prospective, quasi-experimental, pre-post study. J Pain Symptom Manage 40: 652-660. [Crossref]
- Strömgrem AS, Niemann CU, Tange UB, Farholt H, Sonne NM, et al. (2014) Quality of life and symptoms in patients with malignant diseases admitted to a comprehensive cancer centre. Support Care Cancer 22: 1843-1849. [Crossref]
- Tai SY, Lee CY, Wu CY, Hsieh HY, Huang JJ, et al. (2016) Symptom severity of patients with advanced cancer in palliative care unit: longitudinal assessments of symptoms improvement. BMC Palliat Care 15: 32. [Crossref]
- Lavdaniti M, Fradelos EC, Troxoutsou K, Zioga E, Mitsi D, et al. (2018) Symptoms in advanced cancer patients in a Greek hospital: a descriptive study. Asian Pac J Cancer Prev 19: 1047-1052. [Crossref]


